Abstract
Schizophrenia is a disorder of a neurodevelopmental origin manifested symptomatically after puberty. Structural neuroimaging studies show that neuroanatomical aberrations precede onset of symptoms, raising a question of whether schizophrenia can be prevented. Early treatment with atypical antipsychotics may reduce the risk of transition to psychosis, but it remains unknown whether neuroanatomical abnormalities can be prevented. We have recently shown, using in vivo structural magnetic resonance imaging, that treatment with the atypical antipsychotic clozapine during an asymptomatic period of adolescence prevents the emergence of schizophrenia-like brain structural abnormalities in adult rats exposed to prenatal immune challenge, in parallel to preventing behavioral abnormalities. Here we assessed the preventive efficacy of the atypical antipsychotic risperidone (RIS). Pregnant rats were injected on gestational day 15 with the viral mimic polyriboinosinic-polyribocytidylic acid (poly I:C) or saline. Their male offspring received daily RIS (0.045 or 1.2 mg/kg) or vehicle injection in peri-adolescence (postnatal days [PND] 34–47). Structural brain changes and behavior were assessed at adulthood (from PND 90). Adult offspring of poly I:C–treated dams exhibited hallmark structural abnormalities associated with schizophrenia, enlarged lateral ventricles and smaller hippocampus. Both of these abnormalities were absent in the offspring of poly I:C dams that received RIS at peri-adolescence. This was paralleled by prevention of schizophrenia-like behavioral abnormalities, attentional deficit, and hypersensitivity to amphetamine in these offspring. We conclude that pharmacological intervention during peri-adolescence can prevent the emergence of behavioral abnormalities and brain structural pathology resulting from in utero insult. Furthermore, highly selective 5HT2A receptor antagonists may be promising targets for psychosis prevention.
Keywords: risperidone, prevention, structural MRI, schizophrenia, prenatal poly I:C, animal model
Introduction
Schizophrenia is a severe neuropsychiatric disorder whose clinical course is characterized by the onset of symptoms after puberty and whose pharmacotherapy remains unsatisfactory. While much evidence indicates that schizophrenia is associated with a brain insult early in development,1 there is increasing evidence from longitudinal magnetic resonance imaging (MRI) studies that progressive structural brain aberrations occur in this disorder and indeed precede the onset of symptoms, intensifying prior to transition to psychosis.2–6 These data have raised a crucial question of whether schizophrenia can be prevented.2–4,7–12 Studies in individuals in the early clinical stages of the disorder yet prior to the development of the full clinical phenotype have been encouraging in showing that preventive treatment with atypical antipsychotic drugs (APDs) may reduce the risk of progression to first-episode psychosis in some of the patients7–10,13 but controversies remain. Clearly, identification and treatment of individuals who are vulnerable to and/or at current risk of psychosis present diagnostic, ethical, and methodological limitations, the latter including small underpowered samples and non-blind designs.11,14–19 To date, it remains unknown whether progressive structural brain aberrations can be halted by preventive treatments. Given the clinical and methodological challenges of imaging studies in patients,4,6,20,21 getting such information remains a major challenge.
Given the growing importance of early prevention and the difficulties of investigating this question in patients, valid animal models would be invaluable in exploring this question. Neurodevelopmental animal models of schizophrenia, which mimic the clinical course of this disorder whereby the deleterious functional consequences of early insult do not arise until after puberty, are particularly suitable for evaluating the feasibility of prevention. Such models capture the expectation of the “neuroprogressive” perspective of schizophrenia that the underlying pathophysiological and neuropathological mechanisms are progressive in nature and thus allow the investigation of preventive interventions. Based on this rationale, Richtand et al.19 showed that treatment with the atypical APD risperidone (RIS) on postnatal days (PND) 35–56 prevented excessive amphetamine-induced hyperactivity on PND 57 in rats that sustained a neonatal ventral hippocampal lesion. More recently, Meyer et al.22 have reported that treatment with the atypical APD clozapine and the typical APD haloperidol on PND 35–65 prevented the emergence of disrupted latent inhibition (LI) in adulthood (90 days) in mice exposed to maternal gestational immune activation.22 However, because in the study of Richtand et al.19 the target behavior was measured 24 h after treatment cessation and in the study of Meyer et al.22 the treatment was extended to early adulthood, a period during which the assessed behaviors (eg, LI) are likely to have been already abnormal, in both studies, there is a possibility that treatment was exerting acute therapeutic rather than long-term preventive action.
