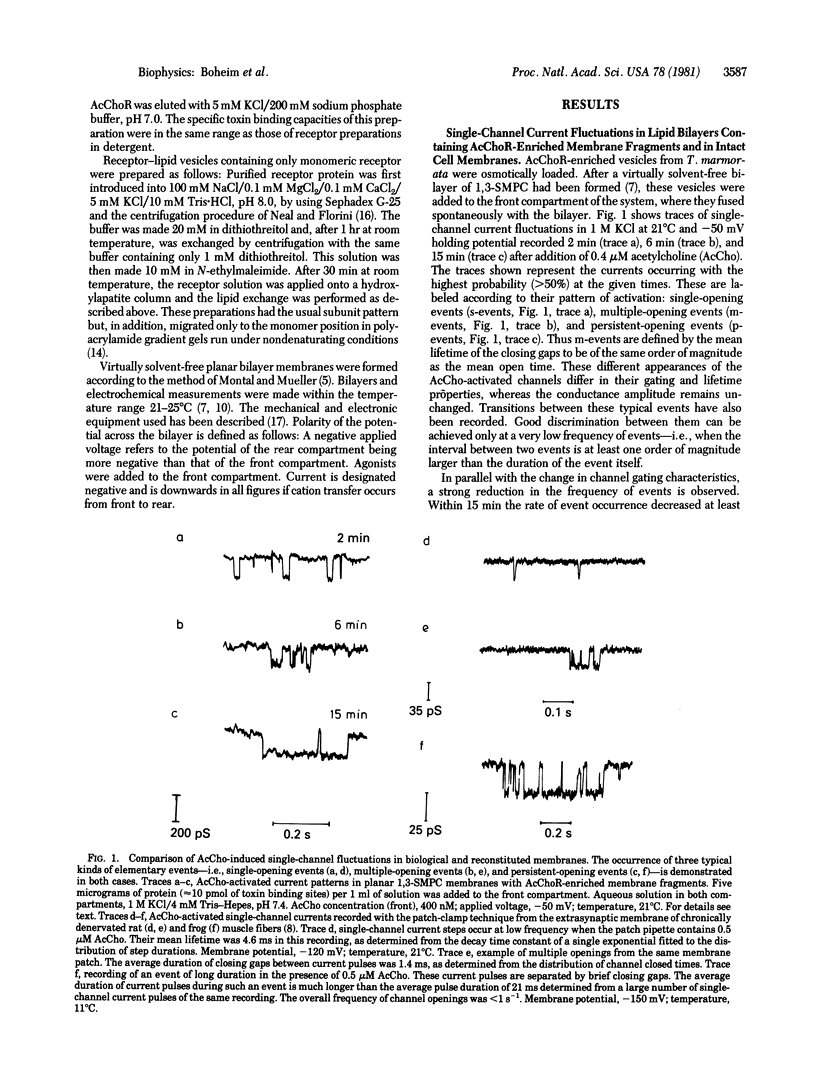
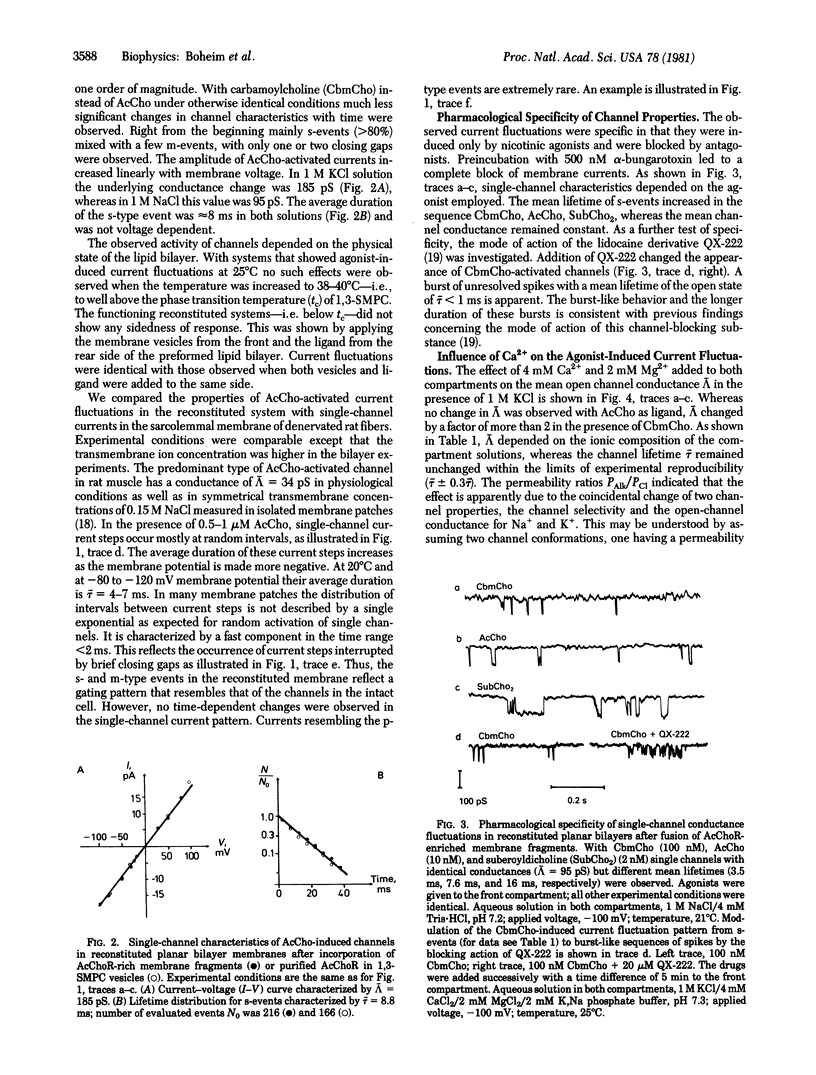
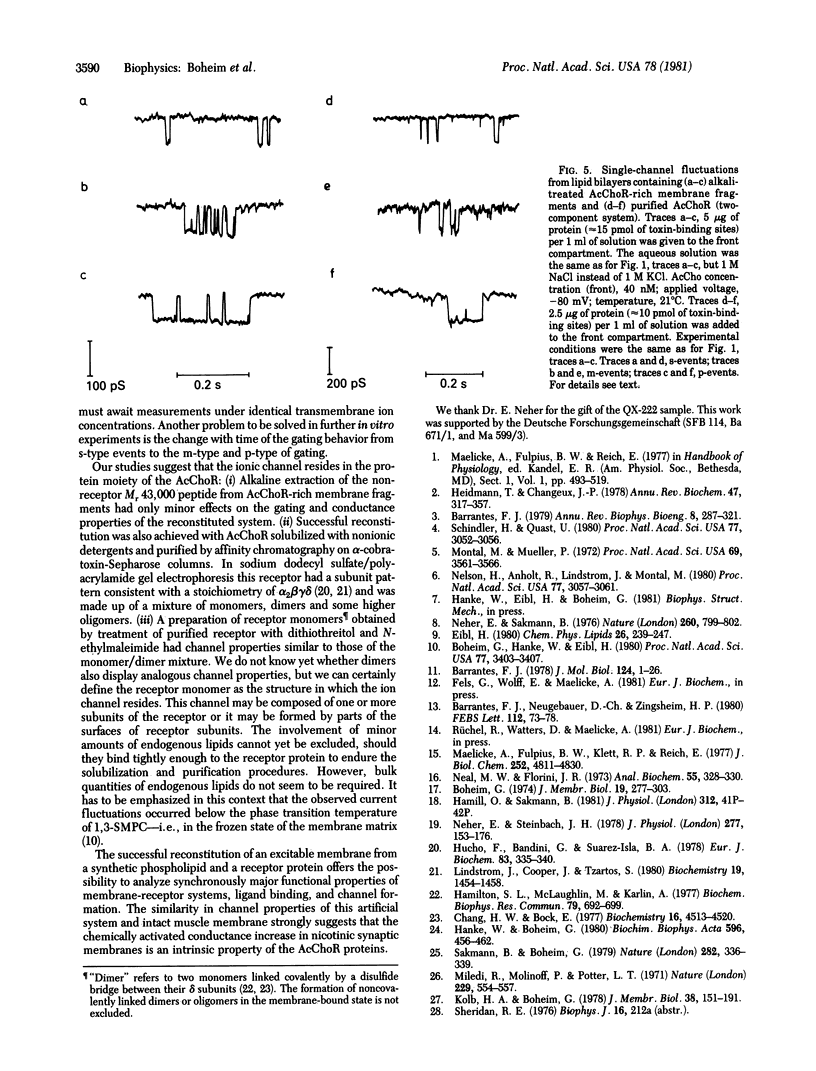
Abstract
Planar lipid bilayers were formed with the mixed chain phospholipid 1-stearoyl-3-myristolglycero-2-phosphocholine. Acetylcholine receptor membrane fragments or the purified receptor protein was incorporated into these bilayers by fusing receptor-containing vesicles with the planar membranes a few degrees below the lipid phase transition temperature. Single-channel currents activated by nicotinic agonists in the reconstituted system resembled those observed in intact rat and frog muscle membrane as measured by the patch clamp technique. The observed channel characteristics did not depend on the degree of receptor purification. Thus, the receptor-enriched fragments and those depleted of nonreceptor peripheral peptides, the purified receptor monomer/dimer mixtures, and the isolated receptor monomer as defined by gel electrophoresis all shared similar electrochemical properties in the synthetic lipid bilayer. The agonist-activated ionic channel