Background: Mutations causing paclitaxel resistance stimulate microtubule detachment from centrosomes.
Results: Depletion of mitotic centromere-associated kinesin (MCAK) reverses microtubule detachment and paclitaxel resistance.
Conclusion: MCAK plays a pivotal role in the mechanism of microtubule detachment and drug resistance.
Significance: The ability of MCAK to reverse paclitaxel resistance identifies modulators of microtubule detachment as important new drug targets.
Keywords: Anticancer Drug, Cytoskeleton, Drug Resistance, Kinesin, Microtubules, Tubulin, MCAK, Class III β-Tubulin, Kif2c, Microtubule Detachment
Abstract
Paclitaxel has powerful anticancer activity, but some tumors are inherently resistant to the drug, whereas others are initially sensitive but acquire resistance during treatment. To deal with this problem, it will be necessary to understand the mechanisms of drug action and resistance. Recent studies indicate that paclitaxel blocks cell division by inhibiting the detachment of microtubules from centrosomes. Here, we demonstrate that mitotic centromere-associated kinesin (MCAK), a kinesin-related protein that destabilizes microtubules, plays an important role in microtubule detachment. Depletion of MCAK altered mitotic spindle morphology, increased the frequency of lagging chromosomes, and inhibited the proliferation of WT CHO cells, confirming that it is an essential protein for cell division. In contrast, MCAK depletion rescued the proliferation of mutant paclitaxel-dependent cell lines that are unable to divide because of defective spindle function resulting from altered α-tubulin or class III β-tubulin overexpression. In concert with the correction of mitotic defects, loss of MCAK reversed an aberrantly high frequency of microtubule detachment in the mutant cells and increased their sensitivity to paclitaxel. The results indicate that MCAK affects cell sensitivity to mitotic inhibitors by modulating the frequency of microtubule detachment, and they demonstrate that changes in a microtubule-interacting protein can reverse the effects of mutant tubulin expression.
Introduction
Paclitaxel is a powerful agent for treating breast, ovarian, head and neck, lung, and other forms of cancer. At the doses typically given to patients, the drug interferes with microtubule assembly, thereby prolonging the mitotic checkpoint and causing tumor cells to die by apoptosis (1). Despite its effectiveness, resistance to paclitaxel therapy often develops and leaves patients with a poor prognosis.
In an effort to understand the molecular basis for drug resistance, a number of laboratories have isolated resistant mammalian cells in culture (2, 3). Early studies demonstrated that alterations in α- and β-tubulin could confer resistance and that the altered tubulin acted to modulate microtubule assembly in a manner that counteracted the presence of the selecting drug (4–6). These mutants shared a common set of properties. Cells selected for resistance to a microtubule-destabilizing drug such as vinblastine were cross-resistant to many other destabilizing drugs but were more sensitive to stabilizing drugs such as paclitaxel. The converse was also found to be true, i.e. paclitaxel-resistant cells were cross-resistant to epothilone A and docetaxel but were more sensitive to colcemid, vinblastine, and other microtubule-destabilizing drugs. Changes in microtubule polymer levels also followed a predictable pattern: paclitaxel-resistant cells had less polymer than normal, and colcemid- and vinblastine-resistant cells had more polymer (7).
A similar set of properties were found in paclitaxel-resistant cells created by overexpression of specific β-tubulin genes. The mammalian β-tubulin gene family contains at least seven members that differ principally in their C-terminal 15 amino acids, but they have additional sporadic differences throughout their sequences (8). The distinctive C-terminal tails have allowed seven isotypes of β-tubulin to be described (β1, β2, β3, β4a, β4b, β5, and β6) (9). Some of these isotypes are ubiquitously expressed, whereas others (e.g. β3, β4a, and β6) are restricted to specific tissues (10, 11). In addition, most cultured cell lines express a small subset of these isotypes; for example, CHO cell microtubules are composed of 70% β1, 25% β4b, and 5% β5 (12). Transfection of CHO cells with cDNAs encoding each of the different isotypes of β-tubulin has shown that altered expression of some (but not all) isotypes is capable of conferring resistance to paclitaxel. Overexpression of the β1, β4b, and β2 isotypes had no effect on microtubule assembly or cell sensitivity to paclitaxel (13); but overexpression of β3, a brain-specific isotype, reduced microtubule assembly and conferred resistance to the drug (14). The similar phenotypes observed in cells with overexpression of β3 and in cells with mutations in their endogenous tubulin suggest that β3 acts like a mutant form of tubulin to produce drug resistance.