We have recently shown for the first time, using schizophrenia-relevant behavioral assessment and in vivo structural MRI, that brain neuropathology consequent to early insult in animals can be prevented by early treatment with atypical APD.23 We used the maternal gestational immune activation model,24–26 which is based on the well-documented association between maternal exposure to viral infection in pregnancy and increased risk of schizophrenia in the offspring.1,27,28 In the model, injection of pregnant rats or mice with the viral mimic polyriboinosinic-polyribocytidylic acid (poly I:C) leads to a wide spectrum of schizophrenia-relevant functional and neuropathological deficits in the adult offspring.24,26,29–31 As in schizophrenia, prenatal poly I:C–induced behavioral abnormalities exhibit maturational delay, emerging in adult but not peri-adolescent offspring26,30.
Using structural imaging, we showed that in utero exposure to poly I:C led in the offspring to postpubertal emergence of hallmark brain structural abnormalities associated with schizophrenia, enlarged lateral ventricles (LV) and smaller hippocampus.2–6,32 Specifically, there were no differences in LV and hippocampal volumes between 35-day-old poly I:C and saline offspring, but they were clearly evident at 90 days of age.23 This pattern of postpubertal emergence paralleled our findings with schizophrenia-relevant behavioral manifestations, which were normal at 35 but abnormal at 90 days.26 Both of the volumetric abnormalities were prevented in the poly I:C offspring that received treatment with clozapine during an asymptomatic period of peri-adolescence (PND 34–47). The latter was paralleled by prevention of behavioral abnormalities phenotypic of schizophrenia, attentional deficit, and hypersensitivity to amphetamine23.
Given that our preventive treatment was administered during an asymptomatic period, a long time elapsed between treatment cessation and behavioral and imaging tests (at least month and a half and 2 months and a half, respectively), and prevention of behavioral abnormalities was paralleled by prevention of brain structural abnormalities, our results have provided the first clear indication that preventive treatment with atypical APDs may exert disease-modifying, as opposed to symptomatic, effects.23 The latter in turn suggests that the poly I:C model possesses predictive validity for identifying effective treatments for prevention of first-episode psychosis. In the light of the rapidly growing focus on early detection and pharmacological intervention in the treatment of schizophrenia, recently reflected in the debate surrounding the newly proposed “psychosis risk syndrome” in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-V33, further investigations of the feasibility of prevention in a neurodevelopmental model that has both etiological (based on a known risk factor) and predictive validity like the poly I:C model are important. Identification of treatments preventing behavioral and brain structural alterations using such a model would be useful in refining hypotheses regarding effective treatments for psychosis prevention as well as identifying a small subset of compounds with greatest potential for study in human clinical trials. Of equal importance is the elucidation of the effects of preventive treatments in control animals that might highlight possible risks and benefits associated with early pharmacological intervention22.
In view of the above, here we sought to further evaluate the predictive validity of the poly I:C model by testing whether the LV and hippocampal volumetric abnormalities as well as accompanying behavioral abnormalities in adult poly I:C offspring would be prevented by treatment in peri-adolescence with RIS. We chose RIS because this drug is widely used in young children with pervasive developmental disorders (PDD) and autism,34,35 used in high-risk individuals,8,13 and effective in a neurodevelopmental animal model.19 Of particular interest was a comparison between the preventive efficacy of low and high doses of RIS because Richtand et al.19 found that a low RIS dose, which is a selective 5HT2A antagonist, was more effective than a high dose with high D2 antagonism, suggesting that selective 5HT2A receptor antagonism may be effective in psychosis prevention.
Methods
Animals
Adult (350–400 g) male Wistar rats were housed 3–4 to a cage under reversed cycle lighting (lights on: 1900–0700 h) with ad lib food and water, except for the LI experiment. All experimental protocols conformed to the guidelines of the Institutional Animal Care and Use Committee of Tel-Aviv University, Israel, and to the guidelines of the National Institutes of Health (NIH) (animal welfare assurance number A5010-01, expires on 9.30.2011).
