Abstract
Viral hemorrhagic fevers are characterized by enhanced permeability. One of the most affected target organs of hantavirus-induced hemorrhagic fever with renal syndrome is the kidney, and an infection often results in acute renal failure. To study the underlying cellular effects leading to kidney dysfunction, we infected human renal cell types in vitro that are critical for the barrier functions of the kidney, and we examined kidney biopsy specimens obtained from hantavirus-infected patients. We analyzed the infection and pathogenic effects in tubular epithelial and glomerular endothelial renal cells and in podocytes. Both epithelial and endothelial cells and podocytes were susceptible to hantavirus infection in vitro. The infection disturbed the structure and integrity of cell-to-cell contacts, as demonstrated by redistribution and reduction of the tight junction protein ZO-1 and the decrease in the transepithelial resistance in infected epithelial monolayers. An analysis of renal biopsy specimens from hantavirus-infected patients revealed that the expression and the localization of the tight junction protein ZO-1 were altered compared to renal biopsy specimens from noninfected individuals. Both tubular and glomerular cells were affected by the infection. Furthermore, the decrease in glomerular ZO-1 correlates with disease severity induced by glomerular dysfunction. The finding that different renal cell types are susceptible to hantaviral infection and the fact that infection results in the breakdown of cell-to-cell contacts provide useful insights in hantaviral pathogenesis.
INTRODUCTION
Hantaviruses are emerging rodent-borne viruses that cause hemorrhagic fever in humans. Typical symptoms of viral hemorrhagic fevers are enhanced vascular permeability, thrombocytopenia, and plasma leakage (23). The permeability of epithelial and endothelial monolayers is regulated by multiprotein complexes comprising specific transmembrane and cytosolic proteins. Two junctional regions are formed at the site of cell-to-cell contacts: tight and adherens junctions. In podocytes a multiprotein complex called a glomerular slit diaphragm, a podocyte-specific variant of adherens and tight junctions, is responsible for the glomerular barrier function (19, 60, 63). Tight junctions selectively regulate the permeability for ions and solutes through the paracellular route. The permeability of the tight junction is regulated by intra- and extracellular stimuli (10, 27, 64, 74, 76, 81). The endothelium and epithelium represent a barrier for pathogens and, during infection, the monolayer integrity is often compromised due to effects of viral replication and immune defense. However, the epithelial and endothelial function is often influenced without observing histomorphologically visible disruption or massive damage of the monolayer in the affected tissue (5, 13, 15, 35, 57, 73, 85). The pathomechanisms that contribute to the alteration in a barrier function, leading to an organ dysfunction in a hantaviral infection, are not completely understood.
Hantavirus pathology is characterized by capillary leakage and organ failure. Its severity varies from mild disease to fatal outcome and may be influenced by intraindividual risk factors. Gender and genetic predispositions, such as tumor necrosis factor alpha (TNF-α) polymorphism or HLA-B8-DR3 haplotype, have been discussed as key determinants of disease severity (33, 37, 48). Furthermore, the course of the disease differs within strains, and the organ spectrum of illness depends on the tropism of the virus. The infection with New World hantaviruses causes hantaviral pulmonary syndrome (HPS). The target organ that is mainly affected in its function by the infection with a pathogenic Old World hantavirus is the kidney, leading to the hemorrhagic fever with renal syndrome (HFRS) (14, 42, 46, 85). Pulmonary findings in Old World hantavirus and renal involvement in New World hantavirus infection may be observed as well (44, 58, 86). Furthermore, both viruses can affect several other organs (28, 39). Nevertheless, the acute renal failure with frequent massive proteinuria is a symptom hallmark of Old World hantavirus infection (14, 32, 45, 53, 66, 67). Differences in organ tropism may account for the pronounced renal involvement in Old World hantavirus infection. However, the detection of hantavirus in the tissue of infected patients is difficult: in renal biopsy specimens from hantavirus-infected patients, the viral antigen was only detectable in the cytoplasm of tubular epithelial cells (25, 28, 29, 36, 78). Studies in a hantavirus infection model with macaques detected viral RNA in tubular epithelial cells and rarely in the glomerulus (71). Experimental infection of deer mice with Sin Nombre virus revealed the expression of N protein in the glomerulus (41). The detected viral antigens colocalize in the areas with tubular damage (36, 71). The renal function depends on the integrity of the tubular epithelium and the glomerular apparatus. Histopathological damage of the glomerular endothelium is not observed, despite enhanced glomerular permeability, as revealed by often severe nonselective proteinuria (1, 51, 71). The molecular mechanism, whereby the tubular reabsorption and glomerular filtration function is disturbed, and to what extent both participate in the outcome of the clinical picture of hantavirus infection is not known. Infectious diseases may be associated with a disruption of barrier function due to direct or immune mediated effects on the intercellular integrity. The massive proteinuria observed in hantavirus-infected patients indicates that glomerular and tubular cells are affected (1, 47, 68). However, the susceptibility of human renal cells and the possible effects of hantaviral infection on the barrier function have thus far not been elucidated. In the present study, we examined the infection of human renal cells in vitro and analyzed the integrity of cell-to-cell contacts of infected cells in vitro and in renal biopsy specimens from hantavirus-infected patients hospitalized in our department.
MATERIALS AND METHODS
Cells and tissues.
Human renal proximal epithelial cells (HREpC) were obtained from Promocell (Heidelberg, Germany) and maintained in renal epithelial cell growth medium 2 (Promocell). Human renal glomerular endothelial cells (HRGEnC) were obtained from ScienCell (Carlsbad, CA) and maintained in endothelial cell medium ECM (ScienCell). Only HREpC and HRGEnC from passages 2 to 6 were used.
The human podocyte cell line was derived from human normal podocytes conditionally transformed with a temperature-sensitive mutant of the simian virus 40 (SV40) large T antigen. Growing at the permissive temperature of 33°C allows the cells to proliferate. Thermoswitching to the nonpermissive temperature of 37°C to inactivate the SV40 T antigen results in a growth arrest and promotes the cell to differentiate. Cells were grown for a period of 14 days at 37°C to ensure differentiation (62).
Frozen archival renal biopsy specimens of seven patients with acute hantavirus infection (confirmed by positive IgM serology for Puumala virus antigen) and, as controls, four samples with normal morphology from nephrectomies were used. Biopsy specimens from hantavirus patients were taken between day 5 and 12 after onset of symptoms. This study was approved by the Ethics Committee of the University Hospital of Heidelberg, and it adhered to the Declaration of Helsinki. Written informed consent was obtained from all patients.
Virus and infection.
The stocks of hantaviral Hantaan virus, strain 76-118 (HTNV) or Puumala virus, strain Vranica (PUUV), were propagated on Vero E6 cells. Virus inocula, HTNV or PUUV, at a multiplicity of infection (MOI) of 0.01 were added to HREpC, HRGEnC, or differentiated podocytes. After incubation for 1 h at 37°C, the unbound virus was removed by a triple washing, and the cells were incubated for the indicated time points at 37°C. The infection was monitored by using immunofluorescence or the Western blot analysis of hantaviral N protein expression with mouse monoclonal anti-nucleocapsid protein (Progen, Heidelberg, Germany) or rabbit polyclonal anti-nucleocapsid protein antibody. An equal loading was verified by the detection of tubulin on the same membrane. For reinfection, Vero E6 cells were inoculated with cell-free supernatants of infected renal cells and monitored for infection for 6 days postinfection (dpi) (HTNV) or 14 dpi (PUUV).
Immunofluorescence and Western blot analysis.