The close association between reduced microtubule levels and paclitaxel resistance led us to propose that any treatment able to inhibit microtubule assembly should cause a resistance phenotype. Mitotic centromere-associated kinesin (MCAK),2 a microtubule motor protein that is also known as Kif2c, has been shown to catalyze microtubule disassembly and therefore provides a good test of that hypothesis (15). Recently, we reported that overexpression of MCAK in wild-type CHO cells reduces microtubule content and confers resistance to paclitaxel (16). We now demonstrate that depletion of MCAK is able to reverse paclitaxel resistance caused by mutant tubulin or by β3-tubulin overexpression, and we further show that the reversal of drug resistance is associated with a reduced frequency of microtubule detachment from centrosomes.
EXPERIMENTAL PROCEDURES
Cell Lines, Antibodies, and Drugs
CHO cells were maintained in α-minimum essential medium (Mediatech, Inc., Manassas, VA) supplemented with 5% fetal bovine serum (Gemini Bio-Products). Drugs and mouse anti-α-tubulin monoclonal antibody DM1A were from Sigma-Aldrich, rabbit anti-MCAK polyclonal antibody was from Cytoskeleton, Inc., and Alexa-conjugated goat secondary antibodies were from Invitrogen.
Construction of Plasmid Expressing shRNAs Specific for MCAK
To silence the expression of CHO MCAK, we used plasmid pBS/U6 (42) to express shRNA. Nucleotides 657–676 from CHO MCAK (GenBankTM accession number U11790) were introduced into pBS/U6 using oligonucleotides 5′-AAT TCA AAA AGG GCC CAG AAC TCG GAA ATA ACA AGC TTG ATT ATT TCC GAG TTC TGG GCC-3′ and 5′-GGC CCA GAA CTC GGA AAT AAT CAA GCT TGT TAT TTC CGA GTT CTG GGC CCT TTT TG-3′. To allow selection of CHO cells containing shRNA, a pTOP-hygro plasmid (43) was modified by replacing its CMV with the U6 promoter to create plasmid pHygro/U6 for use as an empty vector control or with the U6 promoter plus the MCAK shRNA-encoding sequence to create plasmid pHygro/U6-MCAK.
RT-PCR
Expression of endogenous MCAK was assessed using the following primers to selectively amplify the C-terminal region of the cDNA: forward primer MCAK1733F, 5′-GGC ATA AGC TCC TGT G-3′; and reverse primer MCAK2120R, 5′-GCA GGG CTG AGA AGT GC-3′. As a control, a portion of CHO β1-tubulin was also amplified using forward primer 5′-AAT GCC ACC CTG TCT GTC C-3′ and reverse primer 5′-GGG AAC TAA GTA GCC TG-3′.
Transfection and Live Cell Microscopy
Cells were transfected in 35-mm dishes using Lipofectamine (Invitrogen). For live cell imaging, cells were transfected with GFP-MAP4 (44) and grown for 16–48 h. The medium was then replaced with McCoy's 5A medium containing 25 mm HEPES (Mediatech, Inc.) and images were captured every 5 s at 37 °C using a DeltaVision Core microscope (Applied Precision, Inc.). Microtubule detachment was measured using cells in which the centrosome was clearly positioned under the nucleus to provide maximum contrast. Detachments were counted in 10–20 individual cells, each observed for 4 min.
Immunofluorescence
Cells were seeded onto glass coverslips, grown to 50–70% confluence, and rinsed in PBS. To minimize background fluorescence, the cells were pre-extracted before fixation with methanol at −20 °C by incubating them for 1 min at 4 °C with microtubule buffer (20 mm Tris-HCl (pH 6.8), 1 mm MgCl2, 2 mm EGTA, and 0.5% Nonidet P-40) containing 4 μg/ml paclitaxel. Fixed cells were washed in PBS, incubated with 1:100 dilutions of DM1A and anti-MCAK antibodies for 2 h at 37 °C, washed again in PBS, and incubated with 1:100 dilutions of Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-rabbit IgG that included 1 μg/ml DAPI. Cells were viewed using an Optiphot microscope (Nikon Corp.) equipped with a MagnaFire digital camera (Optronics).
Electrophoresis and Western Blotting
Cellular proteins solubilized in 1% SDS were precipitated with 5 volumes of acetone and resuspended in sample buffer containing 0.0625 m Tris-HCl (pH 6.8), 2.5% SDS, 5% 2-mercaptoethanol, and 10% glycerol. The proteins were then separated on 7.5% polyacrylamide minigels and transferred to nitrocellulose membranes. Membranes were blocked with 2% milk in PBS containing 0.05% Tween 20 and incubated in a 1:1000 dilution of anti-MCAK antibody and a 1:30,000 dilution of anti-actin monoclonal antibody C4 (Chemicon) for 1 h, followed by 1:2000 dilutions of Alexa 647-conjugated goat anti-rabbit and anti-mouse IgGs (Invitrogen). Bands were detected by fluorescence emission using a Storm imager (Molecular Dynamics, Inc.).
