Abstract
Cedar pollens cause severe allergic disease throughout the world. We have previously characterized allergenic pollen glycoproteins from mountain cedar (Juniperus ashei) that bind to allergen-specific immunoglobulin E (IgE). In the present report, we investigated an alternative pathway of mast cell activation by mountain cedar pollen extract through IgE-independent mechanisms. We show that mountain cedar pollen directly induces mast cell serotonin and IL-4 release and enhances release induced by IgE cross-linking. Concomitant with mediator release, high levels of intracellular reactive oxygen species (ROS) were generated, and both ROS and serotonin release were inhibited by anti-oxidants. These findings suggest that alternative mechanisms exist whereby pollen exposure enhances allergic inflammatory mediator release through mechanisms that involve ROS. These mechanisms have the potential for enhancing the allergenic potency of pollens.
Keywords: pollen, cytokines, mast cells, interleukin-4, reactive oxygen species, allergy, serotonin
Introduction
Basophils and mast cells play a central role in inflammatory allergic responses [16;26;30;37]. Mediator release occurs following antigen mediated cross-linking of IgE. Alternative mechanisms have been shown to induce mediator release in mast cells and basophils through IgE independent pathways by N-formyl Met-Leu-Pro (fMLP), phospholipase A2 and complement components, C3a and C5a[10;12;13;17;31;45]. Alternative pathways of activation may contribute to the augmentation of inflammation in a variety of disorders such as autoimmunity and atherosclerosis [9;23].
Reactive oxygen species (ROS), superoxide anions, hydrogen peroxide (H2O2), hydroxyl radicals, and nitric oxide are small diffusible molecules produced by virtually all cell types via membrane NADPH oxidase and mitochondrial pathways with subsequent conversion by Fenton and Haber-Weiss reactions [29]. Previous studies have demonstrated that intracellular reduction-oxidation (redox) reactions participate in mast cell activation leading to mediator release [38;39;42;44;47]. Increased levels of intracellular ROS induced through exposure to exogenous agents may enhance or suppress mast cell mediator release [33;44]. High concentrations of H2O2 (2-10 mM) have been shown to induce or enhance degranulation and IL-4 mRNA expression in mast cells [41;44;46]. However, some studies have shown that low concentrations of H2O2 do not induce degranulation and may, in fact, inhibit degranulation induced by IgE cross-linking [41], while very high levels of H2O2 can have deleterious effects leading to cellular injury [25].
In this report we describe an alternative mechanism of pollen -induced activation of allergic inflammatory responses. We have investigated the effects of intracellular ROS induction by mountain cedar pollen extract on mast cell function. We demonstrate that pollen extracts induce mast cell intracellular ROS and degranulat ion, and enhances IL-4 release induced by IgE-cross-linking. Furthermore, we demonstrate that intracellular ROS and mediator release is inhibited by anti-oxidants and is not dependent on the influx of extracellular calcium.
Materials and methods
Reagents
Bovine serum albumin (BSA), N-acetylcysteine (NAC), tetramethylthiourea (TMTU), and dimethylsufoxide (DMSO), diphenyleneiodonium (DPI), quinacrine, anti-dinitrophenol (DNP) IgE (clone SPE-7) were purchased from Sigma (St. Louis, MO, USA) and DNP-BSA from Biosearch Technologies, Inc. (Novato, CA). Dry pollens of mountain cedar (Juniperus ashei), short ragweed (Ambrosia artemisiifolia), pecan (Carya illinoinensis), pigweed (Amaranthus albus) and Timothy grass (Phleum pretense) were purchased from Hollister-Stier (Spokane, WA).
Cell culture
RBL-2H3 cells (obtained from American Type Cell Collection, ATCC) were cultured in adherent cultures Dulbecco’s Modified Essential Medium supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL, Grand Island, NY, USA) at 37 °C in a humidified atmosphere with 5% CO2. HMC-1 cells (a kind gift of JH Butterfield) were cultured in suspension cultures in Iscove’s Modified Dulbecco’s Medium with 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin, and 1.2mM alpha-thioglycerol.
Determination of cell viability
The trypan blue dye-exclusion method was used to determine cell viability. Briefly, equal volumes of trypan blue solution (0.25% w/v in phosphate buffered saline, PBS) and cell suspension were mixed together, and the cells counted on a hemocytometer. The numbers of unstained live cells and stained dead cells were counted and the cell viability determined. The RBL-2H3 cells used for the present work had >98% viability in all experiments. Incubation of RBL-2H3 cells with pollen extracts had no effect on cell viability.
Preparation of mountain cedar pollen
Mountain cedar pollen was extracted in 0.125 M ammonium bicarbonate (NH4HCO3), 0.05 M PBS, 0.125 M sodium bicarbonate (NaHCO3)or 0.125 M ammonium chloride (NH4CL), pH 8.0 at 4 °C for 48 h. Extracts were clarified by centrifugation at 10,000 rpm at 4 °C for 10 min. Pollen supernatants were stored immediately at −20 °C. Extracts were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the total protein concentration estimated by Coomassie blue staining and densitometry.
For direct pollen grain experiments, a 10 mg/ml suspension of pollen grains in HBSS was prepared and serially diluted. 100 μl of the dilutions were used in the experiments. One hundred μl of a 10 mg/ml suspension contained a total of approximately 3.5 × 105 pollen grains.
