Abstract
In addition to its role in development and cell proliferation, β-catenin has been implicated in neuronal synapse regulation and remodeling. Here we review basic molecular and structural mechanisms of synaptic plasticity, followed by a description of the structure and function of β-catenin. We then describe a role for β-catenin in the cellular processes underlying synaptic plasticity. We also review recent data demonstrating that β-catenin mRNA and protein phosphorylation are dynamically regulated during fear memory consolidation in adult animals. Such alterations are correlated with a change in the association of β-catenin with cadherin, and deletion of the β-catenin gene prevents fear learning. Overall, the extant data suggest that β-catenin may function in mediating the structural changes associated with memory formation. This suggests a general role for β-catenin in synaptic remodeling and stabilization underlying long-term memory in adults, and possible roles for dysfunction in the β-catenin pathway in disorders of memory impairment (e.g. Alzheimer’s Disease) and in disturbances in which emotional memories are too strong or resistant to inhibition (e.g. fear learning in Posttraumatic Stress Disorder). Further understanding of the β-catenin pathway may lead to better appreciation for the structural mechanisms underlying learning and memory as well as provide novel therapeutic approaches in memory related disorders.
Keywords: fear, amygdala, synaptic plasticity, ptsd, gene, anxiety
1. Introduction
The search to understand the mechanisms underlying learning and memory has been a topic of interest for more than a century. Dating back to the nineteenth century, Cajal proposed that learning requires the formation of new neuronal connections(Ramon y Cajal, 1894). Later, based on these and other early speculations, Konorski and Hebb independently suggested that alterations in synaptic strength, as well as formation of new synapses, are responsible for information storage (Hebb, 1949; Konorski, 1948). The ability of connections between neurons to change as a function of learning is commonly referred to as synaptic plasticity, and this is accepted today as a mechanism underlying memory formation. Such alterations in synaptic connections can be produced through artificial processes, such as long-term potentiation (LTP), or more natural processes, such as behavioral learning in animals. Understanding molecular mechanisms of learning, memory and synaptic plasticity promise to further our understanding and treatment approaches for disorders of decreased memory such as Alzheimer’s and other dementias, as well as disorders of dysregulated emotional memory such as posttraumatic stress disorder, depression and bipolar disorders.
1.1 Molecular mechanisms of synaptic plasticity
Synaptic plasticity in mature neurons is often initiated by neural activity and an influx of calcium. Calcium influx can lead to alterations in synapse structure and function through a process involving post-translational modification, protein synthesis and gene transcription. Following presynaptic activity and postsynaptic depolarization, calcium enters the postsynaptic neuron through N-methyl-D-aspartic acid (NMDA)-type glutamate receptors (NMDARs) and voltage-gated calcium channels (VGCCs) (Collingridge and Bliss, 1995; Magee and Johnston, 1995; Sabatini et al., 2001). Elevated intracellular calcium levels then activate additional signaling pathways, including calmodulin (CaM)-dependent protein kinases (CaMKs) (Lisman et al., 2002; Tanaka and Nishizuka, 1994). Activation of these protein kinases, subsequently leads to the phosphorylation of AMPA-type glutamate receptors (AMPARs), along with the activation of protein synthesis, and initiation of new gene transcription. These cellular processes, which contribute to synaptic plasticity, begin within a few minutes following neural activity, but can persist for several hours.
2.1 Morphological changes associated with synaptic plasticity
Rapid structural changes, involving the rearrangement of the cytoskeleton at the synapse, can also occur with synaptic plasticity. Most excitatory synapses in the brain terminate on dendritic spines (Yuste and Bonhoeffer, 2004). Dendritic spines are tiny protrusions on the shaft of dendrites, that represent sites where new contacts between cells can be created, and existing contacts strengthened. The cytoskeleton of dendritic spines contains high concentrations of filamentous actin (F-actin) (Fifkova and Delay, 1982). The dynamic nature of the actin permits the spine to change shape within seconds to minutes, thereby contributing to synaptic plasticity. The resulting change in spine shape can last for hours or even days, and may influence synaptic transmission.
In addition to actin, spines also contain a multi-protein complex, the postsynaptic density (PSD), which includes receptors, channels, cell adhesion proteins, and other signaling molecules (Okabe, 2007). All of these components play a role in mediating spine changes. Such changes may be characterized by an increase in the number of spines, or in the overall shape of the spine (Steiner et al., 2008b). Changes in spine number and form have been observed following many different experimental and behavioral conditions. For example, induction of LTP in hippocampal slice cultures has been shown to produce new spine formation, increase the size of the spine head, and shorten the length of the spine neck (Engert and Bonhoeffer, 1999; Fifkova and Anderson, 1981; Yang et al., 2008). Similarly, changes in spine morphology have been shown in vivo with trace eyeblink conditioning (Leuner et al., 2003) and fear conditioning (Ostroff et al., 2010).
2.2 Cell adhesion molecules and synaptic plasticity
Along with structural changes in dendritic spines, synaptic plasticity also results in the formation of new synaptic contacts. During this process, the adhesion between the pre- and postsynaptic neurons is altered. The strengthening and weakening of these contacts can be modulated by cell adhesion molecules. These molecules are bound to the membrane and contain an extracellular domain that engages in either homophilic or heterophilic interactions with similar cell adhesion molecules, or other proteins in the extracellular matrix, respectively. They also contain an intracellular domain that interacts with the cytoskeleton and triggers signaling pathways that can regulate spine and synapse formation (Dityatev et al., 2004). Examples of such molecules include neurexins and neuroligins, the SALM family of adhesion molecules, synaptic cell adhesion molecules (SynCAM), neural cell adhesion molecules (NCAM), and cadherins (see Giagtzoglou et al., 2009 for review). In this review, we will focus on the role of the cadherin, and more specifically, its partner β-Catenin, in synapse formation and organization.
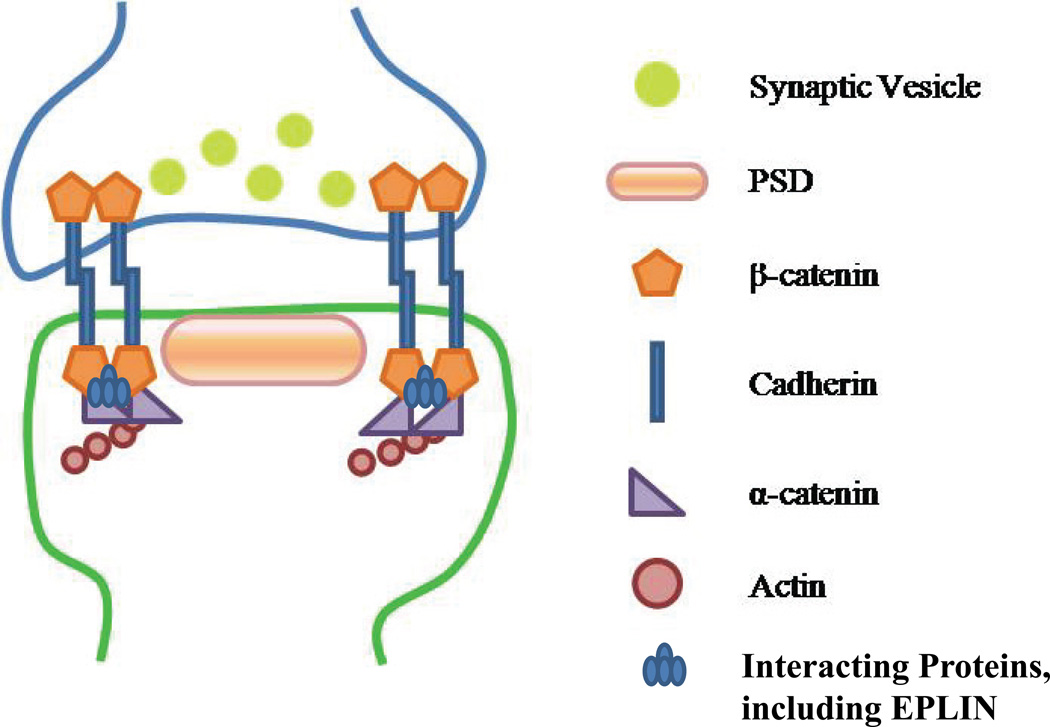
Cadherins are homophilic, calcium-dependent cell adhesion molecules that have been shown to play a role in synapse assembly, synaptic plasticity, and memory formation (Bruses, 2006; Takeichi, 2007). They contain an extracellular domain which provides a link between opposing cells, promoting structural stability, and an intracellular domain which provides a link to the actin cytoskeleton, promoting spine dynamics. Considerable attention has been given to the latter. The intracellular domain of cadherins binds to β-Catenin which then links cadherins, along with an intermediate protein complex, to the actin cytoskeleton via α-catenins (Abe and Takeichi, 2008) as illustrated in Figure 1. This cadherin-catenin complex is localized in synaptic junctions, and alterations in this complex are thought to influence not only synapse development, but also synaptic connectivity and activity (Takeichi and Abe, 2005). Although α-catenin links to the actin cytoskeleton, the role of β-catenin in this cadherin-catenin complex is more pronounced and has been shown to be a prerequisite for adhesion.