Statistics
Data are expressed as the mean ± S.D. Statistical significance of differences between groups was determined using Student's t test.
RESULTS
Depletion of MCAK Causes Mitotic Defects in Wild-type CHO Cells
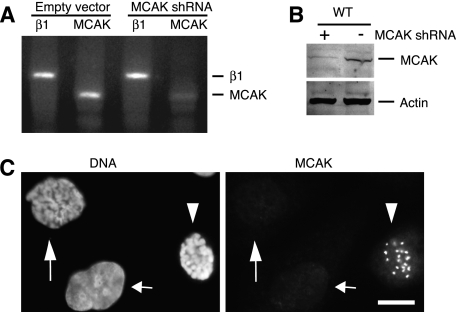
To deplete CHO cell MCAK, we designed a 21-nucleotide sequence that would produce a shRNA and cloned the sequence into the pHygro/U6 vector. Cells transiently transfected with this vector were depleted of MCAK mRNA as detected by semiquantitative RT-PCR (Fig. 1A). MCAK protein was also depleted (Fig. 1B) and resulted in a loss of the centromere and spindle pole immunostaining (Fig. 1C, long arrows) that is normally seen in untransfected mitotic cells (Fig. 1C, arrowheads) (17). An interphase cell lacking MCAK staining was also present (Fig. 1C, short arrows), but it is unclear whether it represents a transfected cell depleted of MCAK or a cell in early G1 phase, which would also be expected to have little, if any, MCAK staining because the protein is degraded at the metaphase-to-anaphase transition (18).
FIGURE 1.
Effects of shRNA on MCAK production. Cells were transiently transfected with pHygro/U6-MCAK to produce MCAK shRNA or with empty vector pHygro/U6. A, RT-PCR was carried out using primers specific for β1-tubulin or MCAK. B, Western blot analysis of transfected WT CHO cells using an antibody specific for MCAK. An antibody was also included to monitor actin as a loading control. C, immunofluorescence of cells transiently transfected with pHygro/U6-MCAK. Cells were stained with anti-MCAK antibody and with DAPI to label the DNA. The long arrows indicate a transfected prophase cell depleted of MCAK. The arrowheads show an untransfected prophase cell in the same field with prominent MCAK staining. The third nucleus (short arrows) is from an interphase cell. Scale bar = 10 μm.
MCAK depletion did not cause major disruptions in CHO spindle morphology, but it did result in more abundant and pronounced astral fibers that were 1.5–2-fold longer than the controls (Fig. 2A), a condition previously called “hairy spindles” (19). In addition, a large increase in the frequency of lagging chromosomes (Fig. 2B, arrow) was seen in cells depleted of MCAK. Quantification of these changes is summarized in Fig. 2C. The lagging chromosomes caused a time-dependent increase in cells with abnormal nuclear morphologies, ranging from 3% in untransfected cells to 10% at 12 h and 50% at 2 days following transfection with shRNA. The extent of multinucleation underestimates the effects of MCAK depletion because the transfection efficiency was only 70%, but the results nonetheless confirm studies in other cell lines indicating that MCAK is essential for faithful chromosome segregation during mitosis (20).
FIGURE 2.
MCAK depletion increases astral microtubule density and increases the frequency of lagging chromosomes. Wild-type CHO cells were transfected with the empty vector (Control) or with the same vector engineered to express shRNA for MCAK. A, after 24 h, the cells were stained with an antibody to α-tubulin. Metaphase spindles are shown. Cells transfected with shRNA produced mitotic spindles that had a greater abundance of astral fibers that were, on average, 1.5–2-fold longer than their normal counterparts. The insets show 2-fold enlargements of the area around one spindle pole. B, cellular DNA stained with DAPI. Chromosomes that appeared to lack a connection to the spindle poles, did not segregate with the others during anaphase, or remained apart from the others during telophase (arrow) were scored as lagging chromosomes. Scale bar = 10 μm. C, the percentage of transfected mitotic cells having hairy spindles and lagging chromosomes was determined in three independent experiments and plotted. **, p < 0.005.
Proliferation of Paclitaxel-dependent Mutants Is Rescued by Depleting MCAK
Cells selected for resistance to paclitaxel frequently have mutations in tubulin that reduce microtubule assembly (5, 6). In more extreme cases, these mutations perturb microtubule assembly to such an extent that the cells are unable to divide unless a microtubule-stabilizing agent such as paclitaxel is present in the growth medium, thus producing a paclitaxel-dependent phenotype (21, 22). We reported recently that paclitaxel-dependent cells have a greatly elevated frequency of microtubules detaching from centrosomes and spindle poles and that paclitaxel suppresses the detachment frequency at the same drug concentrations that rescue cell division (23). We also reported recently that overexpression of MCAK increases the frequency of microtubule detachment in WT cells and makes the cells resistant to paclitaxel (16). On the basis of those observations, we predicted that cell division in paclitaxel-dependent cells might be rescued by depleting MCAK.