seems, therefore, to be contained within the receptor monomer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrantes F. J. Agonist-mediated changes of the acetylcholine receptor in its membrane environment. J Mol Biol. 1978 Sep 5;124(1):1–26. doi: 10.1016/0022-2836(78)90144-4. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J. Endogenous chemical receptors: some physical aspects. Annu Rev Biophys Bioeng. 1979;8:287–321. doi: 10.1146/annurev.bb.08.060179.001443. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Neugebauer D. C., Zingsheim H. P. Peptide extraction by alkaline treatment is accompanied by rearrangement of the membrane-bound acetylcholine receptor from Torpedo marmorata. FEBS Lett. 1980 Mar 24;112(1):73–78. doi: 10.1016/0014-5793(80)80131-1. [DOI] [PubMed] [Google Scholar]
- Boheim G., Hanke W., Eibl H. Lipid phase transition in planar bilayer membrane and its effect on carrier- and pore-mediated ion transport. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3403–3407. doi: 10.1073/pnas.77.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Hanke W., Boheim G. The lowest conductance state of the alamethicin pore. Biochim Biophys Acta. 1980 Mar 13;596(3):456–462. doi: 10.1016/0005-2736(80)90134-0. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hucho F., Bandini G., Suárez-Isla B. A. The acetylcholine receptor as part of a protein complex in receptor-enriched membrane fragments from Torpedo californica electric tissue. Eur J Biochem. 1978 Feb;83(2):335–340. doi: 10.1111/j.1432-1033.1978.tb12099.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Cooper J., Tzartos S. Acetylcholine receptors from Torpedo and Electrophorus have similar subunit structures. Biochemistry. 1980 Apr 1;19(7):1454–1458. doi: 10.1021/bi00548a029. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Anholt R., Lindstrom J., Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1980 May;77(5):3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Boheim G. Alamethicin-induced single channel conductance fluctuations in biological membranes. Nature. 1979 Nov 15;282(5736):336–339. doi: 10.1038/282336a0. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]