For immunofluorescence, acetone-fixed cells or frozen sections of renal biopsy specimens were stained with primary and appropriate fluorescently labeled secondary antibodies. The following antibodies were used: mouse or rabbit anti-ZO-1 (Invitrogen, Karlsruhe, Germany), mouse anti-CD31 (Dako, Hamburg, Germany), goat anti-synaptopodin P-19 (Santa Cruz, Heidelberg, Germany), and mouse anti-cytokeratin 18 (Millipore, Schwalbach/Ts, Germany). Integrin was detected with mouse anti-integrin αVβ3 (clone LM609; Millipore). To confirm the specificity of anti-integrin αVβ3 antibody LM609, fixed cells were incubated with anti-integrin αVβ3 antibody that was pretreated with recombinant human integrin αVβ3 (R&D Systems, Wiesbaden-Nordenstadt, Germany). Recombinant protein was added to a final concentration of 0.04 μg/μl to integrin αVβ3 antibody LM609 (final concentration, 0.01 μg/μl). Images were taken using a Nikon DXM1200C camera attached to a Nikon Eclipse 80i upright microscope (Nikon, Düsseldorf, Germany). The quantification of ZO-1 expression was performed on slides that were all immunolabeled with the mixture of antibodies on the same day. Images on these slides were captured using a constant exposure time. The fluorescence intensity of the selected areas in 32 glomeruli of seven patients and of 18 glomeruli of two uninfected control kidneys was measured with Nikon NIS Elements Software. Mean intensities of glomerular ZO-1 staining in renal biopsy specimens of hantavirus patients and controls were statistically compared by using a Student t test. For Western blot analysis, cells were lysed and, after being boiled in sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-10% PAGE, transferred to a nitrocellulose membrane. The protein detection was performed after the incubation with first and peroxidase-conjugated secondary antibodies using the SuperSignal Pico detection kit (Pierce, Bonn, Germany) according to the manufacturer's instructions. The following antibodies were used: mouse anti-ZO-1 (Invitrogen), mouse anti-α-tubulin DM 1A (Sigma, Deisenhofen, Germany), and rabbit anti-integrin β3 (H-96; Santa Cruz).
Flow cytometry.
For flow cytometry, cells were washed, scraped, and stained with allophycocyanin (APC)-conjugated mouse anti-CD31 antibody (clone AC128; Milteny Biotec, Bergisch Gladbach, Germany) and mouse phycoerythrin (PE)-conjugated anti-integrin αVβ3 antibody (clone LM609). Controls were incubated with APC- and PE-conjugated mouse isotype antibodies. After 1 h, the cells were washed and then analyzed by flow cytometry with FACSCalibur (BD Pharmingen).
Measurement of transepithelial resistance and viability assay.
To establish polarized monolayers, HREpC (2 × 105 cells) were plated on 0.4-μm-pore-size 12-well transwell culture system filters (Greiner Bio-One, Frickenhausen, Germany). The integrity of the monolayers was assessed by measuring the transepithelial electrical resistance (TER) with a Millicell-ERS voltmeter (Millipore). At the TER plateau level, cells were infected with HTNV at an MOI of 0.01 or left uninfected, followed by incubation for 1 h at 4°C. Monolayers were washed, and fresh medium was added. The cells were incubated at 37°C, and the TER was monitored at the indicated time points. Uninfected and HTNV-infected HREpC were lysed at day 6 postinfection. The number of viable cells was determined by measuring the amount of ATP using CellTiter-Glo luminescent cell viability assay (Promega, Mannheim, Germany).
RESULTS
Human renal cells express the hantaviral receptor integrin αVβ3 and are susceptible to hantavirus infection.
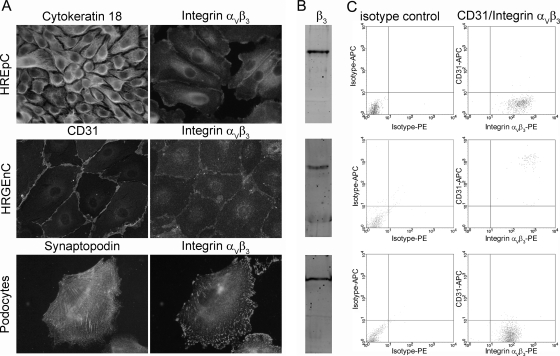
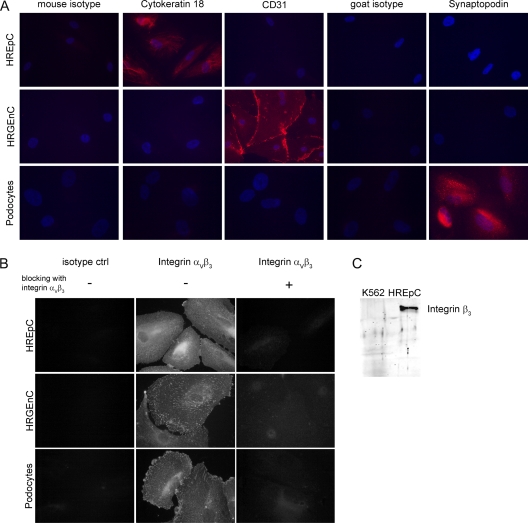
The hantaviral receptor integrin αVβ3 is expressed in endothelial and epithelial cells of different organs and species (18, 65, 69, 70). The Vero E6 cell line of renal tubular epithelial origin, primary human umbilical vein endothelial cells, or hamster tracheal epithelial cells were used to identify integrin as receptor for Old and New World hantaviruses (20, 22, 43, 59, 61). We analyzed human renal cell types for their susceptibility to hantavirus infection by the detection of the hantaviral receptor integrin αVβ3: primary human renal tubular epithelial cells (HREpC), glomerular endothelial cells (HRGEnC), and podocytes. First, we controlled cells for the expression of cell type-specific marker proteins: HREpC were positive for the epithelial marker protein cytokeratin 18, HRGEnC expressed the endothelial marker CD31, and differentiated podocytes were positive for the podocyte-specific protein synaptopodin. The cell populations showed a homogeneous expression of their respective markers (Fig. 1A). Markers that were not specific for the cell type were not expressed in the cell population (Fig. 2A). All three renal cell types expressed the receptor for hantavirus infection, as was shown by immunofluorescence analysis for integrin αVβ3 (Fig. 1A). To confirm the specificity of the anti-integrin αVβ3 antibody LM609, we used a mouse isotype control, along with neutralizing the integrin antibody with recombinant integrin αVβ3. In contrast to cells incubated with integrin antibody, cells incubated with isotype control or with preincubated antibody did not show any specific staining. (Fig. 2B). The expression of the β3-integrin subunit was analyzed by Western blot analysis with a rabbit polyclonal anti-β3-integrin antibody. The subunit was detected in all three renal cell types. (Fig. 1B). The specificity of the rabbit polyclonal anti-β3-integrin antibody was confirmed by the absence of this band in a lysate of the chronic myelogenous leukemia cell line K562 that does not express integrin β3 (Fig. 2C). The surface expression of integrin αVβ3 on the three renal cell types was analyzed by flow cytometry (Fig. 1C). Human renal glomerular endothelial cells were double positive for the endothelial marker CD31 and for integrin αVβ3. In contrast, tubular epithelial cells and podocytes showed surface expression of integrin αVβ3, whereas the endothelial marker CD31 was absent. Together, results from immunofluorescence staining, Western blot, and flow cytometry demonstrate the expression of integrin in the three different human renal cell types.
Fig. 1.
Expression of marker proteins and the hantaviral receptor integrin αVβ3 on renal cell types. (A) Human primary cells HREpC, HRGEnC, and human podocytes were stained with antibodies against marker proteins for renal cell types and with anti-integrin αVβ3 antibody. (B) Lysates of renal cell types were analyzed for the expression of integrin β3 by Western blot analysis. (C) Flow cytometric analysis of cell surface protein expression of integrin αVβ3 and the endothelial marker CD31.
Fig. 2.