Figure 1. β-catenin at the synapse.
Schematic diagram showing that the intracellular domain of cadherin binds to β-Catenin, along with a number of intermediate proteins, which then links cadherin to the actin cytoskeleton via α-catenin. Please note that this is an extremely over-simplified diagram. The cadherin adhesion complex's interaction with the actin cytoskeleton is a dynamic structure that includes other intermediate proteins that are outside of the scope of this review.
3 β-CATENIN STRUCTURE AND FUNCTION
3.1 Structure of β-catenin
β-Catenin belongs to the armadillo family of proteins, and is composed of three domains: an N-terminal domain, a central domain, and a C-terminal domain (Pokutta and Weis, 2007). The central domain is the core region and contains 12 copies of a 42 amino acid sequence motif known as an armadillo repeat. Originally identified in Drosophila, this armadillo repeat domain is specialized for protein-protein binding, and forms a superhelix of helices that features a long, positively charged groove (Huber et al., 1997). β-catenin’s binding partners, including cadherins, adenomatosis polyposis coli (APC), and T-cell factor (TCF), are negatively charged, and are proposed to interact with this groove.
3.2 Functional roles of β-catenin
As mentioned earlier, β-catenin provides an essential, structural component of the cadherin/catenin adhesion complex. It is necessary to prevent the rapid degradation of the cadherin cytoplasmic domain (Huber et al., 2001) and to recruit α-catenin to sites of cell-cell contact (Drees et al., 2005; Yamada et al., 2005). Without the interaction between β-catenin and cadherin, cell-cell adhesion would be compromised (For recent review see: Heuberger and Birchmeier (2010) and Nelson (2008)).
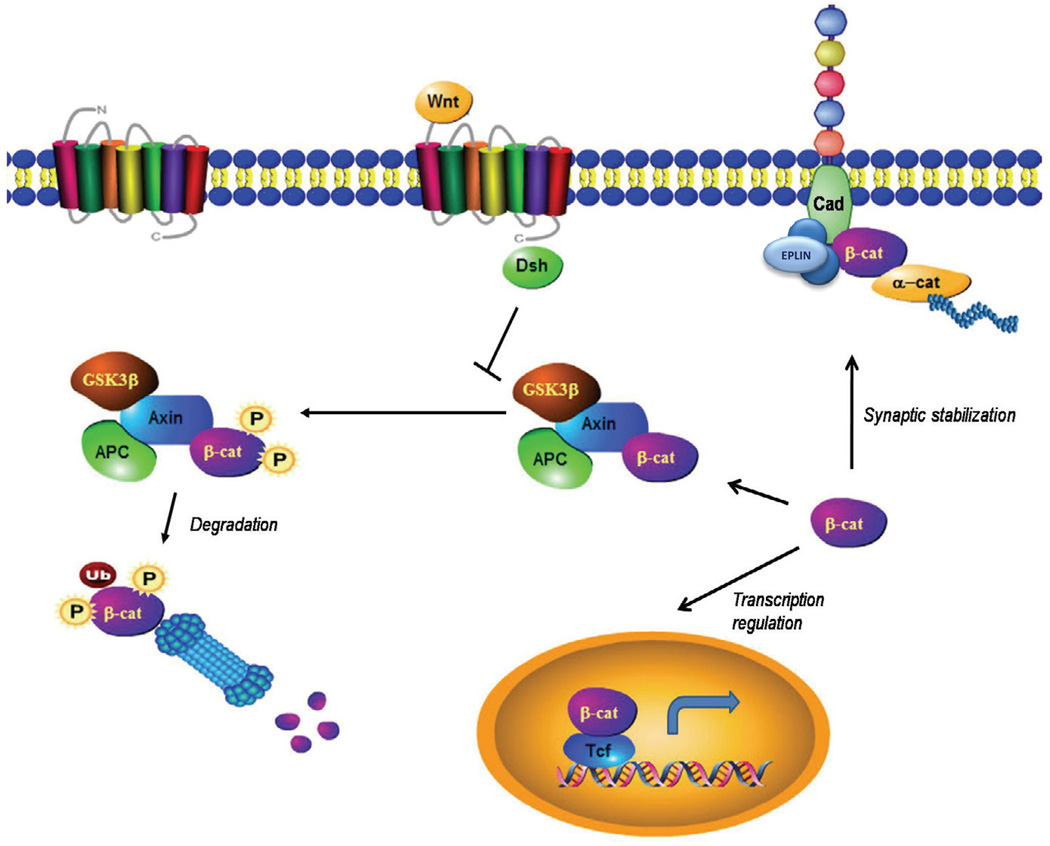
In addition to β-catenin’s role in cell adhesion, it also is a central player in the Wnt/β-catenin signal transduction pathway (For recent review see: MacDonald et al (2009); Rao and Kuhl (2010); Verkaar and Zaman (2011)). Wnts are highly conserved secreted glycoproteins that regulate cell-cell communication, and are involved in a diverse array of cellular processes including development. When Wnt proteins bind to Frizzled (Fz) and low-density lipoprotein-related protein (LRP) receptors, disheveled (Dsh) is recruited to the membrane. Activation of Dsh by Fz inhibits glycogen synthase kinase-3β (GSK-3β), a kinase that phosphorylates β-catenin and marks it for degradation by the proteasome pathway. Through the inhibition of GSK-3β, β-catenin is stabilized, enters the nucleus, and then forms a complex with the TCF/lymphocyte enhancer factor (LEF) family of transcription factors to regulate the expression of Wnt target genes (Logan and Nusse, 2004; Moon et al., 2004). This process is important for cell proliferation, survival, migration, and differentiation, and as suggested by recent research, modulation of synaptic plasticity (Chen et al., 2006). Recent work also suggests a role for β-catenin in learning and memory processes in animals (Maguschak and Ressler, 2008). Figure 2 illustrates these different functional roles of β-catenin.
Figure 2. β-catenin in Wnt signaling and the cadherin complex.
In the resting state, β-catenin is phosphorylated by GSK-3β and rapidly degraded by the proteasome pathway. Upon activation of Wnt signaling, β-catenin is stabilized through the inhibition of GSK3β and translocates to the nucleus to regulate the expression of Wnt target genes. β-catenin also associates with the cytoplasmic domain of cadherin and directly links to the actin cytoskeleton through α-catenin. Note that the cadherin adhesion complex is a dynamic structure that also includes other intermediate proteins (such as EPLIN, Abe K & Takeichi M., 2008.