Release of biogenic amines
Release of biogenic amines from RBL-2H3 cells was assessed by the release of 3H-serotonin. Using this method, Taurog et al. [40] demonstrated that histamine release closely paralleled serotonin release from mast cells. In our studies, RBL-2H3 cells (1×104) were suspended in 100 μl of culture medium containing 1 μCi/ml of 3H-serotonin and were placed into 96-well flat-bottom micr otiter plates for 18 h at 37°C and 5% CO2. Labeled cells were washed twice with pre-warmed (37 °C) assay buffer, Hank’s buffered salt solution (HBSS, Gibco BRL, Grand Island, NY) with 0.1% BSA, then 40 μl of pollen extract was added to the appropriate wells. To assess mediator release to IgE crosslinking, RBL-2H3 cells were plated as above, incubated for 1 hr with 500 ng/ml anti-DNP IgE, washed, and then DNP-BSA with or without pollen extracts added to the wells. Cells were incubated for 30 min at 37°C and the reaction stopped with 100 μl of cold PBS. Cell supernatants were removed, centrifuged for 5 min to eliminate detached cells, and the radioactivity measured by scintillation spectroscopy (Becton, Dickinson and Company, San Jose, CA). The percent serotonin release was calculated as: [(experimental release − spontaneous release) / Total release] × 100.
As an alternative method to interrogate release of mediators from mast cell granules, β-hexosaminidase release was assessed. In this method, cells were prepared as in the 3H-serotonin assay above. Cells were washed with Tyrode’s buffer pH 7.3 (137 mM NaCl, 5.6 mM glucose, 2.7 mM KCl, 0.5 mM NaH2PO4, 1 mM CaCl2, 10 mM HEPES), followed by incubation in experimental conditions diluted in Tyrode’s buffer. After 30 min at 37°C, the reaction was stopped with an equal volume of ice cold Tyrode’s buffer. The supernatants were harvested and any cells present removed by centrifugation at 100 × g for 5 min. Total release was obtained by treating cells with 1.2% Triton X100 in Tyrode’s buffer. β-hexosaminidase in supernatants was quantified by incubating for 2 h at 37°C with the chromogenic substrate, p-nitrophenyl-N-acetyl-β-D-glucopyranoside. The reactions were stopped by addition of 1 M NaOH and the OD405 was determined on a FLUOstar Optima automated ELISA reader (BMG Labtechnologies, Durham, NC). The percent release was calculated as above.
Assessment of intracellular reactive oxygen species
Changes in intracellular ROS levels were measured using the redox-sensitive dye, 2′,7′-dihydrodichlorofluorescein diacetate (DCFH-DA) (Molecular Probes, Eugene, OR, USA), which is converted intracellularly to (DCFH) and oxidized to the fluorescent dichloroflourescein (DCF) by intracellular H2O2, hydroxyl radical, and peroxynitrite [11;20;22]. RBL-2H3 cells were cultured in 96-well flat-bottomed plates until approximately 70% confluent. Cells were loaded with 10 μM DCFH-DA in HBSS (pH 7.3) for 30 min at 37 °C. After washing two times with PBS, cells were treated with 100 μl mountain cedar pollen extract or with pollen grains diluted in HBSS. HMC-1 cells were loaded with DCFH-DA as above, washed and 1 × 105 cells mixed directly with pollen grains. DCF fluorescence was quantified in an automated fluorometer (Packard Fluorocount™, Packard Bioscience Company, Downer Grove, IL, USA) (excitation at 480 nm and emission at 530 nm) [25,26]. To examine the effects of antioxidants, cells were incubated for one hr with NAC, TMTU and DMSO prior to exposure to pollen extract. Data were expressed as a ratio of fluorescent levels to cells placed in medium alone or in total relative fluorescence units. To assess ROS generation by cedar extract alone, increasing dilutions (1:3, 1:12, 1:48, 1:192 and 1:768) of cedar extract was mixed with 10 μM (final concentration) DCFH-DA in 100 μl (total volume) of HBSS. Changes in DCF fluorescence were determined as above.
RNase Protection Assay
RNase protection assays (RPA) were performed according to the manufacturer’s instructions (RiboQuant™, BD Biosciences Pharmingen, San Diego, CA). Briefly, 106 cells were plated onto 3.5 cm cell culture plates overnight and then stimulated with pollen extracts. Cells were washed with PBS and lysed with TRIzol® reagent (Invitrogen, Carlsbad, CA). After chloroform separation, total RNA was precipitated with ethanol. 1 μg of RNA was incubated for16 h with α-32P labeled RPA probes and then treated with RNase A for 45 min. Protected probes were separated from RNase using proteinase K and phenol-chloroform extraction, precipitated with ethanol, and resolved on a 5% denaturing polyacrylamide gel. Dried gels were exposed to a phosphorescent screen and quantified on a Molecular Imager FX (Bio-Rad Laboratories, Inc., Hercules, CA). Data were expressed as ratios to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Cytokine protein assays
Cytokines released into culture supernatants were quantified using a Bio-Plex Rat Cytokine 9-Plex A Panel multiplex assay (Bio-Rad) to detect IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, GM-CSF, IFN-γ, and TNF-α [14]. In this assay, a mixture of fluorescent microspheres conjugated with a capture antibody is mixed with each sample. A secondary fluorescein isothiocyanate (FITC)-conjugated (detection) antibody reacts with the captured antigen. The samples were then analyzed on a Bio-Plex™ Suspension Array System (Bio-Rad), where they were passed through a detector (flow cytometer), and fluorescence intensity for each bead-type deconvoluted, quantified, and compared to a standard curve for each analyte.
Statistical analysis
For differences between individual groups of data a paired 2-sample for means t test was performed, with a 95% confidence interval (p < .05), or 99% confidence interval (p<.01). Comparisons of ROS after direct pollen treatment in HMC cells were analyzed using a Kruskal-Wallis One Way Analysis of Variance on Ranks with post hoc pairwise comparisons performed using the Student-Newman-Keuls method.
