Abstract
Neuronal cultures, including motoneuron cultures are established from embryonic animals. These approaches have provided novel insights into developmental and possibly disease mechanisms mediating cell survival or death. Motoneurons isolated from mouse models of disease such as the SOD1G93A mouse demonstrate subtle abnormalities that may contribute to pathology. Nonetheless, in the animal model, pathological events become more prominent as the animal mature, but the ability to isolate individual cells to investigate these events is limited. Here, we describe a protocol derived and modified from previously published protocols to isolate motoneurons from mature animals. While the yield of cells is low, the ability to examine mature motoneurons will provide a new platform to investigate pathological changes associated with motoneuron disease.
Keywords: Neuronal cultures, neurodegenerative diseases, motoneuron disease, trophic factor, cell death/apoptosis, mitochondria, axonal transport
1. Introduction
The development of motoneurons (MNs) including their dependence on target derived trophic support has been characterized in the spinal cords of developing animals, most notably the chick embryo (1–3). The development and utilization of in vitro culture allows specific effects of trophic factors on neurite extension and cell survival to be determined (4–7). In vitro culture of MNs has provided insight into specific cell death pathways and stress responses (8–12). Furthermore, culture of embryonic MNs has been used to determine potential disease specific processes related to the pathogenesis of motoneuron diseases such as ALS. For example, caspases were shown to be involved in MN cell death during development both in vivo and in vitro (13), and subsequently they were suggested to also play a role in MN degeneration in the mouse model of ALS (14,15). Nonetheless, drawing conclusions on disease processes using embryonic cultures is riddled with experimental caveats, and the most obvious is that embryonic neurons generally do not exhibit the pathological events that occur much later in the adult animal.
Culturing MNs from postnatal, especially adult, animals was considered difficult, if not impossible, in part due to the length of the MN axon, cell-cell interactions and thousands of synapses. Nonetheless, adult neurons and neurospheres can be isolated from adult animals and a detailed protocol was recently published (16). This approach involves a stepwise-Optiprep gradient that allows separation of debris and myelin from neurons and glial cells. Our lab routinely isolates and cultures embryonic MNs (17). We have used combined the protocols to culture MNs from older animals to investigate specific events observed in MNs in a mouse model of ALS. The results are encouraging and appear to provide a foundation for future studies to examine pathophysiological processes in mature MNs. The protocol described in this chapter has been used successfully to isolate viable postnatal day (P) 30 mouse MNs from the lumbar spinal cord.
2. Materials
2.1. Cleaned and sterile 13 mm diameter glass coverslips
Preparation of glass coverslips:
Wash coverslips in 95% absolute ethanol and 5% acetic acid. Rinse several times in Milli-Q water.
Let coverslips dry on filter paper. It is necessary for coverslips to be completely dry before autoclaving, otherwise they will stick together and not come apart.
Transfer coverslips to a glass petri-dish and autoclave.
Set coverslips in tissue culture plates. We have used grierner dishes with a small drop of vacuum grease to hold the coverslip in place (see Note 1 and Figure 1). Alternately, coverslips can be placed into a 24 well tissue culture plate; however, this makes coating them to limit where cells can attach more difficult (see Note 2).
Coverslips should be coated with poly-ornithine (3µg/ml in sterile dH2O) for several hours (at room temp.) to overnight (at 4°C). Poly-ornithine coated coverslips can be stored at 4°C for several weeks.
Wash coverslips several times with PBS +Ca+2,+Mg+2.
Coat coverslips with Laminin (3µg/ml in PBS +Ca+2,+Mg+2) for 3–5 hours at 37°C, 5% CO2.
Before plating cells, wash coverslips several times with PBS +Ca+2,+Mg+2.