Characterization of human renal cells and specificity of the anti-integrin antibodies. (A) HREpC, HRGEnC, and podocytes were analyzed for the presence or absence of the epithelial marker cytokeratin 18, the endothelial marker CD31, and the podocyte-specific protein synaptopodin, along with the specific isotype control antibodies. (B) Analysis of the specificity the of anti-integrin αVβ3 antibody LM609. Fixed renal cells were incubated with isotype control antibody, with anti-integrin αVβ3 LM609 or with anti-integrin αVβ3 antibody LM609 that was preincubated with recombinant human integrin αVβ3. (C) Specificity of the anti-integrin β3 antibody was confirmed by the absence of an integrin-specific band in K562 lysate lacking integrin expression (30).
Hantavirus Hantaan infects human tubular and glomerular cells.
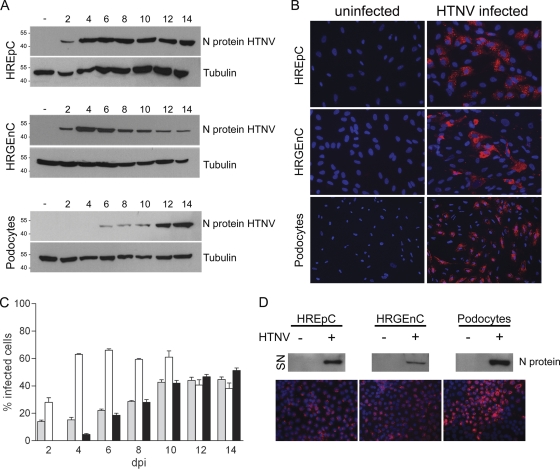
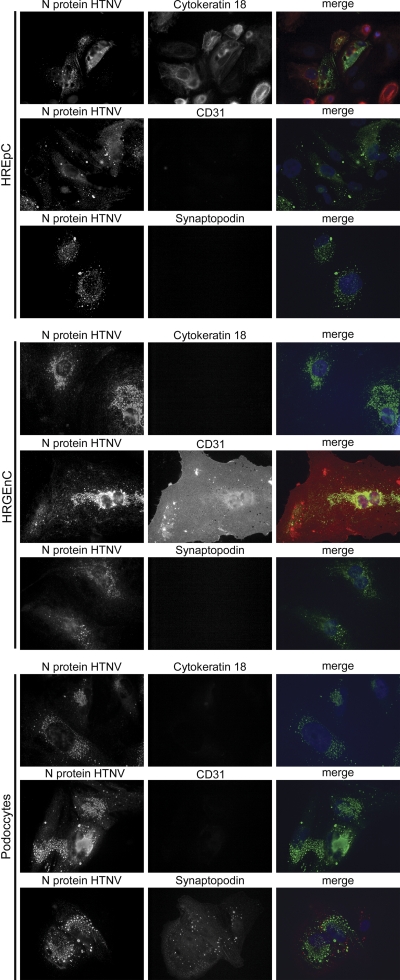
In a next step, we analyzed the susceptibility of human renal cells to the HFRS-causing hantavirus Hantaan 76-118 (HTNV 76-118) in vitro. The infection was monitored by the detection of viral N protein by Western blotting (Fig. 3A) and immunofluorescence (Fig. 3B). Infected tubular epithelial and glomerular endothelial cells were detectable by immunofluorescence 2 days postinfection. An infection of podocytes was visible at 4 dpi. The number of infected human renal epithelial cells and podocytes was continuously increasing and after 14 days, ca. 40% of the cells were positive for hantaviral N protein. In contrast, already 4 dpi more than 60% of the human glomerular endothelial cells were infected (Fig. 3C). The identity of infected cell types was confirmed by immunofluorescence analysis of infected cells for specific marker proteins (Fig. 4). The nuclei of uninfected and infected cells appeared intact with no signs of apoptosis (Fig. 3B). To examine the productivity of infection, we analyzed the supernatants (SN) of renal infected cells for the presence of viral N protein by Western blot analysis (Fig. 3D). The release of infectious particles from susceptible renal cells was confirmed by staining the N protein in Vero E6 cells incubated with supernatants of infected renal cells (Fig. 3D). These results demonstrate the productive infection of different renal cell types that contribute to kidney function.
Fig. 3.
Infection of human renal cells with the hantavirus Hantaan (HTNV). HREpC, HRGEnC, and podocytes were infected with hantavirus HTNV. (A) At the indicated time points, cells were lysed and assessed for expression of viral N protein and tubulin. (B) Cells at 14 days postinfection (dpi) were fixed and immunostained for N protein with anti-N protein and a Cy3-conjugated anti-mouse immunoglobulin secondary antibody. Nuclei were stained with Hoechst 33342. (C) Cells were inoculated with HTNV. At the indicated time points, the cells were fixed and infected cells were quantified by immunostaining for N protein expression. HREpC, gray bars; HRGEnC, white bars; podocytes, black bars. The data are representative of three independent experiments (mean ± the standard deviation [SD]). (D) Cell-free supernatants of uninfected and HTNV-infected renal cells were collected and analyzed for the expression of N protein by Western blot analysis. Vero E6 cells were incubated with supernatants of infected renal cells and stained for hantaviral N protein (red) at 6 dpi.
Fig. 4.
Expression of cell type specific marker proteins in HTNV-infected renal cells. Cells infected with HTNV were analyzed for the expression of hantaviral N protein (first column) and the presence or absence of cell type specific marker proteins (second column) at day 12 postinfection.
Hantaviral infection causes the redistribution and decrease of ZO-1.
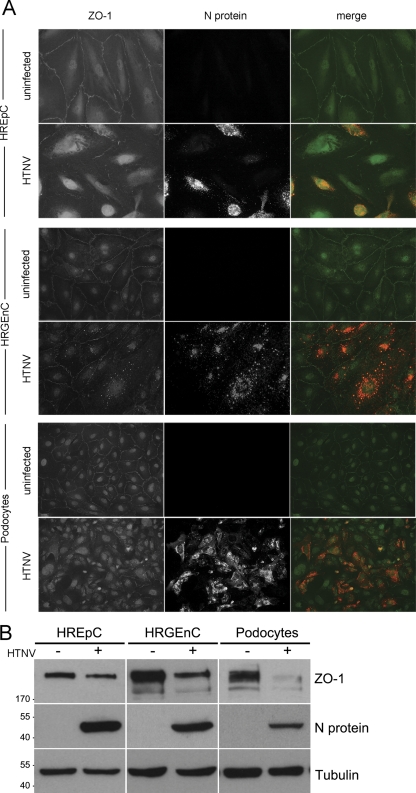
We examined the impact of a hantavirus infection of renal cells on the integrity of their cell-to-cell contacts by analyzing the localization and the expression levels of ZO-1. The junctional marker protein ZO-1 is present in tight junctions of endothelia, in epithelia, and in the glomerular slit diaphragm of podocytes. In addition to the junctional localization, ZO-1 shuttles between the nucleus and cytosol (4, 7). The localization was examined in infected and uninfected cells. In uninfected cells, the immunostaining displayed the localization of ZO-1 along the contacts of adjacent cells and in the nucleus. In cells infected with hantavirus, ZO-1 exhibited a weaker and discontinuous staining at their margins and a redistribution of the protein to the cytoplasm (Fig. 5A).
Fig. 5.
Hantavirus affects localization and expression levels of ZO-1. (A) Uninfected monolayers (upper panels) and monolayers infected with HTNV (lower panels) were stained for the tight junction protein ZO-1 (green) and hantaviral N protein (red) at day 6 postinfection. (B) Uninfected and infected cells were lysed and analyzed for the expression of ZO-1, N protein, and tubulin. Shown is a representative Western blot of three independent experiments.