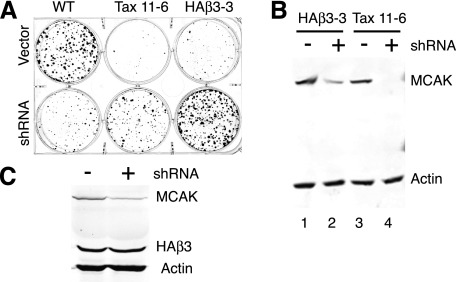
To test that hypothesis, we examined two paclitaxel-dependent CHO cell lines. Tax 11-6 is a cell line with an E77K substitution in α-tubulin that was shown previously to have reduced microtubule polymer and an elevated frequency of microtubule detachment (23). HAβ3-3 is a cell line that was created by transfecting wild-type CHO cells with a plasmid encoding HA-tagged β3-tubulin, an isotype whose expression is normally restricted to brain (10, 11). The transfected cells were found to have reduced microtubule polymer and to be paclitaxel-resistant and paclitaxel-dependent (14). To determine whether MCAK depletion would rescue cell division in these two cell lines, wild-type, Tax 11-6, and HAβ3-3 cells were transfected with a control vector or with a vector that would produce shRNA to deplete MCAK. The transfected cells were then plated at low density to monitor colony formation. As shown in Fig. 3, wild-type cells transfected with the control vector formed numerous colonies, but the shRNA-transfected cells formed significantly fewer colonies that grew poorly, as would be expected for the loss of a protein that is essential for normal cell proliferation. In contrast, the paclitaxel-dependent cell lines transfected with the control vector had difficulty forming colonies when paclitaxel was omitted from the growth medium, but the shRNA-transfected cells produced robust colonies, suggesting that MCAK depletion had reversed the paclitaxel-dependent phenotype. The results further indicated that tubulin mutations or expression of β3-tubulin can compensate for the loss of MCAK and allow the cells to survive the loss of an essential microtubule-interacting protein. To confirm that MCAK had been depleted, endogenous MCAK levels were compared in the paclitaxel-dependent cell lines before transfection and after survival in paclitaxel-free medium following transfection with MCAK shRNA (Fig. 3B). A partial reduction of MCAK was seen in HAβ3-3 cells, and a more complete reduction was seen in Tax 11-6 cells. This result is consistent with the observation that the paclitaxel-dependent phenotype is less severe in HAβ3-3 cells, but to rule out the possibility that some of the HAβ3-3 cells survived without paclitaxel because they lost expression of HA-β3-tubulin, we also probed the cell lysates with an antibody specific for the HA tag (Fig. 3C). In contrast to the reduction observed for MCAK, there was no loss of the HA-β3-tubulin. We thus conclude that both mutant cell lines were rescued from their paclitaxel dependence by the decrease or loss of MCAK.
FIGURE 3.
Effect of MCAK depletion on the proliferation of paclitaxel-dependent cells. A, WT CHO cells and paclitaxel-dependent cell lines Tax 11-6 and HAβ3-3 were transfected with pHygro/U6 (Vector) or pHygro/U6-MCAK (shRNA) and plated for colony formation in hygromycin but without paclitaxel. B, extracts from untransfected HAβ3-3 and Tax 11-6 cells (lanes 1 and 3) and from the same cells that survived in the absence of paclitaxel following transfection with pHygro/U6-MCAK (lanes 2 and 4) were examined by Western blotting with antibodies to MCAK. An antibody to actin was included as a loading control. C, HAβ3-3 cells before transfection (−) and after survival following MCAK depletion (+) were also examined by Western blotting with antibodies to MCAK and the HA tag to ensure that loss of HAβ3-tubulin was not the reason they lost paclitaxel dependence.
Loss of Paclitaxel Dependence Is Associated with Reduced Microtubule Detachment
Previous studies showed that Tax 11-6 cells have an elevated frequency at which microtubules detach from centrosomes and spindle poles and that similar concentrations of paclitaxel are able to suppress detachment and rescue cell division (23). To determine whether paclitaxel dependence in HAβ3-3 cells is also associated with elevated microtubule detachment, live cell imaging studies were carried out. As shown in Fig. 4A, frequent examples of microtubules detaching from centrosomes were seen in these cells. To determine whether MCAK depletion reduced detachment in addition to reversing the paclitaxel-dependent phenotype, the detachment frequencies of control and shRNA-transfected cells were compared. The results indicated that both mutant cell lines transfected with the control vector had an elevated frequency of microtubule detachment compared with wild-type cells, but those frequencies returned to near-normal levels in cells that were able to proliferate in paclitaxel-free medium following MCAK depletion (Fig. 4B). Thus, paclitaxel dependence conferred by a tubulin mutation or by expression of β3-tubulin was similarly associated with an increased frequency of microtubule detachment, and a treatment that reduced the frequency of detachment by depleting MCAK allowed both cell lines to divide without paclitaxel. The results strongly indicate that an elevated frequency of microtubule detachment forms the basis for the paclitaxel-dependent phenotype and that MCAK is likely to be at least one of the proteins involved in the mechanism of detachment.