There has been much discussion as to whether there is crosstalk between cadherin-mediated cell adhesion and canonical Wnt signaling through β-Catenin. Experiments involving genetic modification or overexpression in embryos have suggested that the same pool of β-catenin is involved in both cell adhesion at the plasma membrane and signal transduction in the nucleus (Reiss et al, 2005). However, others have shown that there are different pools of β-Catenin: 1) a monomeric, intramolecularly folded-back form that is generated by Wnt signaling and binds only to TCF transcriptional complexes, and 2) a separate pool that exists as a heterodimer with α-catenin, and preferentially binds to cadherins (Gottardi and Gumbiner, 2004). One explanation for this potential selectivity may be different requirements for cadherin and TCF to bind β-catenin. X-ray crystallography studies have shown that cadherins require all 12 armadillo repeats of β-catenin, while TCF only requires the central eight armadillo repeats (Xu and Kimelman, 2007). Since Wnt signaling generates a folded-back form of β-catenin, it is possible that some of the repeats may no longer be exposed, thus blocking the binding site for cadherin. Although many of the details of these differential interactions and pathways remain unknown, it is clear that β-catenin plays a critical function as a ‘hub’ of neuronal plasticity, mediating intracellular signaling that results both in structural changes underlying synaptic strength and in regulating expression of activity-related genes.
3.3 Posttranslational modifications regulate β-catenin function
In addition to structural changes in β-catenin, posttranslational modifications have also been reported to affect the interaction between β-catenin and its binding partners. For example, phosphorylation of β-catenin at tyrosine 654 (located on the 12th armadillo repeat) by src and EGFR, decreases its affinity for cadherin binding, and reduces its adhesive functions (Hoschuetzky et al., 1994; Roura et al., 1999; Takahashi et al., 1997). Cadherin/β-catenin binding may also be influenced by kinases involved in the signaling form of β-catenin, including casein kinase 1 (CK1; Dupre-Crochet et al., 2007), GSK3β (Maher et al., 2009), and CK2 (Wu et al., 2009; Bek and Kemler 2002).
Aside from being bound to cadherin at the membrane, β-catenin can also be found in the cytosol. This less stable pool of β-catenin is continuously phosphorylated by a dual kinase mechanism. First, CK1α phosphorylates β-catenin at serine 45. This event primes β-catenin for further phosphorylation by GSK3β at residues serine 33, serine 37, and threonine 41 (Liu et al., 2002; Yost et al., 1996). Upon phosphorylation at these sites, β-catenin is ubiquitinated and rapidly degraded by the 26S proteasome. As mentioned earlier, Wnt signaling can inhibit this phosphorylation-dependent degradation by inhibiting GSK3β activity. Consequently, there is an increase in the unphosphorylated form of β-catenin, which has been shown to accumulate more readily in the nucleus, and is associated with increased transcriptional activity.
Once β-catenin enters the nucleus, it binds to TCF, and recruits complexes that promote transcriptional activation. These complexes can be recruited by both the N- and C-termini of β-catenin. Phosphorylation of β-catenin by the Met receptor at N-terminal residue tyrosine 142 (Y142), promotes the association between BCL9-2 and β-catenin. BCL9-2 has been shown to increase nuclear location of β-catenin, and as a consequence, increase signaling (Brembeck et al., 2004). Since BCL9-2 cannot colocalize with the stable pool of β-catenin bound to cadherin at the plasma membrane, BCL9-2 may play a role in determining whether β-catenin molecules favor the cell adhesion or Wnt signaling pathways.
Phosphorylation of β-catenin at the C-terminal can also affect transcriptional activation. It has been shown that AKT phosphorylates β-catenin at residue serine 552, resulting in its dissociation from cell-cell contacts and an accumulation of β-catenin in both the cytosol and nucleus (Fang et al., 2007). Similarly, PKA-mediated phosphorylation of β-catenin at residue serine 675 has been shown to enhance the transcriptional activity of β-catenin (Taurin et al., 2006).
The aforementioned studies suggest that posttranslational modifications can impact the interaction of β-catenin with both cadherin and TCF, or other transcriptional co-activators. In turn, alterations in the affinity of β-catenin for these binding partners may influence β-catenin’s ability to take part in cell adhesion, Wnt signaling, or both. Nevertheless, both processes require β-catenin, and have been shown to modulate synaptic plasticity, thus suggesting that β-catenin may be a candidate molecule to study learning and memory.
3.4 Role of β-catenin in presynaptic structure and function
β-catenin is expressed in the developing and adult CNS. It can be found in both pre- and postsynaptic cells and appears to be present before a synapse becomes functional. Due to the localization of this protein prior to synapse formation, β-catenin stands out as a protein that may play a role in synapse assembly. Over the past several years, there has been much evidence suggesting that β-catenin may be acting both pre- and postsynaptically to regulate synapse formation and function (see Table 1 for an overview).
Table 1.
Effects of loss of function or overexpression of β-catenin on presynaptic and postsynaptic function
| Manipulation | Effect | Reference |
|---|---|---|
| Presynaptic Role | ||
| LOF | Increase in diffusion of vesicles along synapse | Bamji et al., 2003 |
| LOF | Decrease in number of synaptic vesicles per synapse | Bamji et al., 2003 |
| LOF | Impairment in response to prolonged repetitive stimulation | Bamji et al., 2003 |
| LOF | No change in PSD95 | Bamji et al., 2003 |
| Postsynaptic Role | ||
| LOF | Increase in thin, elongated spines | Okuda et al., 2007 |
| LOF | Decrease in dendritic arborization | Yu & Malenka, 2003; Gao et al. 2007 |
| LOF | Decrease in mEPSC amplitude | Okuda et al. 2007 |
| O/E | Increase in dendritic arborization | Kirchmar et al., 2006; Peng et al., 2009; Yu & Malenka, 2003 |
| O/E | Decrease in mEPSC amplitude | Peng et al., 2009 |
| O/E | Decrease in AMPAR density | Peng et al., 2009 |
LOF: Loss of function, O/E: Overexpression
The presynaptic axon contains an active zone, where synaptic vesicles dock and fuse to the plasma membrane (Zhai et al., 2001). Presynaptic molecules associated with synaptic vesicle proteins are also recruited to the active zone, and aid in the transformation of nascent presynaptic sites to functional presynaptic structures (Ziv and Garner, 2004). Originally synaptic proteins were thought to be relatively stable in mature synapses; however, more recent studies have suggested that these proteins are highly mobile and shuffle into and out of individual synapses (Bamji et al., 2006; Krueger et al., 2003). The cadherin/β-catenin complex has previously been shown to play a role in the recruitment and localization of synaptic vesicles to synapses (Iwai et al., 2002; Togashi et al., 2002, Stan et al., 2010). More recently, it has been determined that interfering with β-catenin itself can affect synapse assembly (Bamji et al., 2006; Bamji et al., 2003).
β-Catenin is important for controlling the size and localization of vesicle clusters. Deletion of β-catenin in hippocampal pyramidal neurons has been associated with a decrease in the number of synaptic vesicles per synapse, and an increase in the diffusion of these vesicles along the synapse (Bamji et al., 2006; Bamji et al., 2003). This effect does not appear to be due to postsynaptic components since examination of the shape and distribution of postsynaptic density (PSD)-95, a marker of postsynaptic densities, remains unaffected (Bamji et al., 2003). The decrease in the number of synaptic vesicles seems to be specific to undocked vesicles, those vesicles in the reserved/resting pool, as opposed to docked vesicles, those corresponding to the readily releasable pool. A decrease in the reserved/resting pool is complemented by an impaired response to prolonged repetitive stimulation, and corresponds to a dispersion of vesicles along the axon. The C-terminal domain of β-catenin, which contains a PDZ target sequence, may be responsible for presynaptic vesicle localization (Bamji et al., 2003). Evidence suggests that the PDZ binding motif of β-catenin allows it to act as a scaffolding protein to link cadherins to PDZ domain-containing proteins, retaining vesicles at discrete sites. One protein that has recently been shown to interact with the PDZ binding motif of β-catenin is scribble (Sun et al., 2009). The interaction between scribble and β-catenin, whether it is direct or indirect, may mediate β-catenin’s role in synaptic vesicle localization.