To analyze the expression levels of ZO-1 protein in uninfected and infected renal cells, equal amounts of total cell protein were analyzed by Western blotting (Fig. 5B). ZO-1 levels were markedly reduced in hantavirus-infected renal epithelial and endothelial cells and drastically in podocytes. To assess the effects of hantavirus-induced redistribution and reduction of ZO-1 expression on the barrier function, we measured the TER of polarized renal cells. Since podocytes and human renal glomerular endothelial cells in vitro did not form a confluent monolayer with a stable TER, we used renal tubular epithelial monolayers. Primary HREpC were seeded to confluence on transwell filters, and the TERs of HTNV-infected and uninfected polarized monolayers were monitored (Fig. 6A). At early time points after infection, the TERs of infected monolayers were not significantly different from uninfected cells. After 12 h, we observed a decrease in the barrier function of the infected epithelial monolayer that was further reduced to 36.36% ± 2.98% of the TER prior to infection that was set to 100%. In contrast, the TER of the uninfected monolayer did not show a decline in TER. The infection of HREpC caused no significant loss of viability (Fig. 6B). Taken together, these results indicate that the hantaviral infection of polarized monolayers with functional tight junctions leads to a redistribution and decrease in the tight junction protein ZO-1 and subsequently to the breakdown of the epithelial barrier function.
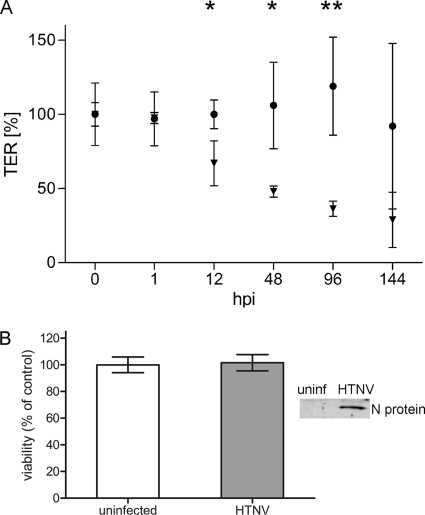
Fig. 6.
Epithelial barrier function is disturbed in hantavirus-infected cells. (A) HREpC were cultured on transwell filters and were infected with HTNV. Transepithelial resistance was measured to analyze the effect of infection on monolayer integrity. The initial TER of the uninfected and infected monolayers was set to 100%. Symbols: •, uninfected cells; ▾. HTNV-infected cells. Shown are data representative of three independent experiments (mean ± the SD). Student t test: *, P < 0.05; **, P < 0.01. (B) HREpC were infected with HTNV at an MOI of 0.01, and infection was monitored by the detection of N protein in cell lysate. Viability was assessed 144 h postinfection (hpi) by measuring the amount of ATP. Control cells remained uninfected. Viability of infected cells is presented as a percentage of uninfected cell control values. The data are representative of three independent experiments (mean ± the SD). Student t test, P=0.818.
Old World hantavirus Puumala infects renal cells and changes the structure of their cell-to-cell contacts.
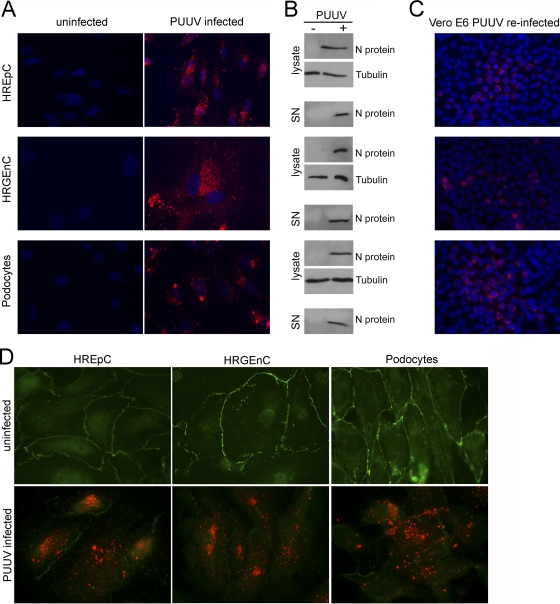
We analyzed whether renal cell types support the productive infection with the pathogenic Old World hantavirus Puumala, which is predominant in Europe and causes a milder form of HFRS called nephropathia epidemica (NE). All three cell types were permissive, as shown by the expression of N protein analyzed by immunofluorescence (Fig. 7A) and Western blot analysis of cell lysates. Infectious particles were produced by all three cell types as demonstrated by the detection of N protein in the supernatant (Fig. 7B) and the reinfection of Vero E6 cells after incubation with supernatants of infected renal cells (Fig. 7C). Furthermore, the alterations of cellular junctions during PUUV infection corresponded to the HTNV-induced changes in ZO-1 localization (Fig. 7D).
Fig. 7.
Productive infection of human renal cells with Puumala virus (PUUV). (A) HREpC, HRGEnC, and podocytes were infected with the Old World hantavirus PUUV and analyzed for expression of N protein by immunofluorescence at 10 dpi. Nuclei were stained with Hoechst 33342. (B) Lysates and cell-free supernatants (SN) of uninfected and PUUV-infected renal cells were analyzed for the presence of N protein by Western blotting. (C) Vero E6 cells were inoculated with cell-free supernatant of infected renal cells. Cells at 14 dpi were analyzed for the expression of Puumala N protein. (D) Localization of the tight junction marker protein ZO-1 was analyzed in uninfected monolayers (upper panel) and PUUV-infected monolayers (lower panel) of different renal cell types. ZO-1 is shown in green and hantaviral N protein in red at day 10 postinfection.
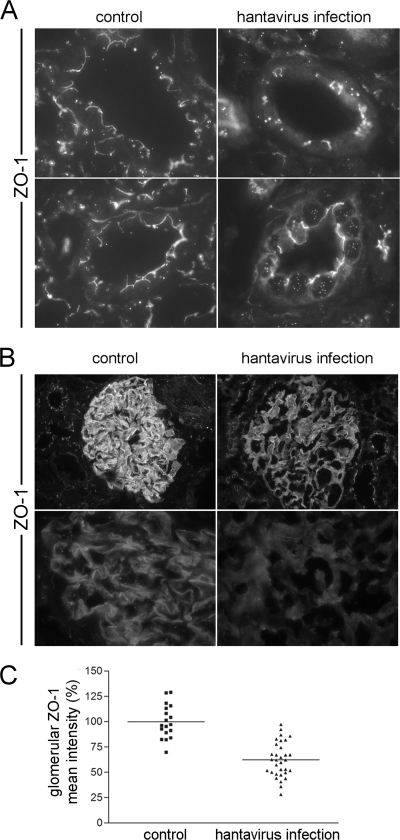
Mislocalization and reduced expression of ZO-1 in hantavirus-infected patients.
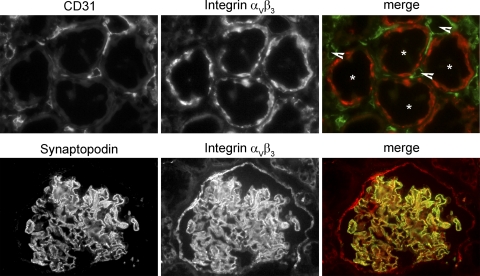
In sections derived from human uninfected adult kidneys, we examined the expression of the receptor for pathogenic hantaviruses, integrin αVβ3. The expression was detected in tubular epithelial cells and in glomerular cells (Fig. 8). Staining of CD31-positive endothelial cells for integrin αVβ3 in peritubular capillaries was not observed. Integrin αVβ3 colocalized with the podocyte marker synaptopodin at the surface of the glomerular basement membrane.
Fig. 8.
Expression of hantaviral receptor integrin αVβ3 in tubules and glomeruli of human kidney. Human renal cryosections were fixed and stained with antibodies against the hantaviral receptor integrin αVβ3 (red) and the marker for endothelial cells CD31 (green, upper row) or the marker for podocytes, synaptopodin (green, lower row). Tubules are marked with asterisks, and peritubular capillaries are marked with arrowheads.