Results
Pollen extracts induce release of biogenic amines in RBL-2H3 cells in the absence of IgE
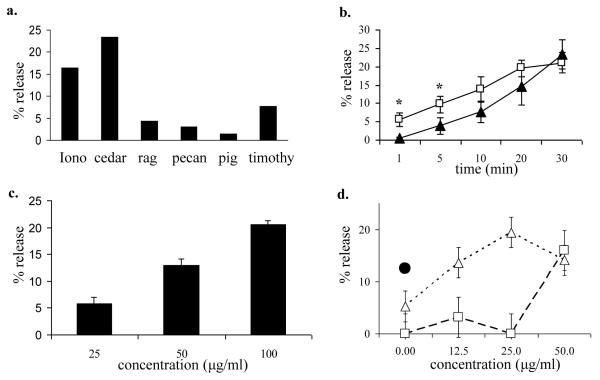
We tested commercial extracts and mountain cedar pollen extracts prepared in our lab for their ability to directly induce biogenic amine release from mast cells using 3H-serotonin (Fig. 1a). Mountain cedar pollen extracted in 0.125M NH4HCO3 buffer (pH 8.0) and standardized to a concentration of 100 μg/ml in NH4HCO3 buffer induced a steady release of serotonin over 30 min (max. 21.1 ± 2.8, n = 3, Fig. 1b) and in a dose-dependent fashion within a range of 25 to 100 μg/ml total protein (Fig. 1c). The total release was similar to that induced by 1 μM ionomycin (max. 23.4 ± 4.0). To assess the interaction between IgE cross-linking and cedar pollen extract, RBL-2H3 cells were sensitized with monoclonal IgE antibodies directed against DNP. Cedar pollen extract in combination with DNP-BSA were additive in their effect on mediator release (Fig. 1d).
Figure 1.
Mast cell serotonin release induced by pollen extract.
a. 3H-serotonin release induced by pollens extracted in NH4HCO3 buffer. Total protein content = 100 μg/ml. Iono = ionomycin (1 μM), cedar = mountain cedar, rag = ragweed, pig = pigweed, and timothy = timothy grass.
b. 3H-serotonin release was determined in RBL-2H3 cells at 1, 5, 10, 20, and 30 minutes after stimulation with cedar pollen extract (open boxes; □) or 1 μM ionomycin (filled triangles; ▲). Data represent the means of 3 independent experiments, * indicates significant differences between ionomycin and cedar extract ( p< .05).
c. 3H-serotonin release was determined in RBL-2H3 cells after incubation with 25, 50, 100, or 200 μg/ml cedar pollen extract for 30 minutes. The results are expressed as mean ± SD (n = 3 separate experiments, each experiment performed in duplicate).
d. 3H-serotonin release in RBL-2H3 cells after exposure to pollen extract plus DNP-BSA 1 ng/ml (open triangles; △) or pollen extract alone (open boxes; □). DNP-BSA 50 ng/ml (filled circle; ●).
Previous work by Schwartz, et al. demonstrated that the majority of β-hexosaminidase is contained within the mast cell granules [36]. To determine if cedar pollen extracts also induced the release of β-hexosaminidase we performed similar experiments as those demonstrating serotonin release. We obtained a similar dose response effect using dilutions of cedar extract, 24.2% release at 1:80 dilution, 22.1% at 1:160, 19.6% at 1:320, 17.4% at 1:640, 13.5% at 1:1280. These data suggest mediators are released, at least in part, from mast cell granules.
Pollen increases ROS levels in mast cells
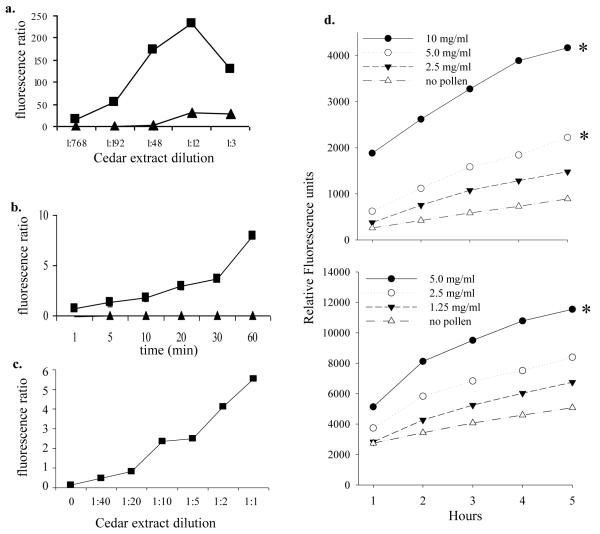
Previous studies have demonstrated the ROS-generating capacity of pollens [5;43]. To determine if cedar extracts possessed inherent ROS-generating capacity, cell-free assays were performed in which cedar pollen extract (prepared in NH4HCO3) were assessed for their ability to directly oxidize DCFHDA (Fig. 2a). The increase in fluorescence indicated an inherent ability of mountain cedar pollen extract to generate ROS capable of oxidizing DCFHDA in the absence of mammalian cellular components or NADPH. To determine if pollen induced intracellular ROS in mast cells, RBL-2H3 cells were loaded with DCFHDA and exposed to pollen grains directly or to pollen extract. Cedar pollen extract induced up to an 8-fold increase in DCF fluorescence (Fig. 2b), and rose with increasing concentrations of pollen extract (Fig 2c). Further, direct application of pollen grains onto RBL-2H3 or the human mast cell line HMC-1 stimulated significant increases in intracellular ROS (Fig. 2d), although the time course was much slower than experiments using pollen extracts. The relative increase in fluorescence was larger in RBL-2H3 cells than HMC-1 but the overall response was similar between the two different cell lines.
Figure 2.