FIGURE 4.
Microtubule detachment from centrosomes. A, HAβ3-3 cells were transfected with GFP-MAP4, and live cell images of fluorescent microtubules were recorded every 5 s. The asterisks indicate the position of the centrosome. The arrowheads mark the position of the minus-end of a microtubule that was seen detaching from the centrosome. Scale bar = 10 μm. B, quantification of the frequency of microtubule detachment per cell per min in WT cells and in paclitaxel-dependent cells (Tax 11-6 and HAβ3-3) transfected with the empty vector (open bars) or with a vector producing shRNA to MCAK and then selected in hygromycin to enrich for cells harboring plasmid DNA (filled bars). ***, p < 0.0001. There was no statistically significant difference in detachment rates between WT and mutant cell lines following depletion of MCAK (filled bars).
Mutant Cells Depleted of MCAK Have Increased Sensitivity to Paclitaxel
Paclitaxel-dependent cells are extreme examples of paclitaxel-resistant cells, i.e. their tubulin alterations decrease microtubule assembly to a greater extent, and the cells have less sensitivity to paclitaxel compared with cells that are resistant to, but not dependent on, the drug (7). The loss of drug dependence would therefore suggest that the cells should have gained increased sensitivity to paclitaxel. To test this prediction, we made use of the observation that defective spindle function in CHO cells causes missegregation of chromosomes and inhibits cytokinesis but the cells slip past the mitotic checkpoint and reenter G1-phase as large multinucleated cells (24–26). Using the appearance of multinucleated cells as a readout for problems encountered during mitosis, we monitored the effects of MCAK depletion on the sensitivity of the mutant cell lines to paclitaxel. Approximately 3% of the wild-type CHO cells were found to be multinucleated during normal growth, but the percentage increased dramatically when paclitaxel reached a concentration that interferes with cell division (Fig. 5, filled circles). In contrast, the percentage of multinucleated cells was high for HAβ3-3 cells (Fig. 5B, filled triangles) and higher still for Tax 11-6 cells (Fig. 5A, filled squares) when the cells were cultured without paclitaxel. Multinucleated cells became much less abundant in both mutant cell lines when paclitaxel was present at normally toxic concentrations, indicating that normal cell division was rescued; but the percentage of multinucleated cells rose again at higher drug concentrations, indicating that the increased amount of drug had again become toxic. The rightward shift in the toxicity portion of the curve compared with wild-type cells reflects the paclitaxel resistance of the drug-dependent cell lines. On the other hand, mutant cells treated with shRNA to deplete MCAK behaved much more like the wild-type cells. Both HAβ3-3 (Fig. 5B, open triangles) and Tax 11-6 (Fig. 5A, open squares) cells had a much lower percentage of multinucleated cells in the absence of paclitaxel, but this percentage increased at the higher toxic drug concentrations. The leftward shift in these curves relative to the untransfected cells with normal levels of MCAK indicates that MCAK depletion made the cells more sensitive to the toxic effects of paclitaxel on cell division. This is especially noticeable for HAβ3-3 cells, which became even more drug-sensitive than wild-type cells following depletion of MCAK, a result that is consistent with the weaker drug-dependent phenotype of this cell line (14).
FIGURE 5.
Effect of MCAK depletion on sensitivity to paclitaxel. The percentage of multinucleated cells (cells that failed to divide) was determined after a 48-h exposure to a series of paclitaxel concentrations. A, WT CHO cells (●), Tax 11-6 cells (■), and Tax 11-6 cells transfected to produce shRNA to MCAK (□) are compared. B, WT CHO cells (●), HAβ3-3 cells (▴), and HAβ3-3 cells transfected to produce shRNA to MCAK (△) are compared.
DISCUSSION
The emergence of drug-resistant tumor cells presents a major barrier to successful chemotherapy. To deal with this problem, it is essential to understand the mechanisms by which resistance arises and to devise strategies to prevent or mitigate its occurrence. A particularly effective class of drugs used in cancer chemotherapy affects microtubule assembly and thereby blocks cell division. These drugs fall into two major groups: those that inhibit and those that promote microtubule assembly. Despite these differing actions, both groups interfere with mitotic spindle function, block cells in mitosis, and prevent cell division. Although the exact mechanism by which these drugs interfere with mitosis is unclear, the current consensus is that they act by suppressing microtubule dynamics (27).