Our data confirm the results of previous immunohistochemistry studies and immunogold analysis that demonstrated the expression of integrin αVβ3 in tubular cells and foot processes of podocytes and the absence from peritubular capillaries (6, 49, 80, 84).
To examine whether the infection with hantavirus leads to an alteration of cell-to-cell contacts in the kidney of hantavirus-infected patients, we examined the localization of the junctional protein ZO-1 in renal biopsy specimens from seven patients suffering from Puumala hantavirus-induced acute renal failure that were treated in our department. Table 1 summarizes patient characteristics. The mean age of the patients was 44.20 ± 12.68 years. The laboratory examinations displayed elevated levels of serum creatinine, lactate dehydrogenase, C-reactive protein, and urea and elevated leukocyte counts. Furthermore, thrombocytopenia and decreased levels of serum albumin were observed in all patients. To analyze the integrity of cell-to-cell contacts in kidneys of hantavirus-infected patients, we performed immunofluorescence stainings for ZO-1 (Fig. 9). The analysis revealed that ZO-1 in tubular epithelial cells of normal control kidneys was expressed in the apical part facing the lumen, indicating that epithelial cells had intact tight junctions. In contrast, tubular ZO-1 in renal biopsy specimens of patients showed an aberrant localization with a rather discontinuous staining at the apical margin and redistribution from the cell-to-cell contacts to the cytoplasm and the nucleus in a diffuse or punctuate pattern. According to these results, tubular tight junctions in patients appeared to be disrupted (Fig. 9A). We analyzed the effects of hantaviral infection on the localization and expression levels of ZO-1 in the glomerular apparatus (Fig. 9B). We observed a weaker staining and a redistribution of ZO-1 in the glomerular tuft. Whereas ZO-1 was concentrated in distinct lines with intense staining along the glomerular capillaries in the control kidneys, the localization in the infected kidneys appeared diffuse cytosolic. A quantification of the mean fluorescence intensity of ZO-1 in the glomeruli revealed a significantly reduced glomerular fluorescence of ZO-1 staining in infected patients (Student t test, P < 0.0001). The mean fluorescence intensity of ZO-1 in the glomerular area of kidneys from infected patients was reduced to 62.33% of uninfected control kidneys (Fig. 9C). In a next step, we analyzed the correlation of glomerular ZO-1 levels with clinical parameters of disease severity (Table 2). Levels of decreased serum albumin correlate with decreased glomerular ZO-1 levels (r=0.951; P=0.006). The correlation of these two parameters provides a mechanistic link between the hantavirus-induced effects on cell-to-cell contacts with the glomerular dysfunction leading to proteinuria.
Table 1.
Characteristics and laboratory findings of hantavirus-infected patients
| Characteristica | Mean (±SD) | Minimum | Maximum |
|---|---|---|---|
| Age (yr) | 44.20 (±12.68) | 28.00 | 55.00 |
| Serum creatinine (mg/dl) | 5.41 (±2.65) | 2.11 | 8.03 |
| Serum albumin (g/liter) | 36.22 (±3.47) | 32.20 | 40.20 |
| Urea (mg/dl) | 132.20 (±41.15) | 78.00 | 176.00 |
| LDH (U/liter) | 331.80 (±62.73) | 273.00 | 413.00 |
| CRP (mg/liter) | 28.06 (±31.02) | 9.20 | 82.80 |
| Platelet count/nl | 344.40 (±134.80) | 209.00 | 534.00 |
| Leukocyte count/nl | 10.22 (±2.33) | 8.05 | 12.99 |
LDH, lactate dehydrogenase; CRP, C-reactive protein.
Fig. 9.
Alteration in the localization and expression of ZO-1 in kidneys of hantavirus-infected patients. (A and B) Cryosections of renal biopsy specimens of patients infected with hantavirus and of control kidneys were fixed with acetone and stained for the tight junction protein ZO-1. (C) Expression of glomerular ZO-1 was quantified by the measurement of the mean fluorescence intensity in the selected glomerular area (the mean ZO-1 fluorescence intensity of glomeruli of control kidneys was set to 100%).
Table 2.
Correlation between glomerular ZO-1 levels and clinical parameters in hantavirus-infected patientsa
| Characteristic | r | CI | P |
|---|---|---|---|
| Age (yr) | 0.081 | -0.863–0.899 | 0.448 |
| Serum creatinine (mg/dl) | 0.361 | -0.765–0.943 | 0.276 |
| Serum albumin (g/liter) | 0.951 | 0.428–0.997 | 0.006 |
| Urea (mg/dl) | 0.644 | -0.552–0.973 | 0.120 |
| LDH (U/liter) | 0.720 | -0.446–0.980 | 0.085 |
| CRP (mg/liter) | 0.549 | -0.646–0.964 | 0.169 |
| Platelet count/nl | -0.016 | -0.886–0.879 | 0.490 |
| Leukocyte count/nl | 0.757 | -0.378–0.983 | 0.069 |
P, Student t test, one-tailed; r, Pearson's correlation coefficient; CI, 95% confidence interval.
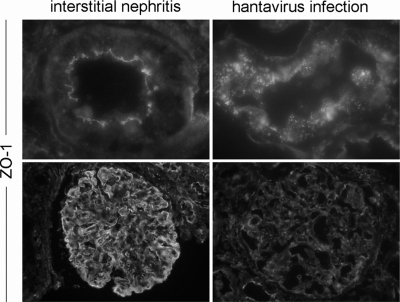
Cell-to-cell contacts are specifically affected in hantavirus-infected patients.
The histomorphological picture in renal biopsy specimens of hantavirus-infected patients corresponds to acute tubulointerstitial nephritis. However, in contrast to the tubulointerstitial nephritis, the hantavirus infection is characterized by often massive proteinuria, but histopathological glomerular injury is absent or moderate despite proteinuria. Therefore, we compared the structure of tight junctions in biopsy specimens from a patient with non-hantavirus-induced acute tubulointerstitial nephritis and from hantavirus-infected patients. The histopathological analysis revealed the typical tubular injury (edema, tubular dilatation, and inflammatory cell infiltrations) in both diseases (data not shown). A comparison of the localization of the tight junction marker protein ZO-1 revealed that, in contrast to hantavirus infection, the tubular and glomerular junctional structure in the interstitial nephritis showed an intact organization with an uninterrupted belt of ZO-1 in tubules and glomeruli. In contrast, in the kidneys of infected patients, staining of ZO-1 was irregular and faint (Fig. 10). Taken together, the results demonstrate a hantavirus-specific clinical picture with a disruption of junctional structures of tubular and glomerular renal cell types, leading to transient massive proteinuria.
Fig. 10.
ZO-1 localization is not altered in patients with nonhantaviral acute interstitial nephritis. Cryosections of renal biopsy specimens of hantavirus-seronegative patients with interstitial nephritis and hantavirus-infected patients were stained for the tight junction protein ZO-1.