ROS generation by pollen extract
a. Fluorescence of cedar pollen extracts incubated with DCFH-DA for 30 min. Cedar pollen was extracted with either NH4HCO3 (filled boxes; ■) or PBS (filled triangles; ▲).
b. DCF fluorescence of RBL-2H3 cells stimulated with 100 μg/ml pollen extracted in NH4HCO3 buffer (■) or 1 μM ionomycin (▲).
c. DCF fluorescence of RBL-2H3 cells incubated with dilutions of pollen extracts (NH4HCO3 buffer) for 30 min. The specific protein content of pollen extracts was not determined in this experiment, but in subsequent. In general, extracts contained approximately 200-300 μg/ml total protein.
d. DCF fluorescence of HMC-1 cells (top, mean of 2 experiments) or RBL-2H3 cells (bottom, single experiment) incubated with dilutions of mountain cedar pollen grains. Data are expressed as relative fluorescence units with the baseline fluorescence of each condition subtracted from subsequent measures at 1-5 hours. Pollen grains in buffer alone showed high baseline levels of autofluorescence that did not change over time (data not shown). Significant differences from the “no pollen” controls are indicated with an asterisk (*).
Different pollen extraction buffers induce varying levels of serotonin release and ROS generation
To determine if different extraction buffers had any effect on the ability of pollen extracts to induce mediator release, mountain cedar pollen was extracted with various buffers; 0.125M NH4HCO3, PBS, 0.125 M sodium bicarbonate (NaHCO3) or 0.125 M ammonium chloride (NH4CL). Extracts were resuspended in those same buffers at a concentration of 100μg/ml. Figure 3 demonstrates no differences in the pattern of major proteins on SDS-PAGE and Coomassie blue staining. The two most highly stained bands in each extract corresponds to the major allergens Jun a 1 (43-kDa) and Jun a 3 (30-kDa), and correspond to previously characterized proteins in mountain cedar pollen extract [27;28].
Figure 3.
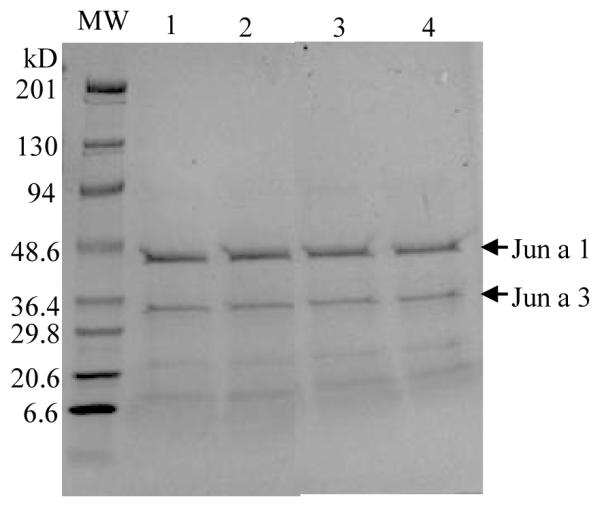
Protein profile of cedar pollen extracts. Cedar pollen extracted in NH4HCO3 (lane 1); NH4Cl (lane 2); NaHCO3(lane 3); or PBS (lane 4) was analyzed by SDS-PAGE stained with Coomassi blue. The major allergens, Jun a 1 (43 kDa) and Jun a 3 (30kDa) (arrows), migrated slightly slower on the gel likely due to carbohydrate moieties.
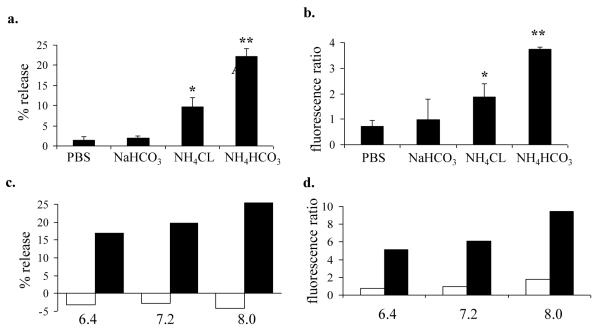
Mountain cedar pollen extracted in different buffers induced divergent mediator release and intracellular ROS. Serotonin release (Fig. 4a) and ROS (Fig. 4b) induced by different buffers followed the pattern of NH4HCO3 > NH4CL > NaHCO3 > PBS (Fig. 4a). Regression analysis between the serotonin release and ROS levels using the different buffers revealed a correlation coefficient, R2, of 99.5%, indicating a very close relationship between the ability to induce both ROS and serotonin release. To establish whether the buffers themselves had an effect on the assays, extracts were dialyzed against PBS to remove excess buffer, and conversely, PBS extracts were dialyzed against NH4HCO3. Neither approach had any effect on enhancing or reducing the ability of extracts to induce degranulation and intracellular ROS production (data not shown).
Figure 4.
Serotonin release and ROS generation in mast cells exposed to cedar pollen extracted in various buffers and pHs. RBL-2H3 cells were incubated with cedar pollen extracts adjusted to 100 μg/ml protein, pH 7.3 for 30 minutes and assessed for 3H-serotonin release with results expressed as percent serotonin release (a. and c.), and DCF fluorescence expressed as mean fluorescence ratios to buffer controls (b. and d.).
a. 3H-serotonin release induced by cedar pollen extracted in PBS, NaHCO3,NH4CL, or NH4HCO3.
* indicates significant difference from PBS (p<.05), ** (p<.01)
b. DCF fluorescence induced by cedar pollen extracted in PBS, NaHCO3, NH4CL, or NH4HCO3.
* indicates significant difference from PBS (p<.05), ** (p<.01)
c. 3H-serotonin release induced by cedar pollen extracted in PBS (open bars) or NaHCO3 (filled bars) at pH of 6.4, 7.2, or 8.0.
d. DCF fluorescence induced by cedar pollen extracted in PBS (open bars) or NaHCO3 (filled bars) at pH of 6.4, 7.2, or 8.0.