In addition to the PDZ binding motif, the phosphorylation state of β-catenin at tyrosine 654 (Y654) may also affect synaptic vesicle localization. As stated earlier, phosphorylation of β-catenin at Y654 decreases its affinity for cadherin(Roura et al., 1999). Recent evidence has shown that phosphorylation of β-catenin at the Y654 residue can be promoted by application of brain-derived neurotrophic factor (BDNF) to cultured hippocampal neurons (Bamji et al., 2006). BDNF is a neurotrophin that is well known to function in synaptic plasticity and regulate synaptic morphology (Greenberg et al., 2009). Upon application of BDNF, the cadherin-β-catenin complex is disrupted, and an enhancement in synaptic vesicle mobility is observed. The dispersal of synaptic vesicles into perisynaptic regions can be abolished by preventing the phosphorylation at this residue by a β-catenin point mutation (Bamji et al., 2006).
An effect on synaptic vesicle localization by phosphorylation of β-catenin at Y654 can also be observed following manipulation of Fer (Lee et al., 2008). Fer is a cytoplasmic tyrosine kinase that is known to act in several signaling pathways, including cell adhesion molecule-regulated signaling (Xu et al., 2004). Depletion of Fer using small hairpin RNAs (shRNAs) in cultured hippocampal neurons results in an increase in the motility of presynaptic clusters, along with an increase in tyrosine phosphorylated β-catenin (Lee et al., 2008). This dispersion of synaptic vesicle clusters could, once again, be prevented by overexpressing a mutant form of β-catenin that prevents phosphorylation at Y654. Overall, these results suggest that β-catenin acts presynaptically to control synaptic vesicle localization.
3.5 Role of β-catenin in postsynaptic structure and function
The role of β-catenin in postsynaptic shape and function has also been studied. As mentioned earlier, the dendritic spines on the postsynaptic neuron are the sites where most excitatory synapses take place. Spines can generally be classified by their shape and volume as thin, stubby, or mushroom-like. The different shapes of dendritic spines are thought to represent strength and maturity, where thin spines are immature, and mushroom shaped are mature. β-catenin has been shown to be important in regulating spine shape and size. Without postsynaptic β-catenin, there is an increase in thin, elongated spines and subsequent decrease in short mushroom-shaped spines (Okuda et al., 2007). Although ablation of postsynaptic β-catenin alters spine morphology, no changes in density of presynaptic markers were observed. Thus, these results suggest that poststynaptic β-catenin ablation does not prevent neurons from maintaining presynaptic inputs.
Alterations in the dendrites themselves have also been observed following β-catenin manipulation. Overexpression of β-catenin in hippocampal neuronal cultures increases dendritic growth and arborization, while decreasing endogenous β-catenin prevents dendritic morphogenesis (Peng et al., 2009; Yu and Malenka, 2003). This function of β-catenin in regulating dendritic growth appears to be due to its role in cell adhesion, and is not dependent on its transcriptional actions.
The above changes have been reported at baseline; however, a similar effect can be observed following neural activation. Neural activity is known to induce changes in dendrite morphology, and this remodeling is critical for neural circuit formation and synaptic function. The effects of neural activity can be mimicked by chronically depolarizing neurons with extracellular potassium, and has been shown to increase dendritic arborization (Peng et al., 2009; Yu and Malenka, 2003).
Interestingly, the effect reported following overexpression of β-catenin is remarkably similar to the observed increase in dendritic arborization following treatment with high potassium, thus suggesting that the two treatments may function through a common signaling pathway. Indeed, depolarization induced by elevated potassium increases Wnt secretion, and one way to increase intracellular β-catenin is through activation of the Wnt pathway. Therefore, it may be that neuronal depolarization increases Wnt activity, which then stabilizes the intracellular pool of β-catenin, ultimately leading to an enhancement in dendritic arborization
β-catenin has also been shown to regulate postsynaptic strength. Miniature excitatory postsynaptic currents (mEPSCs) are events generated in dendrites and generally arise from the spontaneous release of single vesicles. Measuring mEPSCs in β-catenin-ablated neurons gives insight into the effect of β-catenin loss on glutamatergic quantal responses. Loss of β-catenin in hippocampal neurons results in a decrease in the amplitude of mEPSCs, which is largely dependent on AMPA receptors, without affecting the frequency of mEPSCs, which is dependent on the density of functional presynaptic boutons (Okuda et al., 2007). Following rescue with transfection of the wild-type β-catenin gene in these neurons, the mean mEPSC amplitude is restored to levels comparable to control neurons, suggesting that β-catenin plays a role in modulating AMPA-mediated synaptic currents. Similar to the presynaptic role of β-catenin in controlling synaptic vesicle localization, the central armadillo repeats, which bind to cadherins and TCF/LEF transcription factors, along with the C-terminal PDZ-binding motif, are important for regulating synaptic AMPARs (Okuda et al., 2007).
Overexpression of β-catenin also reduces mEPSC amplitudes and is accompanied by a decrease in surface AMPAR cluster size and density. However, the decrease in AMPA receptor density does not appear to coincide with a decrease in synapse density (Peng et al., 2009). This incongruity can be explained by an observed increase in the NMDAR/AMPAR ratio, which is indicative of silent, or inactive, synapses. Furthermore, the physiological changes occur in parallel with the aforementioned increases in dendritic arborization following neural activity (Peng et al., 2009). These results suggest that changes in dendritic morphology may coordinate with excitatory synaptic strength to regulate synaptic scaling. Altogether, these results show that β-catenin may act postsynaptically to couple the structure and function of excitatory synapses.
4 β-catenin, Learning and Memory
4.1 Activity-dependent regulation of β-catenin
Neural activity alters the localization of β-catenin at synapses, thus providing more evidence that β-catenin plays a role in synaptic regulation. Following depolarization with a solution containing a high concentration of KCl, there is an NMDA-dependent redistribution of preexisting β-catenin from dendritic shafts to spines in cultured hippocampal neurons. This redistribution of β-catenin to the spines coincides with an increase in the association of β-catenin with cadherin, and can be mimicked or prevented by application of a tyrosine kinase or phosphatase inhibitor, respectively (Murase et al., 2002).
Similar results were obtained by studying β-catenin point mutations at site, tyrosine 654 (Y654). Point mutations that prevent phosphorylation of β-catenin at this specific site result in a redistribution of β-catenin to the spine, and an increase in the size and density of synapsin-1 and PSD-95, markers of presynaptic and postsynaptic proteins. In addition to changes in synaptic proteins, preventing phosphorylation at Y654 alters synaptic function by increasing the frequency of mEPSCs (Murase et al., 2002). An increase in the frequency of mEPSCs, with no change in the amplitude, may reflect an increase in the probability of neurotransmitter release, or a conversion of silent, or inactive synapses to active synapses (Choi et al., 2000; Gasparini et al., 2000). Nonetheless, manipulating tyrosine phosphorylation and dephosphorylation of β-catenin produces changes in synaptic size and strength. More recent evidence suggests that cyclin-dependent kinase 5 (Cdk5) activity is responsible for this activity-dependent phosphorylation of β-catenin at Y654 (Schuman and Murase, 2003). Together, these results suggest that activity-induced changes in the localization of β-catenin, along with the regulation of tyrosine phosphorylation, are important for synaptic regulation.