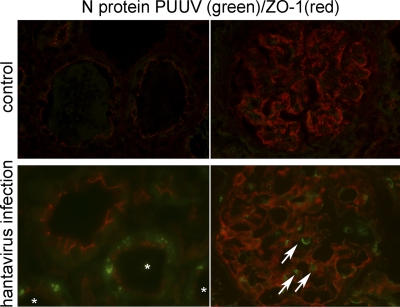
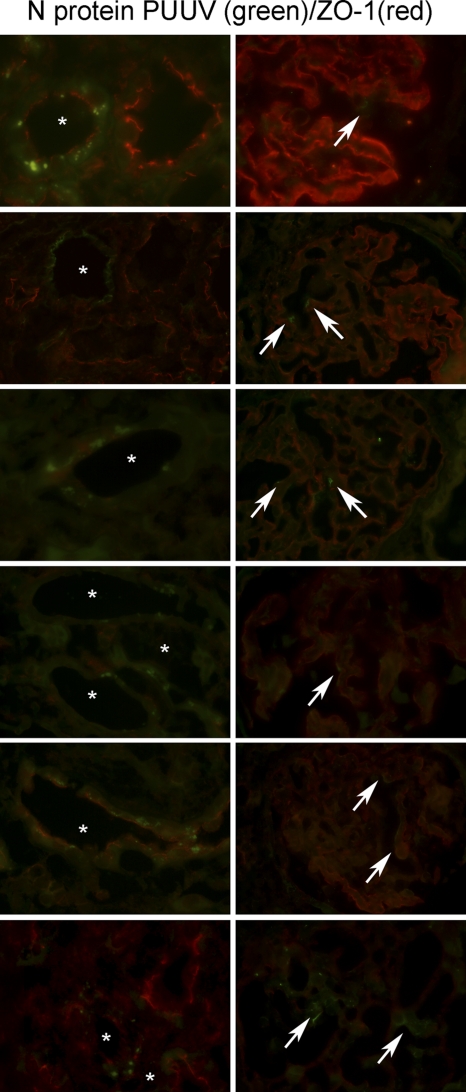
Hantaviral antigen is detected in the glomerular and tubular apparatus.
We compared the in vitro susceptibilities of renal cells with the pattern of viral antigen expression in renal cryosections from Puumala-infected patients. Cryosections of hantavirus-seronegative patients served as controls (Fig. 11). Seven biopsy specimens from seropositive patients were analyzed for the presence of hantaviral antigen by immunofluorescence. Hantaviral N protein was detected in podocytes in the glomeruli and in tubular epithelial cells of all infected patients (Fig. 12). The redistribution of ZO-1 occurred in tubules whose cells expressed hantaviral N protein. Whereas ZO-1 in an adjacent uninfected tubule localized at the apical site facing the tubular lumen, staining of ZO-1 in infected tubules displayed a weaker intensity and a more diffused pattern.
Fig. 11.
Detection of hantaviral antigen in glomerular and tubular cells. Cryosections of renal biopsy specimens of hantavirus-seronegative patients (upper panel) and hantavirus-seropositive patients (lower panel) were stained for the tight junction protein ZO-1 (red) and for the PUUV N protein (green). The image on the left in the lower panel shows infected tubules (asterisks); the image on the right shows infected glomerular cells (arrows).
Fig. 12.
Detection of hantaviral antigen in tubular and glomerular cells. Cryosections of renal biopsy specimens of six hantavirus-seropositive patients were stained for the tight junction protein ZO-1 (red) and for the PUUV N protein (green). The images in the left panels show infected tubules (asterisks), and the images on the right show infected glomerular cells (arrows).
DISCUSSION
The epithelium is a critical barrier for pathogens, and entering the target tissue requires strategies to disrupt the integrity of cell-to-cell contacts. The infection of polarized monolayers is often directly linked to the pathogenesis of disease. The clinical picture of human hantaviral infections differs between Old and New World hantavirus. They may vary in the target organ, in which the infection predominantly manifests. An infection with Old World hantaviruses often leads to acute renal failure with massive proteinuria (1, 51, 71). In recent years reports of cases with pulmonary involvement in Old World hantavirus infection were reported, as well as involvement of other organs (28, 58). The understanding of the susceptibility of human cells for hantavirus infection and the effects of replication on the host cell is crucial for the study of the underlying mechanism of hantaviral pathogenesis. However, the underlying mechanisms of renal dysfunction in HFRS are not yet well understood. The productive infection of renal cells may cause direct effects induced by the viral replication or infection may be responsible for the attack of infected cells by invading immune cells (1, 51, 71). The function of the kidney depends on the glomerular layers (fenestrated endothelium, podocytes, and basement membrane) and the integrity of the tubular epithelium (12, 26, 72). The endothelium of glomerular capillaries differs with its fenestrae from other vascular endothelia that are characterized by tight junction formation, but the fenestrated endothelium works as a molecular filter via its glycocalyx. Podocytes form the barrier via the interdigitated foot processes and their connecting slit diaphragms, specialized multiprotein complexes that share similarities with tight and adherens junctions. A mild dysfunction of the glomerular barrier function can be covered by tubular reabsorption. However, glomerular together with tubular disorder results in proteinuria (11). Our results from cell culture experiments and renal biopsy specimens showed that both systems, glomerular and tubular, show junctional remodeling and may explain the clinical picture of hantavirus-induced acute renal failure that is characterized by massive proteinuria.
Hemorrhagic fever viruses often exert a pronounced tropism for organ-specific epithelia and endothelia. The Nipah virus infects preferentially endothelial cells of small blood and lymphatic vessels corresponding to the expression pattern of the receptor ephrinB2 (54, 82). The measles virus is able to infect respiratory epithelium, dermal capillary, and microvascular endothelial cells in the brain (2, 83). Studies on dengue virus infection demonstrated viral antigen in endothelial cells of the liver, spleen, and alveoli (31). In vitro studies concerning the pathogenesis of viral hemorrhagic disease often make use of human endothelial cells of the umbilical vein (HUVEC). However, endothelial cells of different organs are heterogeneous, since they are specialized for the function in the respective tissue. They exert a typical morphology and protein expression profile (3, 9, 50, 75). An infection with Old World hantaviruses causes HFRS, and the infection with New World hantaviruses leads to HPS. The fenestrated glomerular endothelium of the kidney differs significantly from the continuous endothelium of other organs (24, 50, 52, 56). The well-defined endothelial specialization and heterogeneity are not only apparent in the tropism of pathogens, many human vascular diseases are limited to distinct types of vessels, e.g., autoimmune diseases that mainly manifest in the kidney (34). The strong association between disease and cell type demands the investigation of the underlying molecular pathomechanism in a cell culture model relating to the relevant target organ (79).
We have shown that the hantavirus receptor integrin αVβ3 is expressed on tubular and glomerular cells of the human kidney and that podocytes and glomerular endothelial and tubular epithelial cells are permissive to infection and release infectious particles. In contrast to the comparable replication kinetics of HTNV in epithelial cells and podocytes, the virus infects the glomerular endothelial monolayer much more efficiently. The infection of the glomerular endothelium may allow the entry of the hantavirus into the kidney with subsequent infection of podocytes and tubular cells. The expression of the hantaviral coreceptor CD55 on different renal cell types may also play a role in the susceptibility (8, 40). An analysis of the impact of infection on the integrity of the cell-to-cell contacts revealed structural alterations in tubular and glomerular cells. We also demonstrated the presence of hantaviral antigen in the kidney and the disruption of junctional structures in biopsy specimens of infected patients. The remodeling affects tight junctions of tubular epithelial cells and the glomerular slit diaphragm between the foot processes of podocytes. Further investigations will focus on possible mechanisms that are either direct, since the viral replication could induce a redistribution of junctional proteins, or the disruption of tight junctions could be a consequence of the effects of cytokines. The induction of the innate immune system by hantavirus infection leads to the secretion of cytokines that may be involved in the signaling cascade controlling epithelial and endothelial permeability (10, 27, 74, 76, 81). A stimulation of hantavirus-infected HUVECs with TNF-α or vascular endothelial growth factor (VEGF) results in a higher permeability than a stimulation of uninfected monolayers (21, 55). VEGF and VEGF receptors 1 and 2 are also expressed in renal cells. Since VEGF plays a crucial role in the maintenance of the filtration barrier (16, 17, 77), the infection with hantavirus could enhance the sensitivity for VEGF in renal cells and increase the permeability the same way as in HUVECs. The role for VEGF in virus-induced renal dysfunction was shown in HIV-associated nephropathy, where the infection of podocytes with HIV induces the expression of VEGF and VEGFR2, leading to podocyte dedifferentiation and disease (38). The histopathological changes in hantavirus infection represent mild tubular interstitial changes and moderate interstitial infiltration of mononuclear cells. However, analyzing the cell-to-cell contacts revealed that the hantavirus-induced acute renal failure differs from interstitial nephritis of nonhantaviral origin that displays no redistribution of junctional proteins.