When pollen was extracted in NH4HCO3 at various pHs, the serotonin releasing activity and ROS generation were modestly diminished indicating a slight pH dependence (Fig. 4c and 4d). We conclude that the amount of pollen protein(s) responsible for the increases in mast cell activation are likely present in low abundance and may be selectively extractable in different buffers. Alternatively, certain buffers may activate or inactivate pollen components during the extraction process.
ROS generation and degranulation are decreased by antioxidant treatment but not by removing extracellular calcium
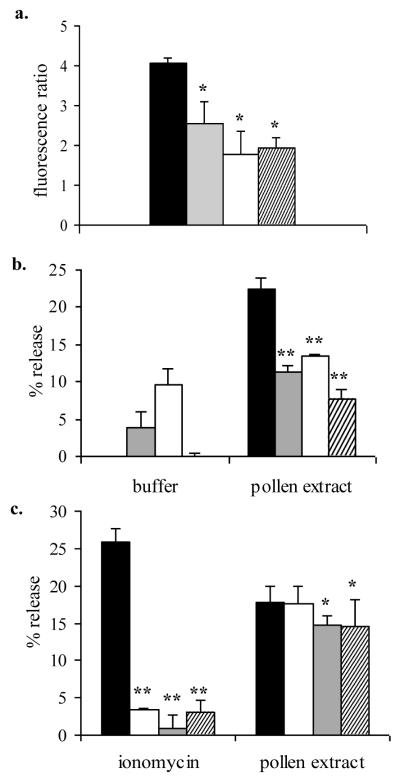
To examine whether the ROS induced by pollen extracts is linked to mediator release, antioxidants NAC (1 to 20 mM), TMTU (0.5 to 20 mM), and DMSO (0.1 to 2%, v/v) were used to inhibit intracellular ROS. Inhibition of ROS generation and degranulation was achieved with 10 mM NAC, 10 mM TMTU and 1% DMSO, which gave a 56.9%, 52.5%, and 37.5% reduction in ROS and 40.2%, 65.7%, and 50.0% reduction in serotonin release, respectively (Fig. 5a and 5b). 100 mM NAC reduced ROS by >99% (data not shown). These results support a role for intracellular ROS in mediator release. Activation of cellular NADPH oxidase and or mitochondrial flavoenzymes was investigated by using the flavoenzyme inhibitors diphenyleneiodonium (DPI) and quinacrine. DPI inhibited DCF fluorescence induced by mountain cedar extract (100 μg/ml) in RBL-2H3 cells by 19.4% at 10 μM and 26% at 100μM and quinacrine inhibited fluorescence by 13.6% at 10μM and 50.9% at 100μM. These data suggest some contribution of NADPH oxidase or mitochondrial flavoenzymes to the generation of ROS.
Figure 5.
Effect of antioxidants and extracellular calcium on serotonin release and ROS generation.
a. and b. RBL-2H3 cells were exposed to either buffer control (filled bars), 1% DMSO (shaded bars), 10 mM NAC (open bars), or, 10 mM TMTU (cross-hatched bars) for 1 hour, washed with medium and then exposed to cedar pollen extract (100 μg/ml protein) for 30 minutes and DCF fluorescence (a.) and 3H-serotonin release (b.) assessed. The results are expressed as mean ± SD (n = 3 experiments). * indicates significant difference (p< .05) from buffer control, ** (p< .01).
c. 3H-serotonin release was assessed in RBL-2H3 cells incubated with ionomycin (1 μM) or 100 μg/ml pollen extract for 30 minutes in the presence or absence of 1.26 mM Ca2+ and/or 5 mM EDTA (indicated below the figure). The results are expressed as mean ± SD (n = 3 experiments).
* indicates significant difference (p< .05) from medium containing Ca2+ and no EDTA, ** (p< .01).
Cross-linking IgE on mast cells leading to degranulation is associated with an influx of extracellular calcium, and is considered a critical mechanism for mediator release. We investigated whether pollen extracts were affected by extracellular calcium depletion. Performing the serotonin release assay in calcium-free medium and/or with addition of ethylene-diamine-tetra-acetic acid (EDTA) to the assay buffer was sufficient to inhibit the serotonin release induced by ionomycin (Fig. 5c), but serotonin release induced by cedar pollen extract was largely unaffected, except that the addition of EDTA caused a slight but statistically significant reduction in release. These data suggest that degranulation induced by pollen extracts largely occurred independently of extracellular calcium influx.
Upregulation of IL-4 mRNA and protein by cedar pollen extract
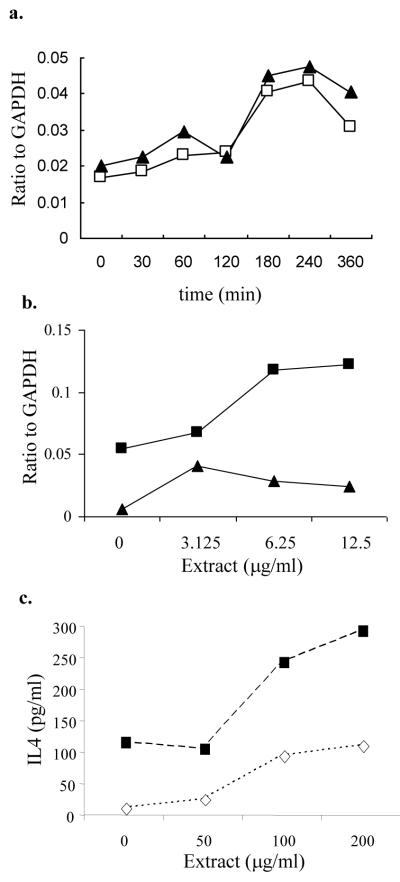
Mast cells have been shown to produce cytokines (IL-4, IL-6, IL-13, TNFα) implicated in the upregulation of immune responses [6;7;18;26;35;46;48]. We used an RNase protection assay to investigate whether cedar pollen extract could activate mast cells to express cytokine mRNA. Stimulation of RBL-2H3 cells for 4 h with cedar pollen extract induced expression of IL-4 and IL-6 mRNA, but there was no change in the basal levels of IL-2, IL-3, IL-8, or TNFα. The combination of pollen extracts with optimal and suboptimal concentrations of DNP-BSA (1 ng/ml) enhanced expression of IL-4 mRNA (Fig. 6b).