β-catenin regulation has also been shown to play a role in activity-dependent gene expression, an important component of synaptic plasticity (Cohen and Greenberg, 2008; Kandel, 2001). In response to NMDAR-dependent activation of calpain, β-catenin is cleaved at the N terminus, making it resistant to GSK-3β mediated degradation (Abe and Takeichi, 2007). This results in an increase in the stabilized form of β-catenin which then translocates to the nucleus to regulate gene transcription. Fosl1 has been identified as one gene that is upregulated following the NMDAR-mediated β-catenin signaling cascade, and interestingly, Fosl1 has also been shown to be upregulated following behavioral learning in rats (Faure et al., 2006). Therefore, β-catenin regulation may be important for the transcription of genes following synaptic activity.
4.2 β-catenin and memory related pathology
Recent evidence points towards a potential role for β-catenin in pathological states of neurotoxicity such as Huntington’s disease (Godin et al., 2010) and Alzheimer’s disease (AD). Alzheimer’s disease is a neurodegenerative disorder characterized by progressive memory loss and cognitive impairment, and at the molecular level, by the presence of neurofibrillary tangles (NFTs) and senile plaques comprised of the insoluble β-amyloid peptide (Aβ) (Hardy, 2006; Hardy and Selkoe, 2002; Lee et al., 1991). Three mutations identified in families affected by Familial Alzheimer’s disease (FAD), presenilin-1 (PS-1), presenilin-2 (PS-2), and the amyloid precursor protein (APP), result in dysfunctional Aβ production (Duff et al., 1996; Price and Sisodia, 1998). There is indirect evidence from AD brains which also supports a role for β-catenin in disease. AD patients with PS-1 mutations have reduced levels of β-catenin (Zhang et al., 1998). PS-1 is a transmembrane protein located at synaptic cell-cell contact sites which forms complexes with β-catenin (Kang et al., 2002; Murayama et al., 1998; Yu et al., 1998; Zhou et al., 1997). This interaction is thought to increase β-catenin stability (Zhang et al., 1998). Mutations in PS-1 decrease β-catenin stability and are associated with an overproduction and aggregation of Aβ peptide (Fraser et al., 2000).
PS-1 is also thought to inactivate GSK3, a negative regulator of β-catenin. GSK3 expression has been shown to be upregulated in the hippocampus of AD patients (Blalock et al., 2004). Similarly, an increase in phosphorylated GSK3 has also been reported in the frontal cortex in AD (Leroy et al., 2007). Since increased GSK3 activity is associated with a decrease in β-catenin stability, these early findings suggest that impairments in β-catenin regulation may be linked to AD pathology.
Aβ neurotoxicity can be produced by the addition of Aβ to neuronal cultures (De Ferrari et al., 2003), conditions which also lower levels of cytoplasmic β-catenin. Lithium, which acts as a positive regulator in the Wnt signaling pathway, by inactivating GSK-3β, can prevent the cytotoxic effects of Aβ (De Ferrari and Inestrosa, 2000; Toledo and Inestrosa). Lithium has also been shown to prevent neurodegeneration and behavioral impairments induced by injections of Aβ fibrils into the dorsal hippocampus of rats (De Ferrari et al., 2003). Lithium also increases β-catenin levels, thus suggesting that the mechanism by which lithium treatment may be acting is due, in part, to the stabilization of β-catenin (De Ferrari et al., 2003).
It has been suggested that AD manifests itself as a perturbation of neuroplasticity prior to overt Aβ –dependent neural degeneration and toxicity (Mesulam, 1999; Selkoe, 2002). β-catenin appears to be required for synaptic plasticity; thus, understanding the physiological role of β-catenin may be critical to inform new treatment and prevention approaches in AD.
5 β-catenin and Learning and Memory in the Adult Brain
The above section suggests a role for β-catenin in adult learning and memory and in disorders of memory function. Thus far, there has been substantial evidence suggesting that β-catenin is involved in neuronal synapse regulation and plasticity; however, the majority of this work has been done in vitro. Note that Bamji and colleagues (2003) used a CaMKII-Cre to specifically remove β-catenin in the hippocampus and found changes in synapse number and synaptic responses. However, there were previously no studies on the role of this intriguing protein in standard behavioral learning and memory assays. The below studies describe the recent examination of a role for β-catenin in long-term memory formation in adults.
5.1 β-catenin is required for Consolidation of Fear Memory Formation
Although it was initially identified for its role in development, β-catenin has also been shown to play a role in neuronal synapse regulation and plasticity. Since alterations in synapse regulation and plasticity are thought to underpin long-term memory formation, β-catenin may play a critical role in this process. However, there has been a scarcity of data exploring this possibility. Thus, we examined the role of β-catenin in amygdala-dependent fear memory. We found that β-catenin is highly expressed in the adult mouse amygdala and is dynamically regulated at both the transcriptional and post-translational levels with fear learning (Figure 3) (Maguschak and Ressler, 2008). We then found that pharmacological stabilization of β-catenin with lithium chloride resulted in enhanced learning, while genetic deletion of the gene that encodes β-catenin, Ctnnb1, in the amygdala resulted in impaired learning. In both cases, the manipulation affected the consolidation, but not acquisition, of the fear memory. Notably, Ctnnb1 deletion did not affect a number of other behaviors, including locomotor, anxiety-related behavior, or hippocampal-dependent memory (Maguschak and Ressler, 2008).
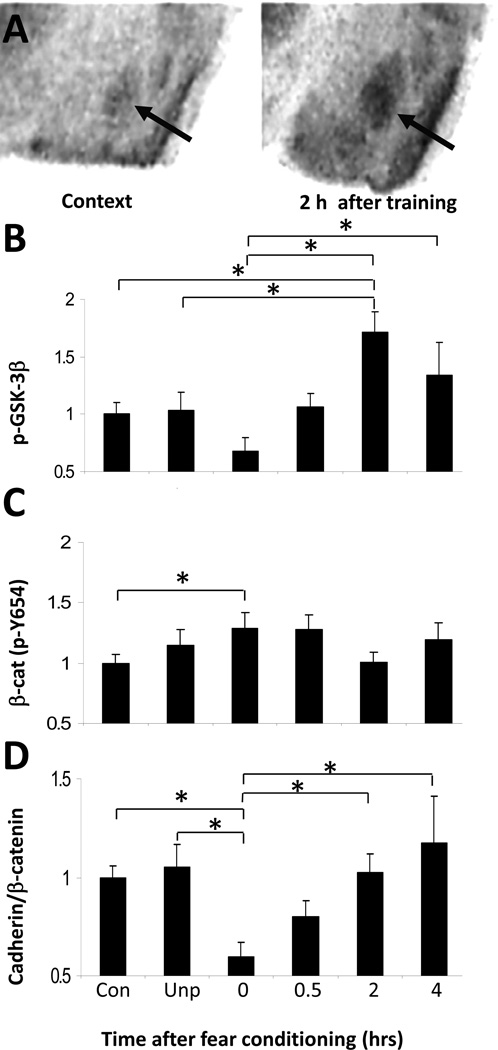
Figure 3. β-catenin and Amygdala-Dependent Fear Conditioning.
(A) β-catenin gene expression in the amygdala is increased following fear conditioning. Qualitative in situ hybridization analysis of β-catenin mRNA in the amygdala in context exposed animals. (B) Additionally, post-translational regulation (phosphorylation state) of β-catenin and GSK-3β are altered following fear conditioning. Mice were exposed to five tone-shock pairings and then sacrificed 0, 0.5, 2, 4 hr after training. Phospho-GSK-3β levels, determined by western blot, are significantly changed across timepoints (p < .001). (C) β-catenin (phospho-Y654) levels, determined by western blot, are significantly changed across timepoints (p < .05). (D) Immunoprecipitation results from a cadherin immunoblot after β-catenin immunoprecipitation. Cadherin interaction with β-catenin is significantly changed across timepoints (p < .05). ‘con’ = context control group, ‘unp’=unpaired shock control group; Bars indicate mean +/− sem; * denotes p < .05 for the different comparisons identified). (adapted from Maguschak & Ressler, 2008).