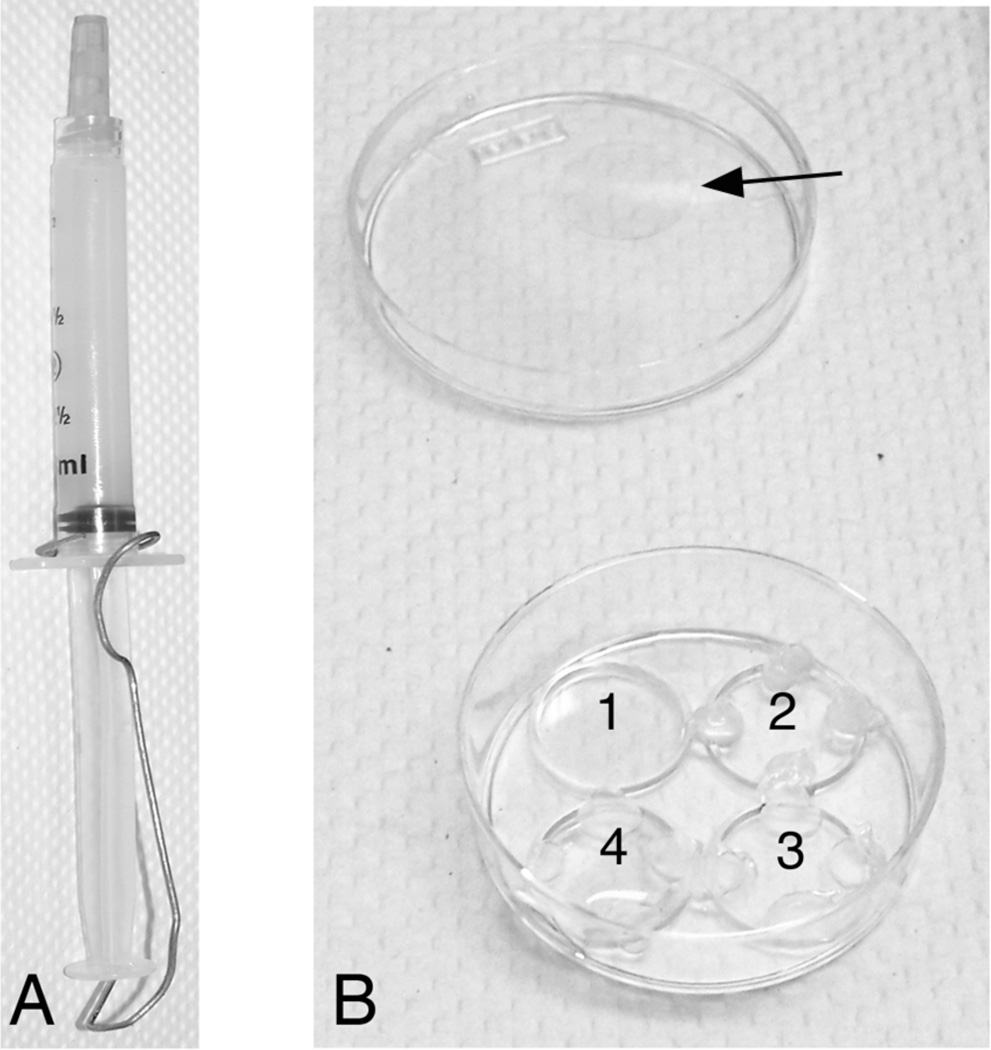
Figure 1.
A. Image of syringe filled with vacuum grease and paper clip to prevent loss during autoclaving. B. Shown is a greiner dish. Well 1 is empty; 2 has vacuum grease applied, 3 has the coverslip and 4 has the coverslip with 100 ul of liquid. Arrow indicates 13 mm glass coverslip.
2.2. 9” siliconized Pasteur pipettes
This procedure must be performed under a fume hood. Using a rubber pipette bulb, Silicoat (Sigma) is aspirated into the glass pipette several times until the majority of the interior of the pipette has been coated. Excess silicoat is expelled and the pipettes allows to air dry on a paper towel overnight in the fume hood. Once completely dry, the pipettes are wrapped in clean paper towels and then foil and autoclaved. Pipettes are stored at room temperature until use.