Figure 6.
Cytokine expression by mast cells exposed to cedar pollen extract.
a. RBL-2H3 cells were stimulated with 100 μg/ml cedar pollen extracts for 4 hours and IL-4 (open boxes; □) and IL-6 (filled triangles; ▲) mRNA relative to GAPDH was measured by RPA.
b. RBL-2H3 cells sensitized with anti-DNP IgE (500 ng/ml) were incubated for 4 hours with cedar extract alone (filled triangles; ▲) or with suboptimal concentrations of DNP-BSA (1.0 ng/ml) (filled boxes; □). IL-4 mRNA relative to GAPDH was measured by RPA. For reference, 50 ng/ml DNP-BSA stimulated an IL-4/GAPDH ratio of 0.1.
c. RBL-2H3 sensitized with anti-DNP-IgE were stimulated with pollen extract plus DNP-BSA at 1.0 ng/ml (◇; open diamonds) or 50 ng/ml (■; filled boxes). Culture supernatants were collected at 24 hours and analyzed for IL-4 by Bioplex. Culture supernatants from cells stimulate with pollen extract alone had undetectable levels of IL-4.
Cytokine protein production was assessed in culture supernatants from RBL-2H3 cells incubated with pollen extracts for 24 h. IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, GMCSF, IFNγ, and TNFα were assessed. No cytokines were detected when RBL-2H3 cells were incubated with pollen alone. IL-4 was detected (Fig. 6c) when pollen extract was combined with mast cells sensitized with anti-DNP IgE and cross-linked with DNP-BSA. Using both optimal (50 ng/ml) and suboptimal (1 ng/ml) concentrations of DNP-BSA, IL-4 increased with increasing concentrations of pollen extract, reaching levels above that seen with optimal concentrations of DNP-BSA alone.
Discussion
Mast cells and basophils are the primary effector cells in immediate hypersensitivity reactions mediated through IgE cross-linking by specific antigens. Here we present evidence of an alternative pathway of mast cell activation by pollen through an IgE-independent mechanism that involves the simultaneous generation of ROS. Our findings are consistent with previous studies demonstrating the ROS generating properties of pollens [3] and the ability of ragweed pollen extracts to activate mast cells in the absence of IgE [8].
The components within pollens responsible for ROS generation are unknown. Boldogh et al.[5] reported that a plant-derived NADPH oxidase may be responsible. This is consistent with our studies which suggest that ROS are generated in pollen extracts independently from mammalian cells, based on the high levels of DCF fluorescence generated in extracts in the absence of cellular components and NADPH. Additional ROS may be generated within mast cells from cellular NADPH oxidase or mitochondrial stress as evidenced by the partial inhibition by DPI and quinacrine, and as reported by others[8].
In our experiments, pollen extracts activated mast cells in the absence of IgE, but also enhanced suboptimal IgE responses. How these responses interact is unclear, but the generation of intracellular ROS may provide insight. Low levels (1.4 fold increase) of intracellular ROS have been shown to be induced by IgE cross-linking [24]. In our experiments, mountain cedar pollen extracts induced a 2 to 8-fold intracellular elevation in ROS levels. High levels of intracellular ROS appear to be necessary for full degranulation by pollen extracts, lower levels of pollen-derived ROS may synergize with suboptimal antigen concentrations to fully activate mast cell mediator release.
How oxidative species contribute to mast cell activation is not fully understood. In our studies, depletion of extracellular calcium failed to inhibit this response. Activation of inositol 1,4,5-triphosphate, which may be redox sensitive, is central in the release of calcium from intracellular stores [4;19;21]. It is possible that the release of intracellular stores of calcium may be essential in ROS-induced degranulation, and that high levels of ROS induced by pollen is sufficient to activate these pathways. An alternative hypothesis is that intracellular pH balance may be altered by the generation of high levels of intracellular ROS, thus catalyzing the release of intracellular stores of calcium through pH sensitive pathways [1;15].
Our data also supports a role for the pollen-enhanced production of IL-4 at the mRNA and protein levels. Others have shown that IL-4 transcription increases with H2O2 via Ref-1/AP-1 translocation [32]. IL-4 and IL-6 mRNA upregulation in mast cells has also been seen after exposure to diesel exhaust particles and formaldehyde, putatively through oxidant stress pathways [35;42].
In summary, pollens, long thought to induce allergenicity solely through IgE cross-linking of allergenic proteins may provide alternative activating signals through pollen-derived ROS. We conducted our studies with mountain cedar (Juniperus ashei) pollen, which is considered a potent allergen [2;34], and our results suggest that oxidants from mountain cedar pollens may enhance its allergenic potency.