Memory formation is thought to involve the weakening and strengthening of synapses, and this process can be modulated by the adhesion between pre- and postsynaptic neurons. One example of this phenomenon involves the role of Aplysia cell adhesion molecules (apCAM’s) in the long-term sensitization of the gill- and siphon-withdrawal reflex, a form of learning in the marine mollusk. Following long-term sensitization with application of serotonin to siphon sensory neurons, apCAMs become rapidly down-regulated in the sensory neurons. This observed dynamic regulation of cell adhesion molecules may be responsible for long-term synaptic plasticity (Mayford et al., 1992). Since β-catenin is important for cell adhesion, and deletion of Ctnnb1 in the amygdala produces deficits in the consolidation of memory, we propose the following model of how β-catenin may function in memory formation (Figure 4).
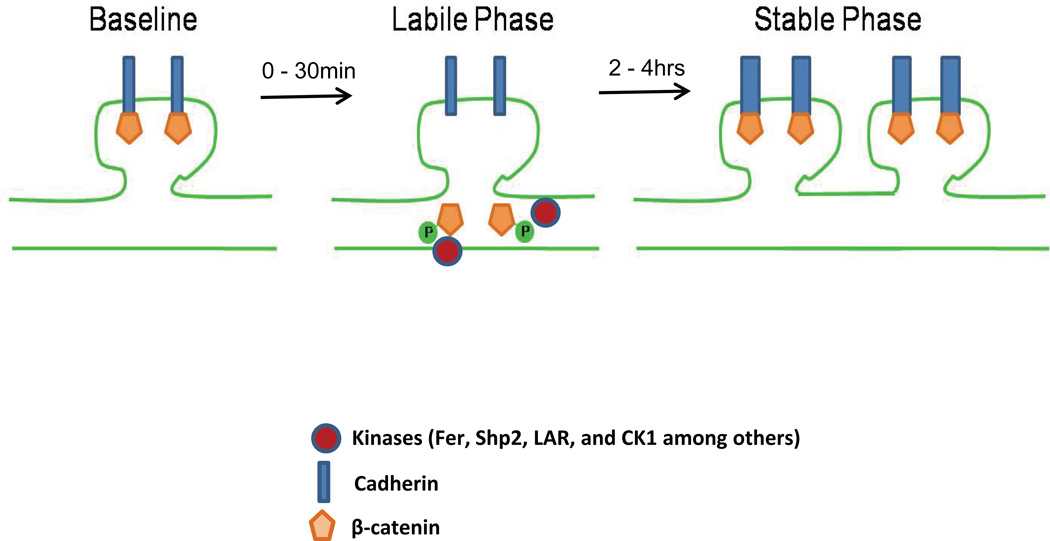
Figure 4. Schematic representation of the role of β-catenin in producing the labile and stable phases of memory formation.
(A) β-catenin is located in a complex with cadherin. Following a learning event, β-catenin becomes phosphorylated and shifts to the dendritic shaft, allowing for synaptic remodeling to take place (labile phase). At some point later, β-catenin redistributes to the dendritic spine, and re-associates with cadherin to strengthen the memory (stable phase). (Thickness of cadherin bar represents relative synaptic structural stability. Note that the cadherin adhesion complex is a dynamic structure that also includes other intermediate proteins, and is modulated by a number of protein kinases, including Fer, Shp2, LAR, and CK1 among others (Piedra et al., 2003; Xu et al., 2004; Grinnell et al., 2010; Dunah et al., 2005; Kypta et al., 1996; and Del Valle-Pérez et al., 2011)).
β-catenin can be found in a complex with cadherin at the plasma membrane of dendritic spines. Immediately following a learning event, β-catenin becomes phosphorylated at site Y654. Similar to previous findings, we found that the increase in phosphorylated β-catenin coincided with a decrease in the interaction between β-catenin and cadherin (Maguschak and Ressler, 2008). This decrease may be required to weaken the bond between the pre- and postsynaptic neurons, allowing for synaptic remodeling to take place. Following a period of β-catenin-cadherin destabilization, β-catenin relocates to the spine and once again forms a complex with cadherin, thereby stabilizing the synapse, and strengthening the memory. A change in the total amount of β-catenin does not appear to be necessary for the stabilization of the synapse. Instead, it may require rapid dynamic changes in the breakdown, redistribution, and replacement of the protein. This proposed model is consistent with previous in vitro work showing that depolarization of hippocampal neurons with KCl causes a redistribution of β-catenin from dendritic shafts to spines, without changing the total amount of the protein (Murase et al., 2002). One difference between the findings in the above study and ours is the rapid destabilization in the β-catenin-cadherin complex prior to the redistribution of β-catenin. This may reflect a difference between studying neuronal cultures and whole animal or may be due to several other factors including the means of inducing neural activity or time course for analysis.
In conditions where β-catenin function may be impaired, the initial labile phase may remain unaffected; however, the stable phase may be comprised. If β-catenin is not present, it will not be able to bind to cadherin and stabilize the synapse. This proposed model suggests that dynamic regulation of β-catenin may be involved in the structural conversion of short-term labile to long-term stable memory traces (Figure 4).
5.2 Dynamic Regulation of Cadherin/β-catenin Interaction
The findings reported above suggest that β-catenin is required for normal consolidation, but not acquisition, of memory. The evidence presented thus far suggests that the decrease and subsequent increase in the interaction between β-catenin and cadherin may be critical for the labile and stable phases of memory formation. Such dynamic regulation has been proposed previously when examining its cellular regulation in hippocampal cultures (Bamji et al., 2006; Tai et al., 2007), but it has not previously been demonstrated in vivo or in behavioral learning and memory paradigms.
Neural activity increases the synthesis and secretion of brain-derived neurotrophic factor (BDNF), which has been shown to play a critical role in synaptic plasticity (Lu et al., 2008). Treatment with BDNF induces synaptic vesicle dispersion, which is associated with an increase in β-catenin tyrosine phosphorylation and a decrease in β-catenin-cadherin interaction (Bamji et al., 2006). Within 30 minutes after the dispersion, phosphorylation decreases, and the β-catenin-cadherin interaction is restored (Bamji et al., 2006). This finding suggests that the disruption and re-stabilization of β-catenin-cadherin complexes may be required for new synapse formation. BDNF has been demonstrated to be important in both amygdala (Rattiner et al., 2004) and hippocampal dependent memory formation (Heldt et al., 2007). These new findings that β-catenin regulation is also involved in these processes raise the question of whether the specific effects of BDNF on memory consolidation are, in part, via the β-catenin pathway.
Similarly, NMDAR-dependent neural activity has also been shown to induce changes in the interaction between β-catenin and cadherin (Tai et al., 2007). NMDAR activation decreases the rate of cadherin endocytosis, increasing the accumulation of cadherin in the plasma membrane. In addition the level of tyrosine phosphorylated β-catenin is decreased, leading to an increase in the interaction between β-catenin and cadherin in dendritic spines (Tai et al., 2007). Furthermore, prolonged stability of the cadherin at the plasma membrane blocks NMDAR-dependent synaptic plasticity, suggesting that the dynamic behavior of β-catenin and cadherin is important for this process.
5.3 NMDA Receptors, BDNF, and β-catenin
There have been previous studies showing that BDNF may modulate NMDAR activity. Upon BDNF binding to and activating the tyrosine receptor kinase TrkB, there is an enhancement in glutamatergic synaptic transmission (Levine et al., 1995) and an increase in the phosphorylation of the NMDAR (Suen et al., 1997). In vitro studies have shown that the phosphorylation of NMDAR occurs within 5 min of exposure to BDNF(Suen et al., 1997). Since BDNF activation of TrkB receptors functions to transiently dissociate the β-catenin from the cadherin, while glutamatergic activation of NMDA receptors functions to increase the association, it is possible that the two systems may interact to produce the transient destabilization and re-stabilization of synapses.