2.3. Dissections
Dissection instruments including #1 dumont forceps, #5 dumont forceps and micro-knife
2.4. Tissue Culture Reagents
Specific Reagents are listed below. While many of the reagents can be obtained from multiple sources, specific sources are provided because these have proven successful for MN culture.
B27 (invitrogen #17504-044)
10% BSA (Tissue Culture grade; Sigma) in HABG (see Culture Media below)
Gentamycin (Invitrogen)
Glutamax (Invitrogen)
Hibernate A (Brain Bits)
Hibernate A w/o calcium (Brain Bits)
Horse Serum (Invitrogen)
Laminin (Sigma)
Neurobasal media (Invitrogen
OptiPrep (Sigma or Accurate Chemical)
Papain (Worthington #L500 3119)Poly-ornithine (Sigma; stock solution of 1 ml/ml in 0.15 M boric acid)13. Trophic factors (CT-1, CNTF, GDNF available from R&D)
2.5. Tissue Culture Media
All reagents should be sterile filtered prior to use.
HABG is an artificial CSF media with supplements to promote neuronal survival: 98 mls HA, 2 mls B27, 10 ul gentamycin, 293 ul Glutamax.
NB medium is formulated to promote neuronal growth and survival in culture: 48 mls NB, 1.0 ml B27, 146 ul Glutamax, 5 ul gentamycin, 1 ml heat inactivated horse serum. Trophic factors (1 ng/ml) are added immediately prior to plating cells.
Papain is a protease with endopeptidase, amidase, and esterase activities. It is used when a more gentle tissue dissociation is required: 12 mg papain, 6 mls HA without calcium, 17.6 ul Glutamax
2.6. Optiprep Gradient
for one 15 ml tube:
| Layer | OptiPrep | HABG | total |
|---|---|---|---|
| 1 (bottom layer) | 173 µl | 827 µl | 1 ml |
| 2 | 127 µl | 876 µl | 1 ml |
| 3 | 99 µl | 901 µl | 1 ml |
| 4 (top layer) | 74 µl | 926 µl | 1 ml |
3. Methods
3.1 Dissection and isolation of spinal cord
Dissections should be performed in a clean and draft-free area. Although dissections can be performed in a laminar flow hood, we usually perform them on a clean lab bench that has been wiped down with 70% ethanol. All instruments and containers for buffers and tissue are sterile.
From an anesthetized mouse, isolate the lumbar spinal cord. Performing a dorsal lamenectomy prior to taking the spinal cord makes actual dissection easier.
Place lumbar spinal cord in cold PBS (- Ca+2/Mg+2). Dissect spinal cord from vertebrae and transfer to cold HABG. Note: it is essential to keep tissue and media cold during dissections. Keep all reagents on ice and place petri dishes for dissections on an ice-pack during dissection.
Using fine forceps, remove nerve roots and meninges.
Using a micro-knife, separate dorsal from ventral spinal cords and discard the dorsal portion.
Using the micro-knife, chop ventral spinal cord into 0.5 mm pieces of tissue.
-
Transfer the tissue pieces to a 50 ml tube containing ±10–15 mls HABG on ice.
Note: place tissue from no more than 3 spinal cords/tube.
3.2 Dissociation to single cell suspension
Allow pieces to settle and come to room temp. Remove HABG and replace with papain (at room temp).
Incubate tissue in papain at 30°C for 30 minutes in a shaking incubator or waterbath. The tube should shake at a speed just high enough to keep the tissue pieces in suspension (e.g., ± 170 rpm).
Allow tissue pieces to settle at room temp. Remove papain and replace with 1.9 mls HABG (at room temp) + 100 ml 10% BSA + 1 µl DNAse.
Using a fire-polished, silicon-coated glass pipette, triturate 8–10 times in approximately 45 seconds. Let intact tissue pieces settle. Remove suspension and transfer to a clean 15 ml tube.
Add 2 mls HABG to tissue pieces and repeat step 4.
Repeat with another 2 mls HABG.
