Abstract
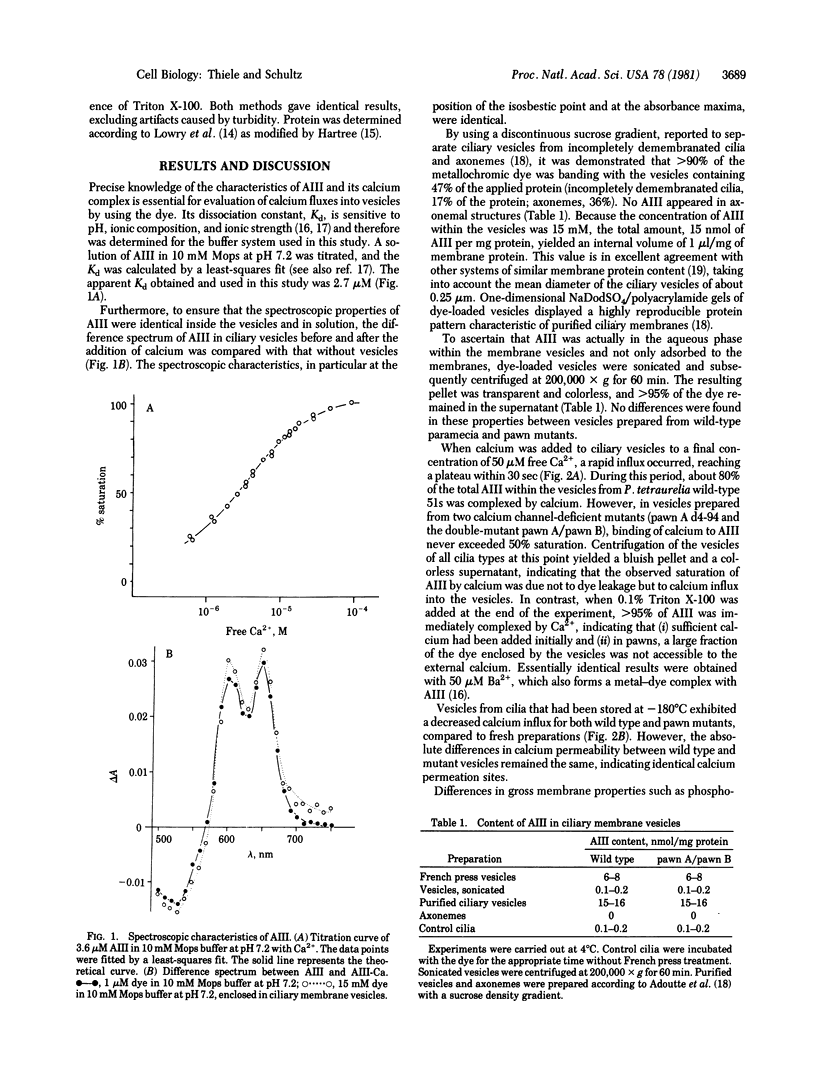
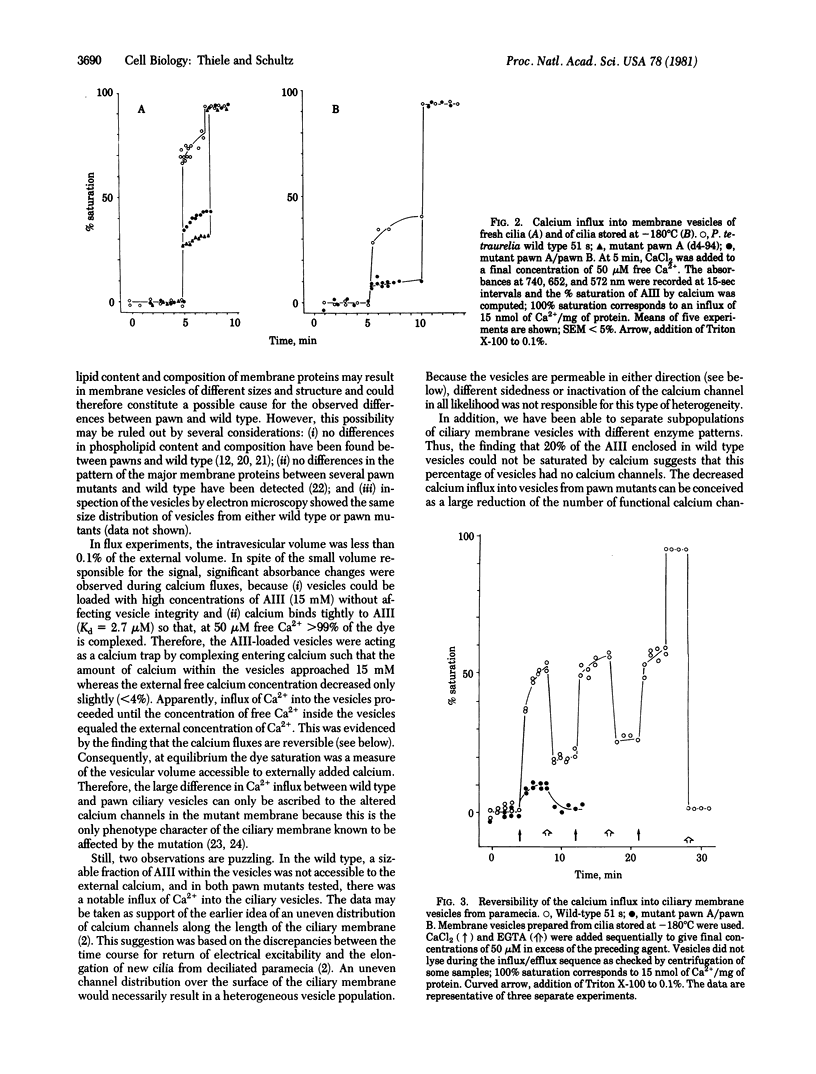
Calcium influx into ciliary membrane vesicles from paramecia was measured by using arsenazo III as metallochromic indicator and as intravesicular calcium trap. Influx was rapid and reversible. In ciliary membrane vesicles from paramecium pawn mutants which lack the voltage-sensitive calcium channel, calcium influx was significantly less than in wild-type paramecia. These data demonstrate that the voltage-dependent calcium channel of paramecia is functional in a cell-free system and available for biochemical studies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Ramanathan R., Lewis R. M., Dute R. R., Ling K. Y., Kung C., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium tetraurelia. III. Proteins of cilia and ciliary membranes. J Cell Biol. 1980 Mar;84(3):717–738. doi: 10.1083/jcb.84.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium tetraurelia. II. Phospholipids of ciliary and other membranes. Biochim Biophys Acta. 1979 Jan 19;550(2):174–187. doi: 10.1016/0005-2736(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Chang S. Y., Van Houten J., Robles L. J., Lui S. S., Kung C. An extensive behavioural and genetic analysis of the pawn mutants in Paramecium aurelia. Genet Res. 1974 Apr;23(2):165–173. doi: 10.1017/s0016672300014786. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. Chemical dissection of cilia. Arch Biol (Liege) 1965;76(2):317–352. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro E. S. Positional distribution of fatty acids in the major glycerophospholipids of Paramecium tetraurelia. J Lipid Res. 1980 Jul;21(5):559–570. [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Chang S. Y., Satow Y., Houten J. V., Hansma H. Genetic dissection of behavior in paramecium. Science. 1975 May 30;188(4191):898–904. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. M., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium. IV. Protein kinase activities of cilia and ciliary membrane. Biochim Biophys Acta. 1980 Oct;615(2):341–353. doi: 10.1016/0005-2744(80)90501-x. [DOI] [PubMed] [Google Scholar]