To summarize, we could demonstrate that renal cells, which are responsible for the function of the kidney, are susceptible to the infection with hantavirus and lose their barrier function by specific remodeling of the structure of cell-to-cell contacts. The disorganization affects both the tubular and the glomerular apparatus, leading to the hantavirus-specific clinical picture that is characterized by renal failure with massive proteinuria.
ACKNOWLEDGMENTS
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (KR 3711/2-1) to E.K.
We thank Heike Ziebart, Vanessa Bollinger, and Charlotte Holler for excellent technical assistance and Claudia Halfen and Michelle Froese for critical reading of the manuscript.
No financial conflicts of interest exist.
Footnotes
Published ahead of print on 20 July 2011.
REFERENCES
- 1. Ala-Houhala I., et al. 2002. Increased glomerular permeability in patients with nephropathia epidemica caused by Puumala hantavirus. Nephrol. Dial. Transplant. 17:246–252 [DOI] [PubMed] [Google Scholar]
- 2. Andres O., Obojes K., Kim K. S., ter Meulen V., Schneider-Schaulies J. 2003. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J. Gen. Virol. 84:1189–1197 [DOI] [PubMed] [Google Scholar]
- 3. Augustin H. G., Kozian D. H., Johnson R. C. 1994. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. Bioessays 16:901–906 [DOI] [PubMed] [Google Scholar]
- 4. Balda M. S., Matter K. 2000. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 19:2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balda M. S., et al. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baraldi A., et al. 1995. β1 and β3 integrin upregulation in rapidly progressive glomerulonephritis. Nephrol. Dial. Transplant. 10:1155–1161 [PubMed] [Google Scholar]
- 7. Bauer H., Zweimueller-Mayer J., Steinbacher P., Lametschwandtner A., Bauer H. C. 2010. The dual role of zonula occludens (ZO) proteins. J. Biomed. Biotechnol. 2010:402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buranda T., et al. 2010. Recognition of decay accelerating factor and αvβ3 by inactivated hantaviruses: toward the development of high-throughput screening flow cytometry assays. Anal. Biochem. 402:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cines D. B., et al. 1998. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91:3527–3561 [PubMed] [Google Scholar]
- 10. Clarke H., Soler A. P., Mullin J. M. 2000. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J. Cell Sci. 113 (Pt. 18):3187–3196 [DOI] [PubMed] [Google Scholar]
- 11. D'Amico G., Bazzi C. 2003. Pathophysiology of proteinuria. Kidney Int. 63:809–825 [DOI] [PubMed] [Google Scholar]
- 12. Deen W. M. 2004. What determines glomerular capillary permeability? J. Clin. Invest. 114:1412–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dejana E., Tournier-Lasserve E., Weinstein B. M. 2009. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16:209–221 [DOI] [PubMed] [Google Scholar]
- 14. Duchin J. S., et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949–955 [DOI] [PubMed] [Google Scholar]
- 15. El-Aouni C., et al. 2006. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J. Am. Soc. Nephrol. 17:1334–1344 [DOI] [PubMed] [Google Scholar]
- 16. Eremina V., Quaggin S. E. 2004. The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 13:9–15 [DOI] [PubMed] [Google Scholar]
- 17. Eremina V., et al. 2003. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frangie C., et al. 2006. Extracellular calpains increase tubular epithelial cell mobility. Implications for kidney repair after ischemia. J. Biol. Chem. 281:26624–26632 [DOI] [PubMed] [Google Scholar]
- 19. Fukasawa H., Bornheimer S., Kudlicka K., Farquhar M. G. 2009. Slit diaphragms contain tight junction proteins. J. Am. Soc. Nephrol. 20:1491–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gavrilovskaya I. N., Brown E. J., Ginsberg M. H., Mackow E. R. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 73:3951–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gavrilovskaya I. N., Gorbunova E. E., Mackow N. A., Mackow E. R. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82:5797–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gavrilovskaya I. N., Shepley M., Shaw R., Ginsberg M. H., Mackow E. R. 1998. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geisbert T. W., Jahrling P. B. 2004. Exotic emerging viral diseases: progress and challenges. Nat. Med. 10:S110–S121 [DOI] [PubMed] [Google Scholar]
- 24. Ghitescu L., Robert M. 2002. Diversity in unity: the biochemical composition of the endothelial cell surface varies between the vascular beds. Microsc. Res. Tech. 57:381–389 [DOI] [PubMed] [Google Scholar]
- 25. Groen J., et al. 1996. Hantavirus antigen detection in kidney biopsies from patients with nephropathia epidemica. Clin. Nephrol. 46:379–383 [PubMed] [Google Scholar]
- 26. Haraldsson B., Nystrom J., Deen W. M. 2008. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88:451–487 [DOI] [PubMed] [Google Scholar]
- 27. Harhaj N. S., et al. 2006. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest. Ophthalmol. Vis. Sci. 47:5106–5115 [DOI] [PubMed] [Google Scholar]
- 28. Hautala T., et al. 2002. Hypophyseal hemorrhage and panhypopituitarism during Puumala virus infection: magnetic resonance imaging and detection of viral antigen in the hypophysis. Clin. Infect. Dis. 35:96–101 [DOI] [PubMed] [Google Scholar]
- 29. Hung T., et al. 1992. Identification of Hantaan virus-related structures in kidneys of cadavers with haemorrhagic fever with renal syndrome. Arch. Virol. 122:187–199 [DOI] [PubMed] [Google Scholar]
- 30. Jacquel A., et al. 2006. A survey of the signaling pathways involved in megakaryocytic differentiation of the human K562 leukemia cell line by molecular and c-DNA array analysis. Oncogene 25:781–794 [DOI] [PubMed] [Google Scholar]
- 31. Jessie K., Fong M. Y., Devi S., Lam S. K., Wong K. T. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411–1418 [DOI] [PubMed] [Google Scholar]
- 32. Jonsson C. B., Figueiredo L. T., Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23:412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanerva M., Vaheri A., Mustonen J., Partanen J. 1998. High-producer allele of tumour necrosis factor-alpha is part of the susceptibility MHC haplotype in severe Puumala virus-induced nephropathia epidemica. Scand. J. Infect. Dis. 30:532–534 [DOI] [PubMed] [Google Scholar]
- 34. Kang D. H., et al. 2002. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 13:806–816 [DOI] [PubMed] [Google Scholar]
- 35. Khaiboullina S. F., Netski D. M., Krumpe P., St Jeor S. C. 2000. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J. Virol. 74:11966–11971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim S., et al. 1993. Localization of Hantaan viral envelope glycoproteins by monoclonal antibodies in renal tissues from patients with Korean hemorrhagic fever H. Am. J. Clin. Pathol. 100:398–403 [DOI] [PubMed] [Google Scholar]
- 37. Klingstrom J., Lindgren T., Ahlm C. 2008. Sex-dependent differences in plasma cytokine responses to hantavirus infection. Clin. Vaccine Immunol. 15:885–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korgaonkar S. N., et al. 2008. HIV-1 upregulates VEGF in podocytes. J. Am. Soc. Nephrol. 19:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krause R., Aberle S., Haberl R., Daxbock F., Wenisch C. 2003. Puumala virus infection with acute disseminated encephalomyelitis and multiorgan failure. Emerg. Infect. Dis. 9:603–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krautkramer E., Zeier M. 2008. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 82:4257–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kucinskaite-Kodze I., et al. 2011. Characterization of monoclonal antibodies against hantavirus nucleocapsid protein and their use for immunohistochemistry on rodent and human samples. Arch. Virol. 156:443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lahdevirta J., Enger E., Hunderi O. H., Traavik T., Lee H. W. Hantaan virus is related to hemorrhagic fever with renal syndrome in Norway. Lancet ii:606. [DOI] [PubMed] [Google Scholar]