3.3 Isolation of neurons
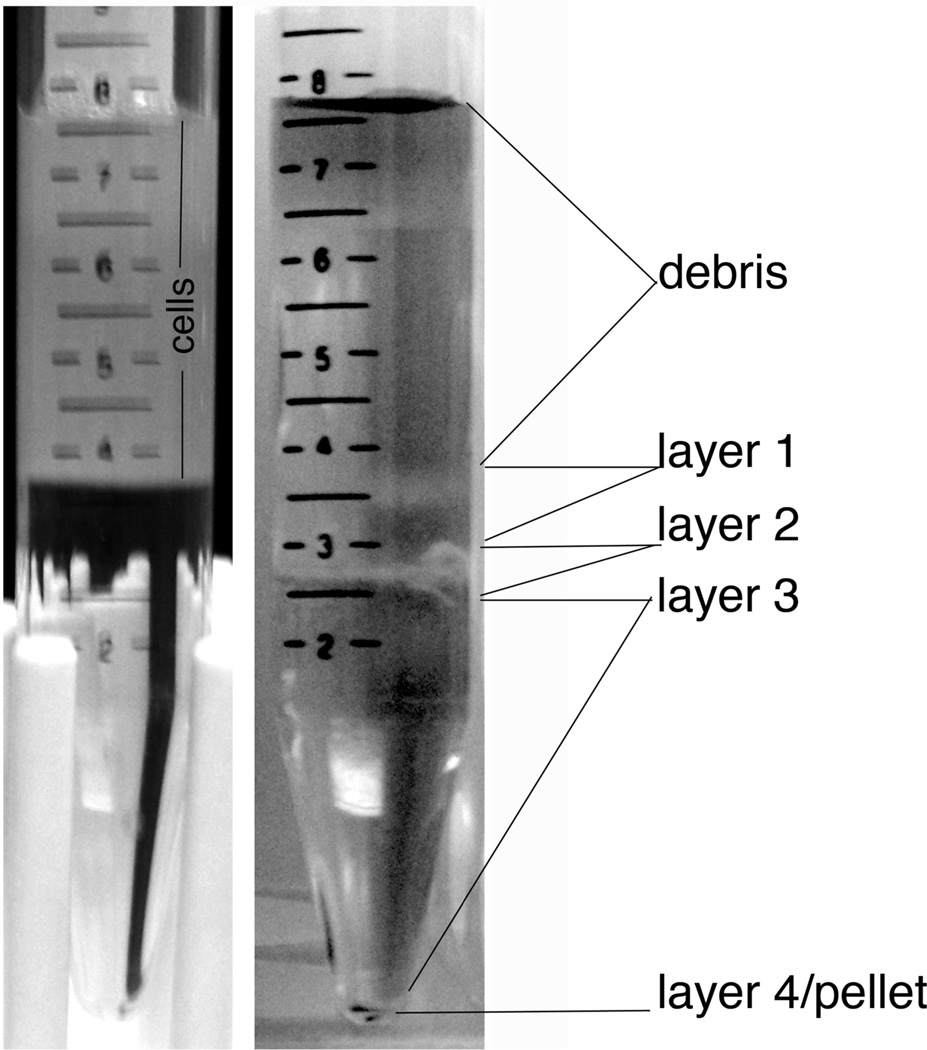
To prepare the gradient make each layer in an individual tube, then create the gradient by adding each layer on top of the previous layer, starting with layer 1 at the bottom. Be sure to add each layer very gently so not to disrupt the gradient (Figure 2A). A hand held glass pipette is useful.
Carefully apply cell suspension on top of the OptiPrep gradient (Figure 2B).
Centrifuge at 1900 rpm (800 g; no brake) for 15 min at room temp
Remove debris layer and fractions 1 and 2 (see Figure 2C). Collect fraction 3 and transfer to a 50 ml tube. Add +10 ml HABG to cells.
Centrifuge cells at 1000 rpm (no brake) for 10 minutes at room temp to remove OptiPrep.