We investigated an alternative pathway of mast cell activation by mountain cedar pollen through IgE-independent mechanisms>Pollen induces oxidative stress, degranulation and cytokine production in mast cells>Pollen enhances IgE-dependent degranulation and cytokine production in mast cells>Antioxidant treatment suppresses the pollen-induced activation of mast cells
Acknowledgments
The authors wish to thank Dr. Chris Collaco for his technical assistance and expert review of the manuscript. This publication was made possible in part by grant number ES06676 from the National Institute of Environmental Health Sciences, NIH, and through support from the Sealy Center for Environmental Health and Medicine at the University of Texas Medical Branch, Galveston, Texas. DH was supported by a predoctoral award from NIEHS T32-07524. TMH was supported by NICHD, sponsored Child Health Research Center New Project Award Grant P30 HD 27841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shuichiro Endo, Department of Otolaryngology-Head and Neck Surgery, University of Yamanashi, 1110, Shimogato, Chuo, Yamanashi, 409-3898 Japan.
Daniel J. Hochman, University of Texas Medical Branch, Graduate School of Biomedical Sciences, 301 University Blvd, Galveston, Texas 77555-1050
Terumi Midoro-Horiuti, University of Texas Medical Branch, Department of Pediatrics, Room 2.300, Clinical and Experimental Immunology and Infectious Disease, 301 University Blvd, Galveston, Texas 77555-0366.
Randall M. Goldblum, University of Texas Medical Branch, Department of Pediatrics, Room 2.300, Clinical and Experimental Immunology and Infectious Disease, 301 University Blvd, Galveston, Texas 77555-0366.
Edward G. Brooks, University of Texas Health Science Center San Antonio, Dept of Pediatrics, Immunology and Infectious Diseases, Rm. 528L-4, 7703 Floyd Curl Drive, MC 7811, San Antonio, Texas 78229-3900.
References
- [1].Alfonso A, Vieytes MR, Botana LM. Calcium-pH crosstalks in rat mast cells: modulation by transduction signals show non-essential role for calcium in alkaline-induced exocytosis. Biochem.Pharmacol. 2005;69:319–327. doi: 10.1016/j.bcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [2].Andre C, Dumur JP, Hrabina M, Lefebvre E, Sicard H. Juniperus ashei: the gold standard of the Cuppressaceae. Allergie et Immunologie. 2000;32:104–106. [PubMed] [Google Scholar]
- [3].Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: Carriers of allergenic proteins and oxidases. J.Allergy Clin.Immunol. 118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- [5].Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J.Clin.Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brown MA, Pierce JH, Watson CJ, Falco J, Ihle JN, Paul WE. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987;50:809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- [7].Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J.Exp.Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chodaczek G, Bacsi A, Dharajiya N, Sur S, Hazra TK, Boldogh I. Ragweed pollen-mediated IgE-independent release of biogenic amines from mast cells via induction of mitochondrial dysfunction. Mol.Immunol. 2009;46:2505–2514. doi: 10.1016/j.molimm.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Christy AL, Brown MA. The Multitasking Mast Cell: Positive and Negative Roles in the Progression of Autoimmunity. J Immunol. 2007;179:2673–2679. doi: 10.4049/jimmunol.179.5.2673. [DOI] [PubMed] [Google Scholar]
- [10].Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127:371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crow JP. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitritein Vitro:Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- [12].da Silva WD, Lepow IH. Complement as a mediator of inflammation: II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967;125:921–946. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dahinden CA. Histamine and sulfidoleukotriene release from human basophils: different effects of antigen, anti-IgE, C5a, f-Met-Leu-Phe and the novel neutrophil-activating peptide NAF. Int.Arch.Allergy Appl.Immunol. 1989;90:113–118. doi: 10.1159/000235011. [DOI] [PubMed] [Google Scholar]
- [14].de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous Detection of 15 Human Cytokines in a Single Sample of Stimulated Peripheral Blood Mononuclear Cells. Clin.Diagn.Lab.Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Smet P, Parys JB, Vanlingen S, Bultynck G, Callewaert G, Galione A, De Smedt H, Missiaen L. The relative order of IP3 sensitivity of types 1 and 3 IP3 receptors is pH dependent. Pflügers Archiv Eur J Physiol. 1999;438:154–158. doi: 10.1007/s004240050893. [DOI] [PubMed] [Google Scholar]
- [16].Ehrlich P. Beitrage zur Kenntniss der granulirten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol. 1879;3:166–169. [Google Scholar]
- [17].Erdei A, Pecht I. Complement peptides and mast cell triggering. Immunol.Lett. 1996;54:109–112. doi: 10.1016/s0165-2478(96)02658-2. [DOI] [PubMed] [Google Scholar]
- [18].Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003;33:2168–2177. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- [19].Guillemette G, Segui JA. Effects of pH, reducing and alkylating reagents on the binding and Ca2+ release activities of inositol 1,4,5-triphosphate in the bovine adrenal cortex. Mol Endocrinol. 1988;2:1249–1255. doi: 10.1210/mend-2-12-1249. [DOI] [PubMed] [Google Scholar]
- [20].Homan-Müller JW. Production of hydrogen peroxide by phagocytizing human granulocytes. The Journal of laboratory and clinical medicine. 1975;85:198–207. [PubMed] [Google Scholar]