Cellular microtubules are continuously nucleated at the centrosome, an organelle located near the nucleus, and extend their growing plus-ends toward the plasma membrane while their minus-ends remain embedded in the centrosome. The plus-ends have been observed to undergo stochastic episodes of growth and shortening interspersed by periods of pause, a behavior known as “dynamic instability” (28). This behavior increases the plasticity of the microtubules, allowing them to rapidly remodel in response to cellular and environmental cues (29). For example, when cells enter mitosis, the cytoplasmic microtubule network is remodeled into a bipolar mitotic spindle apparatus. Spindle microtubules probe the cytoplasm and are believed to become stabilized when they bind to the kinetochore region on chromosomes. These interactions ensure the proper alignment of the condensed chromosomes and the subsequent segregation of their sister chromatids. It makes sense that microtubule growth and shortening would be needed for this “search and capture” activity and that drugs could interfere with spindle function by suppressing this activity.
Despite the logic used to reach this conclusion, we demonstrated recently that drug concentrations 10-fold lower than required to block cell division were sufficient to maximally inhibit microtubule dynamics in a variety of cell types (30). Moreover, paclitaxel-dependent cell lines that require the drug for proliferation had highly suppressed microtubule dynamics at the very drug concentrations that allowed the cells to divide (23). Together, these results argue that microtubule drugs do not inhibit cell division by simply interfering with dynamics. We found instead that drug concentrations able to inhibit mitosis act by altering the frequency at which microtubule minus-ends detach from centrosomes (23, 30).
Currently, little is known about the process of microtubule detachment. A small number of publications have previously described this behavior, but the mechanism by which detachment occurs has yet to be deciphered (23, 30–34). Detachment frequencies appear to be very low in interphase cells (30, 32, 34). However, we reported recently that the frequency increases significantly during mitosis, suggesting that detachment is a cell cycle-regulated process necessary for normal spindle function and cell division (30). A similar conclusion was reached by another laboratory (33), but the authors found that the detachment frequency was elevated during anaphase, whereas we observed stimulation of the process as early as prophase. The role of microtubule detachment in spindle function is unclear. Detached microtubules become fragments of varying size and stability that are able to translocate through the cytoplasm by an unknown mechanism, and one study found evidence that peripheral microtubule fragments become incorporated into the mitotic spindle (35). Other recent studies have indicated that meiotic spindles are formed from overlapping microtubule fragments that may be held together by microtubule motor proteins (36–38). It is thus tempting to speculate that mitotic spindles similarly require a mixture of detached microtubule fragments in addition to continuous microtubules anchored at the spindle poles to function properly (23, 39).
The importance of microtubule detachment is supported not only by its stimulation during mitosis but also by a number of additional observations. 1) Drugs that inhibit microtubule assembly increase the frequency of detachment, whereas drugs that promote assembly and stabilize microtubules decrease the frequency of detachment (23, 30). 2) Tubulin mutations that affect the sensitivity of cells to microtubule drugs alter the concentration of drug needed to modulate the frequency of detachment and produce microtubule fragments (30). 3) Tubulin mutations that block cell division and are correctable by the action of microtubule-stabilizing drugs (paclitaxel-dependent phenotype) have highly elevated frequencies of microtubule detachment that revert to normal following drug treatment (23). 4) Overexpression of β3-tubulin causes paclitaxel dependence and also elevates the frequency of microtubule detachment (Fig. 4).
The ability of drugs, tubulin mutations, and changes in tubulin composition to affect microtubule detachment indicates that the process is essential for normal mitotic progression. The observations that detachment occurs at centrosomes and spindle poles and that the process is cell cycle-regulated suggest that detachment does not simply result from mechanical stress; rather, it seems likely that specific proteins are involved in the process. MCAK is a protein that could be playing such a role. In addition to its location at mitotic centromeres, it is found in interphase centrosomes and at spindle poles (18, 40). Moreover, it is known to stimulate disassembly at both ends of isolated microtubules (15). In support of a role for MCAK in microtubule detachment, we showed recently that overproduction of the protein was able to stimulate detachment, produce microtubule fragments, confer resistance to paclitaxel, and enhance sensitivity to colcemid (16). In addition, depletion of MCAK has been shown to increase the density and length of microtubules at the spindle poles (Fig. 2A) (41), a phenotype consistent with a decreased frequency of microtubule detachment. We have now further shown that depletion of MCAK in paclitaxel-dependent cells harboring an α-tubulin mutation or expressing the β3-tubulin isotype caused a reversal of drug resistance and drug dependence. These changes were accompanied by a reduction in the frequency of microtubule detachment to near-normal values and provide evidence that MCAK is involved in the process of microtubule detachment.