Resuspend cell pellet in 5 mls HABG. Using a 9” glass pipette (not siliconized) add 4% BSA in HABG (1 ml) to the bottom of the tube to create a BSA cushion.
Centrifuge cells at 1000 rpm (no brake) for 10 minutes. Additional cell debris will be removed in the BSA cushion.
Resuspend cells in 1 ml NB media and determine cell yield using a hemocytometer.
Plate 1–4 × 104 cells in 100 µl/coverslip.
Allow cells to attach at 37°C, 5% CO2 for at least 1 hour. This insures that cells attach to the coverslip and are not washed into other areas of the dish.
Add NB + trophic factors to tissue culture plates (2 mls/ 35 mm dish) containing coverslips and return to 37°C/ 5% CO2 incubator (see Note 3).
At this point the investigator can move on to specific experiments.
Figure 2.
A. Optiprep density gradient with dissociated cells on top. B. Individual layers of cells are visible after centrifugation. Layer 3 contains primarily neurons, including motoneurons. Layer 2 also contains neurons (Brewer and Torricelli); however, we did not recover motoneurons from this layer.
3.4 Final Considerations
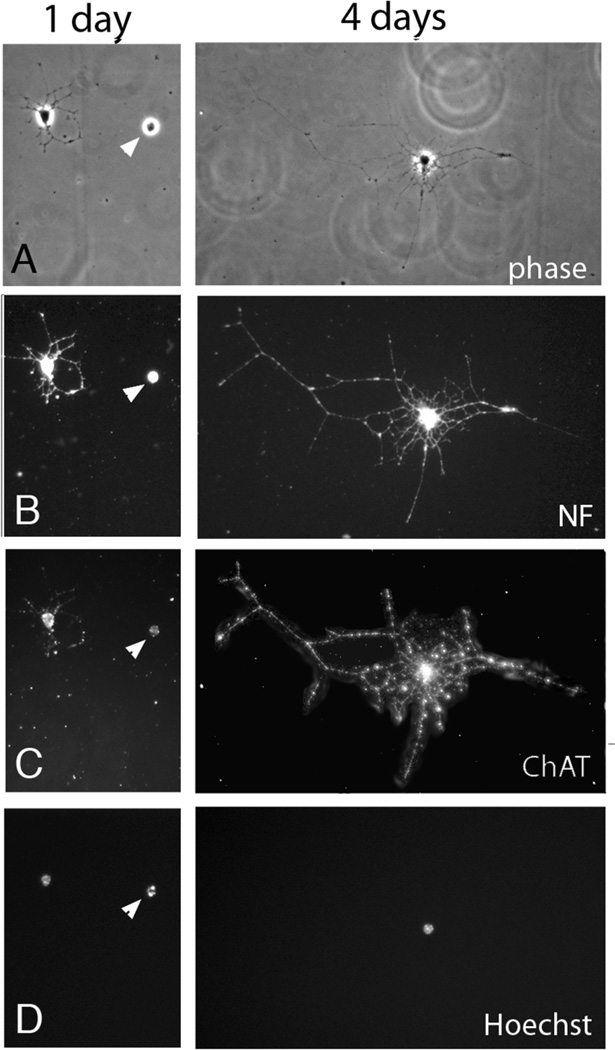
We took the entire lumbar spinal cord for our initial culture, and found that within 24 hours, 57% of the cells were neurofilament (H+L) immunopositive, and 18% of these cells were choline acetyl transferase (ChAT)-immunopositive. The cells had intact and healthy appearing nuclei (as determined by Hoechst 33342) and extensive neurite outgrowth. We next utilized only the ventral lumbar spinal cord. After four days in culture approximately 70% of the cells are neurons, and 93% of these cells are ChAT positive. Within 24 hours the cells extend numerous processes that are quite extensive by 4 days (Figure 3). The cells are healthy as determined by the Live/Dead kit (Molecular Probes) where calcein AM is taken up by healthy cells and hydrolyzed by esterases to a green-fluorescent product. No cell with this morphology had a red-fluorescent nucleus that would indicate disrupted membranes and incorporation of ethidium into DNA (not shown).