Prenatal Poly I:C Treatment
Prenatal treatment was performed as described previously.26,36 At about 3 months of age, rats (Tel-Aviv University Medical School) were mated and the first day after copulation was defined as day 1 of pregnancy. On gestation day (GD) 15, pregnant dams were anesthetized with 3% isoflurane (Minrad, Bethlehem, PA) in 98% O2 and given a single intravenous injection at the tail vein of 4 mg/kg poly I:C (Sigma, Rehovot, Israel) dissolved in saline, or saline (control). The volume of injection was 1 ml/kg. Poly I:C caused weight loss for approximately 1 day without significantly increasing miscarriage rate. At birth, pups were culled to 10, composed of 5 females and 5 males when possible. On PND 21, the pups were weaned and housed 3–4 to a cage by sex and litter and maintained undisturbed until drug injections that commenced on PND 34. Only male offspring were used in the experiments described here.
Preventive Treatment
Preventive treatment was given on PND 34–47, a period considered to represent peri-adolescence or adolescence.37 This period was chosen because poly I:C offspring are behaviorally26 and neuroanatomically23 asymptomatic during this period, and we showed that clozapine administration at this window prevented the emergence of behavioral and brain structural abnormalities in adulthood.23 Here, offspring of poly I:C or saline dams were injected daily intraperitoneally with 0.045 mg/kg RIS (low RIS), 1.2 mg/kg RIS (high RIS), or vehicle (Veh). The volume of injection was 1 ml/kg. RIS (Janssen, Beerse, Belgium) was dissolved in 0.1M tartaric acid (7.5 μl/1 mg) and diluted with saline. The low dose was chosen after Richtand et al.19 who found it to be effective in preventing amphetamine-induced hyperactivity caused by neonatal ventral hippocampal lesion, whereas the high dose was chosen on the basis of binding studies.38,39 RIS combines a potent 5HT2 receptor antagonism with a milder, but still potent, D2 antagonism. 5HT2A and D2 receptor occupancy predominate at lower and higher doses of RIS, respectively, and the difference between the occupancy of 5HT2A and D2 receptors produced by RIS becomes smaller as the dose is increased.38–41 Thus, our low dose exerted a predominantly 5HT2A receptor antagonistic action, with weak D2 dopamine (DA) receptor antagonism, whereas our high dose exerted also strong D2 antagonism. From PND 47, offspring were maintained undisturbed until behavioral testing or imaging at 3–4 months of age.
Magnetic Resonance Imaging
MRI scans were performed under inhalational isoflurane (1–2%; Minrad) anesthesia in 98% O2. Body temperature was maintained by circulating water at 37°C under the “bed” in which the animals were lying during the scans. Respiration was kept at 60–80 breath cycles per minute.
MRI Scan
MRI was performed on a 7.0 T/30 spectrometer (Bruker, Rheinstetten, Germany) using a volume coil for excitation and a rat quadrature coil for acquisition. Coronal T2 maps of the brain were obtained using spin echo with repetition time = 3000 ms and 16 echo times from 10 to 160 ms, field of view of 3 cm, matrix dimension of 256 × 128 (zero filled to 256 × 256), and 12 slices 1.5-mm thick with no gap.
Image Analysis
T2 maps were extracted from the multi-echo signal that was fitted to a mono-exponential decay function on a pixel-by-pixel basis. T2 maps were then coregistered using SPM2 software (Wellcome Department of Imaging Neuroscience, University College of London). Brains were normalized to a rat brain template registered with stereotactic rat brain atlas of Paxinos and Watson.42 The area of the LV, the hippocampus, and the whole brain were obtained from the T2-weighted images using manual segmentation (Medical Image Analysis version 2.4 MATLAB). LV, hippocampal, and whole-brain volumes were calculated by combining all slices where they appeared (approximately 2.20 to −4.52 mm, −2.1 to −6.7 mm, and 6.7 to −9.3 mm from Bregma, respectively42), and multiplied by slice thickness (1.5 mm).
Hippocampal volume was measured from 4 consecutive slices in which the hippocampus was clearly visible. The starting rostral slice was defined by the Cornu Ammonis and dentate gyrus (DG) and coincided with the dorsal hippocampal commissure approximately −2.12 mm from Bregma. The caudal boundary was defined by loss of contrast between the external capsule and the subiculum and the clear separation of the 2 cerebral hemispheres. In addition, the aquaduct opened up and became a clearly visible, round circle. The last hippocampal slice corresponded to approximately −6.7 mm from Bregma, whereas the first non-hippocampal slice corresponded to approximately −8.0 mm from Bregma. The anatomical borders used to draw the contour around the hippocampus in each of the 4 slices were presented in our previous article.23 Following Wolf et al.,43 in order to assess intrarater reliability, the same (well trained and experienced) rater (Y.P.) blind to treatment classification of the animals outlined again the regions of interest of half of the rats (drawn randomly) after 3 weeks. Reliability was measured at the level of the total volume (n = 24) and at the level of the slice (n = 96) using the intraclass Pearson correlation coefficient (ricc). High intrarater reliability (ricc values > 0.87) was obtained for all the MRI-derived volumetric assessments of the regions.