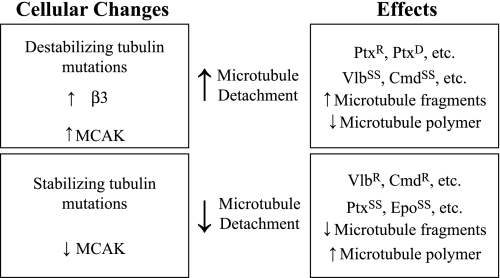
The results summarized in Fig. 6 support a model based on the idea that functional mitotic spindles require a balance between continuous and fragmented microtubules. Changes that disturb this balance by increasing or decreasing the frequency of detachment produce an altered drug response. For example, changes such as tubulin mutations, β3 overproduction, and MCAK overproduction that destabilize microtubules increase microtubule detachment and tip the balance toward too many fragments. These changes produce resistance to paclitaxel, a drug that exerts toxicity by inhibiting microtubule detachment and reducing fragment formation (23). On the other hand, these same changes increase sensitivity to vinblastine and colcemid because these drugs also increase detachment and produce microtubule fragments (30). In contrast, other changes such as microtubule-stabilizing tubulin mutations and MCAK depletion decrease microtubule detachment and tip the balance toward too few microtubule fragments. This increases sensitivity to paclitaxel and confers resistance to vinblastine and colcemid. The model is consistent with all of the experimental observations except for the predicted vinblastine and colcemid resistance in cells depleted of MCAK, which we have not been able to verify. We suggest that this discrepancy may be explained by the fact that MCAK functions not only at spindle poles but also at kinetochores, whereas the microtubule drugs specifically act at the spindle poles to inhibit mitosis. Thus, MCAK overexpression affects sensitivity to both paclitaxel (decreased sensitivity) and vinblastine (increased sensitivity) because MCAK is present to function at the kinetochores, whereas the increased detachment at the spindle poles caused by MCAK overexpression can be compensated by paclitaxel to produce paclitaxel resistance or aggravated by vinblastine to produce enhanced vinblastine sensitivity. On the other hand, depletion of MCAK leaves insufficient MCAK at the kinetochores to allow wild-type cell survival with or without drugs. It is interesting, however, that Tax 11-6 and HAβ3-3 cells were able to survive with moderate to large reductions in MCAK, indicating that, in contrast to microtubule drugs, tubulin mutations may be able to compensate for MCAK loss, even at kinetochores.
FIGURE 6.
Central role of microtubule detachment in resistance to microtubule drugs. Changes that increase or decrease microtubule detachment and the effects that are caused by those changes are summarized. Ptx, paclitaxel; Vlb, vinblastine; Cmd, colcemid; Epo, epothilone A; R, resistant; D, dependent; SS, supersensitive.
Acknowledgments
We thank Joanna Olmsted for contributing the GFP-MAP4 construct and Xiangwei He for the generous use of the DeltaVision microscope.
This work was supported, in whole or in part, by National Institutes of Health Grant CA085935 (to F. C.).
- MCAK
- mitotic centromere-associated kinesin.