Figure 3.
Shown are P30 mouse MNs in culture for 1 or 4 days. Phase contrast images (A), NF immunoreactivity (B) ChAT immunoreactivity (C) and Hoechst staining (D) are shown. The cells had intact and healthy appearing nuclei (as determined by Hoechst 33342) and extensive neurite outgrowth. Arrowhead indicates a dead cell.
The yield for these cultures is very low. We estimate approximately 4 × 104 cells/ventral spinal cord. For this reason, characterization of the culture and subsequent experiments appear limited to assays that allow individual cell analysis. Nonetheless, this procedure has yielded relatively consistent results using postnatal day 30 mice. We were unsuccessful, however, in isolating motoneurons for P100 animals. We believe that the ability to examine mature motoneurons will provide a new platform to investigate pathological changes associated with motoneuron disease. For these reasons, the benefits appear to outweigh the limitations.
Footnotes
Vacuum grease should be autoclaved before use in tissue culture; however, it can expand and leak out of the syringe during autoclaving. To prevent this, Leur Lok 1 or 3 ml syringes are filled with vacuum grease and a large paper clip is used to secure the plunger so that it is not pushed out in the autoclave (Figure 1).
To limit the ability of cells to plate only on the coverslips, coat coverslips with 100 µl only. The liquid will not reach to the edge of the coverslip creating a barrier for cells.
It is very difficult to view cells in culture. For the best viewing process use one of the techniques below. Once the coverslip is flipped onto a microscope slide, cells can be viewed with phase-contrast. Note that this procedure is not sterile so do not put the coverslip back in culture as it will become contaminated.
References
- 1.Hamburger V. Neurosci Res Program Bull. 1977;15 Suppl:iii-37. doi: 10.1007/978-1-4899-6743-5_5. [DOI] [PubMed] [Google Scholar]
- 2.Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Cell Tissue Res. 2000;301:71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheim RW. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 4.Masuko S, Kuromi H, Shimada Y. Proc Natl Acad Sci U S A. 1979;76:3537–3541. doi: 10.1073/pnas.76.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson CE, Huchet M, Changeux JP. Proc Natl Acad Sci U S A. 1981;78:2625–2629. doi: 10.1073/pnas.78.4.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eagleson KL, Bennett MR. Neurosci Lett. 1983;38:187–192. doi: 10.1016/0304-3940(83)90038-1. [DOI] [PubMed] [Google Scholar]
- 7.Bloch-Gallego E, Huchet M, el M'Hamdi H, Xie FK, Tanaka H, Henderson CE. Development. 1991;111:221–232. doi: 10.1242/dev.111.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Milligan CE, Oppenheim RW, Schwartz LM. J Neurobiol. 1994;25:1005–1016. doi: 10.1002/neu.480250809. [DOI] [PubMed] [Google Scholar]
- 9.Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 10.Barnes NY, Li L, Yoshikawa K, Schwartz LM, Oppenheim RW, Milligan CE. J Neurosci. 1998;18:5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Oppenheim RW, Milligan CE. J Neurobiol. 2001;46:249–264. doi: 10.1002/1097-4695(200103)46:4<249::aid-neu1006>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Prevette D, Oppenheim RW, Milligan CE. Mol Cell Neurosci. 1998;12:157–167. doi: 10.1006/mcne.1998.0709. [DOI] [PubMed] [Google Scholar]
- 14.Pasinelli P, Borchelt DR, Houseweart MK, Cleveland DW, Brown RH., Jr Proc Natl Acad Sci U S A. 1998;95:15763–15768. doi: 10.1073/pnas.95.26.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 16.Brewer GJ, Torricelli JR. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AR, Robinson MB, Milligan CE. Nat Protoc. 2007;2:1499–1507. doi: 10.1038/nprot.2007.208. [DOI] [PubMed] [Google Scholar]