- 43. Larson R. S., Brown D. C., Ye C., Hjelle B. 2005. Peptide antagonists that inhibit Sin Nombre virus and Hantaan virus entry through the β3-integrin receptor. J. Virol. 79:7319–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Launay D., et al. 2003. Pulmonary-renal syndrome due to hemorrhagic fever with renal syndrome: an unusual manifestation of Puumala virus infection in France. Clin. Nephrol. 59:297–300 [DOI] [PubMed] [Google Scholar]
- 45. Lee H. W. 1982. Hemorrhagic fever with renal syndrome (HFRS). Scand. J. Infect. Dis. Suppl. 36:82–85 [PubMed] [Google Scholar]
- 46. Lee H. W., Lee P. W., Johnson K. M. 1978. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 137:298–308 [DOI] [PubMed] [Google Scholar]
- 47. Makela S., et al. 2004. Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am. J. Kidney Dis. 43:809–816 [DOI] [PubMed] [Google Scholar]
- 48. Makela S., et al. 2002. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J. Infect. Dis. 186:843–846 [DOI] [PubMed] [Google Scholar]
- 49. Mayer G., Boileau G., Bendayan M. 2003. Furin interacts with proMT1-MMP and integrin αv at specialized domains of renal cell plasma membrane. J. Cell Sci. 116:1763–1773 [DOI] [PubMed] [Google Scholar]
- 50. McGinn S., Poronnik P., Gallery E. D., Pollock C. A. 2004. A method for the isolation of glomerular and tubulointerstitial endothelial cells and a comparison of characteristics with the human umbilical vein endothelial cell model. Nephrology (Carlton) 9:229–237 [DOI] [PubMed] [Google Scholar]
- 51. Miettinen M. H., et al. 2006. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int. 69:2043–2048 [DOI] [PubMed] [Google Scholar]
- 52. Muczynski K. A., Cotner T., Anderson S. K. 2001. Unusual expression of human lymphocyte antigen class II in normal renal microvascular endothelium. Kidney Int. 59:488–497 [DOI] [PubMed] [Google Scholar]
- 53. Mustonen J., et al. 1994. Nephropathia epidemica in Finland: a retrospective study of 126 cases. Scand. J. Infect. Dis. 26:7–13 [DOI] [PubMed] [Google Scholar]
- 54. Negrete O. A., et al. 2005. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436:401–405 [DOI] [PubMed] [Google Scholar]
- 55. Niikura M., et al. 2004. Modification of endothelial cell functions by Hantaan virus infection: prolonged hyper-permeability induced by TNF-α of Hantaan virus-infected endothelial cell monolayers. Arch. Virol. 149:1279–1292 [DOI] [PubMed] [Google Scholar]
- 56. Page C., Rose M., Yacoub M., Pigott R. 1992. Antigenic heterogeneity of vascular endothelium. Am. J. Pathol. 141:673–683 [PMC free article] [PubMed] [Google Scholar]
- 57. Peralta Soler A., Mullin J. M., Knudsen K. A., Marano C. W. 1996. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am. J. Physiol. 270:F869–F879 [DOI] [PubMed] [Google Scholar]
- 58. Rasmuson J., et al. 2011. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 30:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raymond T., Gorbunova E., Gavrilovskaya I. N., Mackow E. R. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent αvβ3 integrin conformers. Proc. Natl. Acad. Sci. U. S. A. 102:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Reiser J., Kriz W., Kretzler M., Mundel P. 2000. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 11:1–8 [DOI] [PubMed] [Google Scholar]
- 61. Rowe R. K., Pekosz A. 2006. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 80:1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saleem M. A., et al. 2002. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13:630–638 [DOI] [PubMed] [Google Scholar]
- 63. Schnabel E., Anderson J. M., Farquhar M. G. 1990. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 111:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schneeberger E. E., Lynch R. D. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213–C1228 [DOI] [PubMed] [Google Scholar]
- 65. Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. 1994. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci. 107(Pt. 2):527–541 [DOI] [PubMed] [Google Scholar]
- 66. Settergren B., Juto P., Trollfors B., Wadell G., Norrby S. R. 1989. Clinical characteristics of nephropathia epidemica in Sweden: prospective study of 74 cases. Rev. Infect. Dis. 11:921–927 [DOI] [PubMed] [Google Scholar]
- 67. Settergren B., Juto P., Trollfors B., Wadell G., Norrby S. R. 1988. Hemorrhagic complications and other clinical findings in nephropathia epidemica in Sweden: a study of 355 serologically verified cases. J. Infect. Dis. 157:380–382 [DOI] [PubMed] [Google Scholar]
- 68. Settergren B., Trollfors B., Fasth A., Hultberg B., Norrby S. R. 1990. Glomerular filtration rate and tubular involvement during acute disease and convalescence in patients with nephropathia epidemica. J. Infect. Dis. 161:716–720 [DOI] [PubMed] [Google Scholar]
- 69. Singh B., Fu C., Bhattacharya J. 2000. Vascular expression of the αvβ3-integrin in lung and other organs. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L217–L226 [DOI] [PubMed] [Google Scholar]
- 70. Singh B., Rawlings N., Kaur A. 2001. Expression of integrin αvβ3 in pig, dog and cattle. Histol. Histopathol. 16:1037–1046 [DOI] [PubMed] [Google Scholar]
- 71. Sironen T., et al. 2008. Pathology of Puumala hantavirus infection in macaques. PLoS One 3:e3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smithies O. 2003. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc. Natl. Acad. Sci. U. S. A. 100:4108–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Staddon J. M., Herrenknecht K., Smales C., Rubin L. L. 1995. Evidence that tyrosine phosphorylation may increase tight junction permeability. J. Cell Sci. 108(Pt. 2):609–619 [DOI] [PubMed] [Google Scholar]
- 74. Stamatovic S. M., Dimitrijevic O. B., Keep R. F., Andjelkovic A. V. 2006. Protein kinase Cα-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J. Biol. Chem. 281:8379–8388 [DOI] [PubMed] [Google Scholar]
- 75. Stevens T., et al. 2001. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am. J. Physiol. Cell Physiol. 281:C1422–C1433 [DOI] [PubMed] [Google Scholar]
- 76. Suzuki T., et al. 2009. PKCη regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. U. S. A. 106:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tao Y., et al. 2007. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int. 72:1358–1366 [DOI] [PubMed] [Google Scholar]
- 78. Temonen M., et al. 1996. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin. Immunol. Immunopathol. 78:47–55 [DOI] [PubMed] [Google Scholar]
- 79. Temonen M., et al. 1993. Susceptibility of human cells to Puumala virus infection. J. Gen. Virol. 74(Pt. 3):515–518 [DOI] [PubMed] [Google Scholar]
- 80. Wei C., et al. 2008. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14:55–63 [DOI] [PubMed] [Google Scholar]
- 81. Wong C., Jin Z. G. 2005. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J. Biol. Chem. 280:33262–33269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wong K. T., et al. 2002. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am. J. Pathol. 161:2153–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yanagi Y., Takeda M., Ohno S. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 87:2767–2779 [DOI] [PubMed] [Google Scholar]
- 84. Yoon S., Gingras D., Bendayan M. 2001. Alterations of vitronectin and its receptor αv integrin in the rat renal glomerular wall during diabetes. Am. J. Kidney Dis. 38:1298–1306 [DOI] [PubMed] [Google Scholar]
- 85. Zaki S. R., et al. 1994. Retrospective diagnosis of a 1983 case of fatal hantavirus pulmonary syndrome. Lancet 343:1037–1038 [DOI] [PubMed] [Google Scholar]
- 86. Zaki S. R., et al. 1995. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552–579 [PMC free article] [PubMed] [Google Scholar]