REFERENCES
- 1. Musacchio A., Salmon E. D. (2007) Nat. Rev. Mol. Cell Biol. 8, 379–393 [DOI] [PubMed] [Google Scholar]
- 2. Cabral F. (2008) in Cancer Drug Discovery and Development: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology (Fojo A. T. ed) pp. 337–356, Humana Press, Totowa, NJ [Google Scholar]
- 3. Orr G. A., Verdier-Pinard P., McDaid H., Horwitz S. B. (2003) Oncogene 22, 7280–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabral F., Sobel M. E., Gottesman M. M. (1980) Cell 20, 29–36 [DOI] [PubMed] [Google Scholar]
- 5. Schibler M. J., Cabral F. (1986) J. Cell Biol. 102, 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minotti A. M., Barlow S. B., Cabral F. (1991) J. Biol. Chem. 266, 3987–3994 [PubMed] [Google Scholar]
- 7. Cabral F. (2000) Drug Resistance Updates 3, 1–6 [DOI] [PubMed] [Google Scholar]
- 8. Sullivan K. F. (1988) Ann. Rev. Cell Biol. 4, 687–716 [DOI] [PubMed] [Google Scholar]
- 9. Lopata M. A., Cleveland D. W. (1987) J. Cell Biol. 105, 1707–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leandro-García L. J., Leskelä S., Landa I., Montero-Conde C., López-Jiménez E., Letón R., Cascón A., Robledo M., Rodríguez-Antona C. (2010) Cytoskeleton 67, 214–223 [DOI] [PubMed] [Google Scholar]
- 11. Luduena R. F. (1998) Int. Rev. Cytol. 178, 207–275 [DOI] [PubMed] [Google Scholar]
- 12. Sawada T., Cabral F. (1989) J. Biol. Chem. 264, 3013–3020 [PubMed] [Google Scholar]
- 13. Blade K., Menick D. R., Cabral F. (1999) J. Cell Sci. 112, 2213–2221 [DOI] [PubMed] [Google Scholar]
- 14. Hari M., Yang H., Zeng C., Canizales M., Cabral F. (2003) Cell Motil. Cytoskeleton 56, 45–56 [DOI] [PubMed] [Google Scholar]
- 15. Desai A., Verma S., Mitchison T. J., Walczak C. E. (1999) Cell 96, 69–78 [DOI] [PubMed] [Google Scholar]
- 16. Ganguly A., Yang H., Cabral F. (2011) Mol. Cancer Ther. 10, 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ems-McClung S. C., Walczak C. E. (2010) Semin. Cell Dev. Biol. 21, 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganguly A., Bhattacharya R., Cabral F. (2008) Cell Cycle 7, 3187–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kline-Smith S. L., Walczak C. E. (2002) Mol. Biol. Cell 13, 2718–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline-Smith S. L., Khodjakov A., Hergert P., Walczak C. E. (2004) Mol. Biol. Cell 15, 1146–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barlow S. B., Gonzalez-Garay M. L., Cabral F. (2002) J. Cell Sci. 115, 3469–3478 [DOI] [PubMed] [Google Scholar]
- 22. Cabral F. R. (1983) J. Cell Biol. 97, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganguly A., Yang H., Cabral F. (2010) Mol. Cancer Ther. 9, 2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abraham I., Marcus M., Cabral F., Gottesman M. M. (1983) J. Cell Biol. 97, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cabral F., Barlow S. B. (1991) Pharmacol. Ther. 52, 159–171 [DOI] [PubMed] [Google Scholar]
- 26. Kung A. L., Sherwood S. W., Schimke R. T. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 9553–9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jordan M. A., Wilson L. (2004) Nature Rev. 4, 253–265 [DOI] [PubMed] [Google Scholar]
- 28. Mitchison T., Kirschner M. W. (1984) Nature 312, 237–242 [DOI] [PubMed] [Google Scholar]
- 29. Kirschner M., Mitchison T. (1986) Cell 45, 329–342 [DOI] [PubMed] [Google Scholar]
- 30. Yang H., Ganguly A., Cabral F. (2010) J. Biol. Chem. 285, 32242–32250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abal M., Piel M., Bouckson-Castaing V., Mogensen M., Sibarita J. B., Bornens M. (2002) J. Cell Biol. 159, 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keating T. J., Peloquin J. G., Rodionov V. I., Momcilovic D., Borisy G. G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5078–5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rusan N. M., Wadsworth P. (2005) J. Cell Biol. 168, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waterman-Storer C. M., Salmon E. D. (1997) J. Cell Biol. 139, 417–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tulu U. S., Rusan N. M., Wadsworth P. (2003) Curr. Biol. 13, 1894–1899 [DOI] [PubMed] [Google Scholar]
- 36. Burbank K. S., Groen A. C., Perlman Z. E., Fisher D. S., Mitchison T. J. (2006) J. Cell Biol. 175, 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srayko M., O'toole E. T., Hyman A. A., Müller-Reichert T. (2006) Curr. Biol. 16, 1944–1949 [DOI] [PubMed] [Google Scholar]
- 38. Yang G., Houghtaling B. R., Gaetz J., Liu J. Z., Danuser G., Kapoor T. M. (2007) Nat. Cell Biol. 9, 1233–1242 [DOI] [PubMed] [Google Scholar]
- 39. Gaglio T., Dionne M. A., Compton D. A. (1997) J. Cell Biol. 138, 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maney T., Hunter A. W., Wagenbach M., Wordeman L. (1998) J. Cell Biol. 142, 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizk R. S., Bohannon K. P., Wetzel L. A., Powers J., Shaw S. L., Walczak C. E. (2009) Mol. Biol. Cell 20, 1639–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sui G., Soohoo C., Affar el B., Gay F., Shi Y., Forrester W. C., Shi Y. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5515–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez-Garay M. L., Chang L., Blade K., Menick D. R., Cabral F. (1999) J. Biol. Chem. 274, 23875–23882 [DOI] [PubMed] [Google Scholar]
- 44. Olson K. R., McIntosh J. R., Olmsted J. B. (1995) J. Cell Biol. 130, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]