Interestingly, both BDNF and NMDA are required for the consolidation of memories. Therefore, we propose that similar mechanisms are taking place in vitro and in vivo to stabilize and strengthen synapses (Figure 5). BDNF is released following a learning event, which phosphorylates β-catenin, decreasing the β-catenin-cadherin interaction. This decrease in the β-catenin-cadherin interaction increases synaptic vesicle mobility, allowing for synaptic plasticity. At about the same time, BDNF may increase the phosphorylation of NMDAR. The activation of the NMDAR then helps to re-stabilize the synapse, by decreasing the rate of cadherin endocytosis and redistributing β-catenin into spines. Once the synapse is stabilized, the memory becomes strengthened. Future studies directly examining this interaction, in vivo, would be important and interesting.
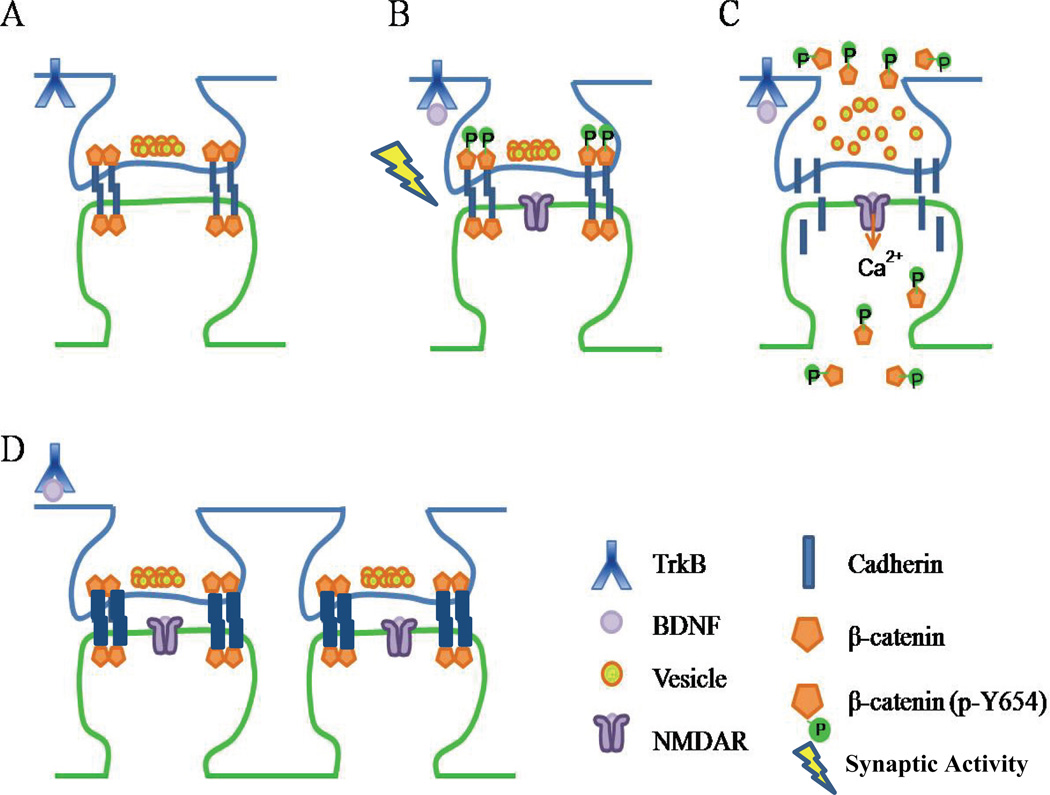
Figure 5. Schematic representation showing a possible interaction between BDNF signaling and NMDAR activation.
(A) β-catenin is located in a complex with cadherin at the plasma membrane of the synapse. (B) Activation of TrkB receptor by BDNF, along with enhanced synaptic activity, results in the phosphorylation of β-catenin at Y654, thus causing a dissociation of β-catenin from cadherin. (C) BDNF activation also results in the phosphorylation of NMDARs. During this time, phosphorylated β-catenin shifts to the dendritic shaft, and synaptic vesicles disperse. (D) The activation of NMDARs, along with the internalization of TrkB re-stabilizes the synapse by bringing β-catenin and cadherin together at the membrane. (Thickness of cadherin bar represents relative synaptic structural stability. Note that the cadherin adhesion complex is a dynamic structure that also includes other intermediate proteins).
5.4 β-catenin, depression, and mood regulation
The focus of this review has been the role of β-catenin pathways in learning and memory; however, there is increasing awareness that many affective disorders, such as depression and bipolar disorder, may also represent states of dysregulated synaptic plasticity. A number of lines of evidence now implicate the β-catenin/GSK-3 pathways in these disorders (Gould et al., 2006; Wada, 2009). This connection was initially made through the recognition of Lithium as a potent regulator of the β-catenin/GSK-3 pathway as described above. One series of studies demonstrated that targeted in-vivo inhibition of GSK-3 produces antidepressive-like behavior (Kaidanovich-Beilin et al., 2004). Among the more direct lines of evidence linking the β-catenin/GSK-3 pathway with lithium’s effect on mood stabilization was the finding that β-catenin overexpression in the mouse brain mimics lithium-sensitive behaviors (Gould et al., 2007). This study demonstrated that β-catenin transgenic mice exhibited behaviors identical to those observed in lithium-treated mice. These data are consistent with the hypothesis that the behavioral effects of lithium are mediated through its direct inhibition of GSK-3 and the consequent increase in beta-catenin. Additionally, recent data implicate the Wnt pathway inhibitor, Dkk-1, in stress-induced hippocampal damage that has been previously associated with affective disorders(Matrisciano et al., 2011). Most recently is a finding connecting DISC1 to this pathway. Numerous studies have associated DISC1 polymorphisms with affective disorders (e.g. Hodgkinson et al., 2004; Henna et al., 2009; Schosser et al., 2010). Recently it was shown that DISC1 inhibits GSK-3 activity through direct physical interaction, which stabilizes β-catenin. These results implicate DISC1 in GSK3/β-catenin signaling pathways and provide a framework for understanding how alterations in this pathway may contribute to the etiology of psychiatric disorders (Mao et al., 2009).
6 Conclusions and Translational Implications
β-catenin is essential for normal embryonic development of the central nervous system as well as normal neuronal functioning in adulthood. Alterations in β-catenin signaling lead to detrimental effects throughout the lifespan. As mentioned earlier, β-catenin knockouts are embryonic lethal (Machon et al., 2003). In contrast, embryonic transgenic mice that express stabilized β-catenin in neural precursors develop gross enlargements of the cerebral cortex, hippocampus, and amygdala (Chenn and Walsh, 2002, 2003). Thus, both down- and up-regulation of β-catenin can alter developmental processes. Such tight regulation of β-catenin function is also important for synapse assembly. Perturbations in β-catenin regulation produce deficits in both pre- and postsynaptic structure and function. Understanding how deregulated β-catenin function interferes with homeostasis of the healthy adult vertebrate brain may provide insight into the etiology of neurodegenerative conditions.
In summary, β-catenin is present from development into adulthood. It plays critical roles in many of the cellular and molecular functions that take place during all aspects of life. Understanding how β-catenin may function both during development, but also during synapse remodeling in adulthood, may help to understand how alterations in its normal regulation can lead to disease. β-catenin has been shown to be involved in synaptic plasticity, particularly involving emotional learning and memory processes. β-catenin appears to be involved in disorders related to decreased memory function (e.g. Alzheimer’s disease) as well as overly strong memory formation (such as fear learning in PTSD). Further understanding of the β-catenin pathway in adulthood may lead to better mechanistic appreciation for the structural mechanisms underlying emotional and declarative learning and memory as well as provide novel therapeutic approaches in memory related disorders.
Highlights.
β-catenin has been implicated in neuronal synapse regulation and remodeling.
We review basic molecular and structural mechanisms of synaptic plasticity.
β-catenin is a ‘hub’ protein in the processes underlying synaptic plasticity.