- [21].Hong-Geller E, Cerione RA. Cdc42 and Rac Stimulate Exocytosis of Secretory Granules by Activating the IP3/Calcium Pathway in RBL-2H3 Mast Cells. J.Cell Biol. 2000;148:481–494. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Keston AS, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal.Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- [23].Lindstedt KA, Mayranpaa MI, Kovanen PT. Mast cells in vulnerable atherosclerotic plaques - a view to a kill. J Cell Mol Med. 2007;11:739–758. doi: 10.1111/j.1582-4934.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsui T, Suzuki Y, Yamashita K, Yoshimaru T, Suzuki-Karasaki M, Hayakawa S, Yamaki M, Shimizu K. Diphenyleneiodonium prevents reactive oxygen species generation, tyrosine phosphorylation, and histamine release in RBL-2H3 mast cells. Biochem Biophys.Res.Commun. 2000;276:742–748. doi: 10.1006/bbrc.2000.3545. [DOI] [PubMed] [Google Scholar]
- [25].Menon IA. Nature of the oxygen species generated by xanthine oxidase involved in secretory histamine release from mast cells. Biochimie et biologie cellulaire. 1989;67:397–403. doi: 10.1139/o89-064. [DOI] [PubMed] [Google Scholar]
- [26].Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- [27].Midoro-Horiuti T, Goldblum RM, Kurosky A, Goetz DW, Brooks EG. Isolation and characterization of the mountain cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin.Immunol. 1999;104:608–612. doi: 10.1016/s0091-6749(99)70331-3. [DOI] [PubMed] [Google Scholar]
- [28].Midoro-Horiuti T, Goldblum RM, Kurosky A, Wood TG, Brooks EG. Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. J Immunol. 2000;164:2188–2192. doi: 10.4049/jimmunol.164.4.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mladenka P, Simunek T, Hubl M, Hrdina The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radical Research. 2006:263–272. doi: 10.1080/10715760500511484. [DOI] [PubMed] [Google Scholar]
- [30].Mota I, Vugnum I. Effects of anaphylactic shock and compound 48/80 on the mast cells of the guinea pig lung. Nature. 1956;177:427–429. doi: 10.1038/177427a0. [DOI] [PubMed] [Google Scholar]
- [31].Murakami M, Hara N, Kudo I, Inoue K. Triggering of degranulation in mast cells by exogenous type II phospholipase A2. J.Immunol. 1993;151:5675–5684. [PubMed] [Google Scholar]
- [32].Nguyen C, Teo JL, Matsuda A, Eguchi M, Chi EY, Henderson WR, Jr., Kahn M. Chemogenomic identification of Ref-1/AP-1 as a therapeutic target for asthma. PNAS. 2003;100:1169–1173. doi: 10.1073/pnas.0437889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohmori H, Komoriya K, Azuma A, Kurozumi S, Hashimoto Y. Xanthine oxidase-induced histamine release from isolated rat peritoneal mast cells: involvement of hydrogen peroxide. Biochem.Pharmacol. 1979;28:333–334. doi: 10.1016/0006-2952(79)90524-0. [DOI] [PubMed] [Google Scholar]
- [34].Ramirez DA. The natural history of mountain cedar pollinosis. J Allergy Clin Immunol. 1984;73:88–93. doi: 10.1016/0091-6749(84)90489-5. The. [DOI] [PubMed] [Google Scholar]
- [35].Saneyoshi K, Nohara O, Imai T, Shiraishi F, Moriyama H, Fujimaki H. IL-4 and IL-6 production of bone marrow-derived mast cells is enhanced by treatment with environmental pollutants. Int.Arch.Allergy Immunol. 1997;114:237–245. doi: 10.1159/000237674. [DOI] [PubMed] [Google Scholar]
- [36].Schwartz LB. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J.Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- [37].Soter NA, Austen KF. The diversity of mast cell-derived mediators: implications for acute, subacute, and chronic cutaneous inflammatory disorders. J Invest Dermatol. 1976;67:313–319. doi: 10.1111/1523-1747.ep12514349. [DOI] [PubMed] [Google Scholar]
- [38].Suzuki Y, Yoshimaru T, Inoue T, Ra C. Discrete generations of intracellular hydrogen peroxide and superoxide in antigen-stimulated mast cells: Reciprocal regulation of store-operated Ca2+ channel activity. Mol.Immunol. 2009;46:2200–2209. doi: 10.1016/j.molimm.2009.04.013. [DOI] [PubMed] [Google Scholar]
- [39].Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- [40].Taurog JD, Mendoza GR, Hook WA, Siraganian RP, Metzger H. Noncytotoxic IgE-Mediated Release of Histamine and Serotonin from Murine Mastocytoma Cells. J Immunol. 1977;119:1757–1761. [PubMed] [Google Scholar]
- [41].Wan BYC, Peh KH, Ho M, Assem ES. Effects of nitric oxide and hydrogen peroxide on histamine release from RBL-2H3 cells. Biochem Pharmacol. 2001;62:1537–1544. doi: 10.1016/s0006-2952(01)00770-5. [DOI] [PubMed] [Google Scholar]
- [42].Wan J, Diaz-Sanchez D. Antioxidant Enzyme Induction: A New Protective Approach Against the Adverse Effects of Diesel Exhaust Particles. Inhalation Toxicology. 2007;19:177–182. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- [43].Wang XL, Takai T, Kamijo S, Gunawan H, Ogawa H, Okumura K. NADPH oxidase activity in allergenic pollen grains of different plant species. Biochem Biophys Res Comm. 2009;387:430–434. doi: 10.1016/j.bbrc.2009.07.020. [DOI] [PubMed] [Google Scholar]
- [44].Wolfreys K, Oliveira DB. Alterations in intracellular reactive oxygen species generation and redox potential modulate mast cell function. Eur J Immunol. 1997;27:297–306. doi: 10.1002/eji.1830270143. [DOI] [PubMed] [Google Scholar]
- [45].Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through Fc[gamma]RI: additive effects of C3a. Clin Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [46].Wu Z, Turner DR, Oliveira DB. IL-4 gene expression up-regulated by mercury in rat mast cells: a role of oxidant stress in IL-4 transcription. Int.Immunol. 2001;13:297–304. doi: 10.1093/intimm/13.3.297. [DOI] [PubMed] [Google Scholar]
- [47].Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, Shimizu K. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin.Exp.Allergy. 2002;32:612–618. doi: 10.1046/j.0954-7894.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- [48].Young JD, Liu CC, Butler G, Cohn ZA, Galli SJ. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc.Natl.Acad.Sci.U.S.A. 1987;84:9175–9179. doi: 10.1073/pnas.84.24.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]