- Machemer H., Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol. 1979 Nov;296:49–60. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Oertel D., Schein S. J., Kung C. Separation of membrane currents using a Paramecium mutant. Nature. 1977 Jul 14;268(5616):120–124. doi: 10.1038/268120a0. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. A method of estimating the amount of calcium bound to the metallochromic indicator arsenazo III. Biochim Biophys Acta. 1979 Aug 22;586(2):217–230. doi: 10.1016/0304-4165(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Satow Y., Kung C. Membrane currents of pawn mutants of the pwA group in Paramecium tetraurelia. J Exp Biol. 1980 Feb;84:57–71. doi: 10.1242/jeb.84.1.57. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Bennett M. V., Katz G. M. Altered calcium conductance in pawns, behavioural mutants of Paramecium aurelia. J Exp Biol. 1976 Dec;65(3):699–724. doi: 10.1242/jeb.65.3.699. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Jantzen H. M. Cyclic nucleotide-dependent protein kinases from cilia of Paramecium tetraurelia: partial purification and characterization. FEBS Lett. 1980 Jul 11;116(1):75–78. doi: 10.1016/0014-5793(80)80532-1. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Klumpp S. Guanylate cyclase in the excitable ciliary membrane of Paramecium. FEBS Lett. 1980 Dec 15;122(1):64–66. doi: 10.1016/0014-5793(80)80402-9. [DOI] [PubMed] [Google Scholar]
- Thomas M. V., Gorman A. L. Internal calcium changes in a bursting pacemaker neuron measured with arsenazo III. Science. 1977 Apr 29;196(4289):531–533. doi: 10.1126/science.850795. [DOI] [PubMed] [Google Scholar]
- Walter M. F., Schultz J. E. Calcium receptor protein calmodulin isolated from cilia and cells of Paramecium tetraurelia. Eur J Cell Biol. 1981 Apr;24(1):97–100. [PubMed] [Google Scholar]