β-catenin mediates structural change associated with emotional memory formation.
Acknowledgements
This work was primarily supported by National Institutes of Mental Health (MH071537) and the Burroughs Wellcome Fund (KJR).
Financial Disclosure Statement: There were no commercial sponsors or commercial relationships related to the current work. Within the last 3 years, Dr. Ressler has received awards and/or funding support related to other studies from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and is a cofounder of Extinction Pharmaceuticals for use of NMDA-based therapeutics with psychotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105(1):13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002;115(Pt 24):4743–4753. doi: 10.1242/jcs.00154. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruses JL. N-cadherin signaling in synapse formation and neuronal physiology. Mol Neurobiol. 2006;33:237–252. doi: 10.1385/MN:33:3:237. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at 'silent synapses'. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Del Valle-Pérez B, Arqués O, Vinyoles M, de Herreros AG, Duñach M. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol. 2011 Jul;31(14):2877–2888. doi: 10.1128/MCB.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8(4):458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, Richardson EC, Fujita Y. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2007;27(10):3804–3816. doi: 10.1128/MCB.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Conde F, Cheruel F, el Massioui N. Learning-dependent activation of Fra-1: involvement of ventral hippocampus and SNc/VTA complex in learning and habit formation. Brain Res Bull. 2006;68:233–248. doi: 10.1016/j.brainresbull.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Anderson CL. Stimulation-induced changes in dimensions of stalks of dendritic spines in the dentate molecular layer. Exp Neurol. 1981;74:621–627. doi: 10.1016/0014-4886(81)90197-7. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PE, Yang DS, Yu G, Levesque L, Nishimura M, Arawaka S, Serpell LC, Rogaeva E, St George-Hyslop P. Presenilin structure, function and role in Alzheimer disease. Biochim Biophys Acta. 2000;1502:1–15. doi: 10.1016/s0925-4439(00)00028-4. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: "vertebrate" and "invertebrate" perspectives. Cold Spring Harb Perspect Biol. 2009;1(4) doi: 10.1101/cshperspect.a003079. a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington's disease. EMBO J. 2010;29(14):2433–2445. doi: 10.1038/emboj.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7(11):1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell KL, Casserly B, Harrington EO. Role of protein tyrosine phosphatase SHP2 in barrier function of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2010;298(3):L361–L370. doi: 10.1152/ajplung.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, Blackwood D, Curtis D, Deary IJ, Harris SE, Isometsä ET, Lawrence J, Lönnqvist J, Muir W, Palotie A, Partonen T, Paunio T, Pylkkö E, Robinson M, Soronen P, Suominen K, Suvisaari J, Thirumalai S, St Clair D, Gurling H, Peltonen L, Porteous D. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14(9):865–873. doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2) doi: 10.1101/cshperspect.a002915. a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, Malhotra AK. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75(5):862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Alvarez A, Godoy J, Reyes A, De Ferrari GV. Acetylcholinesterase-amyloid-beta-peptide interaction and Wnt signaling involvement in Abeta neurotoxicity. Acta Neurol Scand Suppl. 2000;176:53–59. doi: 10.1034/j.1600-0404.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Hirota Y, Ozaki K, Okano H, Takeichi M, Uemura T. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol Cell Neurosci. 2002;19:375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55(8):781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Konorski J. Conditioned Reflexes and Neuron Organization. Cambridge: Cambridge University Press; 1948. [Google Scholar]
- Krueger SR, Kolar A, Fitzsimonds RM. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–957. doi: 10.1016/s0896-6273(03)00729-3. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134(6):1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Peng IF, Ng YG, Yanagisawa M, Bamji SX, Elia LP, Balsamo J, Lilien J, Anastasiadis PZ, Ullian EM, Reichardt LF. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and beta-catenin. J Cell Biol. 2008;183:893–908. doi: 10.1083/jcb.200807188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Leroy K, Ando K, Heraud C, Yilmaz Z, Authelet M, Boeynaems JM, Buee L, De Decker R, Brion JP. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J Alzheimers Dis. 19:705–719. doi: 10.3233/JAD-2010-1276. [DOI] [PubMed] [Google Scholar]
- Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11(11):1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009;186(2):219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Busceti CL, Bucci D, Orlando R, Caruso A, Molinaro G, Cappuccio I, Riozzi B, Gradini R, Motolese M, Caraci F, Copani A, Scaccianoce S, Melchiorri D, Bruno V, Battaglia G, Nicoletti F. Induction of the Wnt antagonist Dickkopf-1 is involved in stress-induced hippocampal damage. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016447. e16447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256(5057):638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, Yasutake K, Nihonmatsu N, Wolozin B, Takashima A. Direct association of presenilin-1 with beta-catenin. FEBS Lett. 1998;433:73–77. doi: 10.1016/s0014-5793(98)00886-2. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36(Pt 2):149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y. beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci U S A. 2007;104:13479–13484. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Cain CK, Bedont J, Monfils MH, Ledoux JE. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci U S A. 2010;107:9418–9423. doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YR, He S, Marie H, Zeng SY, Ma J, Tan ZJ, Lee SY, Malenka RC, Yu X. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron. 2009;61:71–84. doi: 10.1016/j.neuron.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, Duñach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylatio and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23(7):2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- Ramon y, Cajal S. La fine structure des centres nerveux. Proc. R. Soc. Land. 1894;55:444–468. [Google Scholar]
- Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106(12):1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24(4):742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Maravall M, Svoboda K. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2001;11:349–356. doi: 10.1016/s0959-4388(00)00218-x. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schosser A, Gaysina D, Cohen-Woods S, Chow PC, Martucci L, Craddock N, Farmer A, Korszun A, Gunasinghe C, Gray J, Jones L, Tozzi F, Perry J, Muglia P, Owen MJ, Craig IW, McGuffin P. Association of DISC1 and TSNAX genes and affective disorders in the depression case-control (DeCC) and bipolar affective case-control (BACCS) studies. Mol Psychiatry. 2010;15(8):844–849. doi: 10.1038/mp.2009.21. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Murase S. Cadherins and synaptic plasticity: activity-dependent cyclin-dependent kinase 5 regulation of synaptic beta-catenin-cadherin interactions. Philos Trans R Soc Lond B Biol Sci. 2003;358:749–756. doi: 10.1098/rstb.2002.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci U S A. 2010;107(24):11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Fluhrer R, Haass C. Intramembrane proteolysis by gamma-secretase. J Biol Chem. 2008a;283:29627–29631. doi: 10.1074/jbc.R800010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008b;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci U S A. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20(14):3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Suzuki K, Tsukatani Y. Induction of tyrosine phosphorylation and association of beta-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence. Oncogene. 1997;15:71–78. doi: 10.1038/sj.onc.1201160. [DOI] [PubMed] [Google Scholar]
- Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci U S A. 1998;95:9637–9641. doi: 10.1073/pnas.95.16.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 2005;15:216–221. doi: 10.1016/j.tcb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry. 15:272–285. 228. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Verkaar F, Zaman GJ. New avenues to target Wnt/β-catenin signaling. Drug Discov Today. 2011;16(1–2):35–41. doi: 10.1016/j.drudis.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Wada A. Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J Pharmacol Sci. 2009;110(1):14–28. doi: 10.1254/jphs.09r02cr. [DOI] [PubMed] [Google Scholar]
- Wu H, Symes K, Seldin DC, Dominguez I. Threonine 393 of beta-catenin regulates interaction with Axin. J Cell Biochem. 2009;108(1):52–63. doi: 10.1002/jcb.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, Balsamo J. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yu G, Chen F, Levesque G, Nishimura M, Zhang DM, Levesque L, Rogaeva E, Xu D, Liang Y, Duthie M, St George-Hyslop PH, Fraser PE. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta-catenin. J Biol Chem. 1998;273:16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–2090. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]