Behavioral Phenotyping
Behavioral phenotyping commenced 6 weeks after the cessation of the preventive treatment and included attentional deficit and heightened sensitivity to amphetamine. Selective attention deficit, a hallmark cognitive deficit of schizophrenia,44,45 was assessed using the LI and discrimination reversal (DR) tasks. In LI tasks, animals first receive nonreinforced preexposure to a stimulus and are then conditioned with this stimulus. In DR tasks, animals are first reinforced for responding to 1 of 2 stimuli or places and then reinforced for responding to the previously nonreinforced alternative. In both tasks, previously acquired information slows down the acquisition of behavioral control by the altered contingencies. Normal performance in both tasks reflects normal attentional bias to less fully process old inconsequential inputs, and both are disrupted in adult but not peri-adolescence offspring of poly I:C–injected dams.24,26,31 Disrupted LI is a widely used index of the impaired capacity to ignore irrelevant stimuli in schizophrenia46 because it is observed in rats and normal humans treated with amphetamine, in high schizotypal humans, and in acutely psychotic schizophrenia patients, whereas APDs restore disrupted LI in rodents, normal humans, and schizophrenia patients (for the discussion of commonalities between disrupted LI and reversal, see31). It should be noted that both LI and DR include behavioral measures not relevant to modeling attentional deficit in schizophrenia, namely, fear conditioning in the LI task (manifested in the non-preexposed [NPE] groups) and discrimination learning in the DR task.
We also assessed the offspring's sensitivity to the locomotion-stimulating effects of the DA releaser amphetamine (amphetamine-induced activity, AIA), which has been shown to be abnormally elevated in adult but not peri-adolescent offspring of poly I:C–injected dams24,26,31. Increased sensitivity to amphetamine in the poly I:C offspring mimics the well-documented subcortical DA hyperfunction in schizophrenia and, in particular, the exacerbation of psychotic symptoms in response to amphetamine in schizophrenia patients47,48.
Latent Inhibition
LI was conducted as described previously.26 Rats were trained in standard rodent test chambers (Campden Instruments, Loughborough, Leicester, United Kingdom) equipped with a retractable bottle and a drinkometer. They were handled for about 2 min daily for 5 days prior to the beginning of the experiment. A 22 h, water restriction schedule was initiated simultaneously with handling and continued throughout the experiment. During the next 5 days, rats were trained to drink in the experimental chamber for 20 min a day. Water in the test apparatus was given in addition to the daily ration of 1 h given in the home cages. The LI procedure was conducted on days 11–14 and consisted of 4 stages given 24 h apart—Preexposure: With the bottle removed, the preexposed (PE) rats received 40 tone (10 s, 80 dB, 2.8 kHz) presentations with an interstimulus interval of 40 s, whereas the NPE rats were confined to the chamber for an identical period of time without receiving the tone. Conditioning: With the bottle removed, each rat received 2 tone-shock (0.5 mA, 1 s) pairings given 5 min apart, with shock immediately following tone termination. Lick retraining as in initial training. Data of rats that failed to consume 600 licks were dropped from the analysis. Test: Each rat was placed in the chamber with an access to the bottle. When the rat completed 75 licks, the tone was presented. Times to complete 25 licks before and after tone presentation (licks 51–75 and licks 76–100, respectively) were recorded. LI is defined as shorter times to complete licks 76–100 (weaker suppression of drinking) of the PE compared with NPE rats.
Position Discrimination and Reversal
DR was conducted in a T-maze 31,49,50 (width 15.5 cm, height of walls 11 cm, length of stem 70 cm, and length of crosspiece 121 cm) submerged in a circular swimming pool (diameter 137 cm and height 35 cm). A hidden platform (15.5 × 15.5 cm) was located 1 cm below water at the end of one of the arms. The task included 2 stages given 24 h apart. On day 1, rats were required to learn a left-right discrimination with the platform consistently located in one of the arms (left and right sides counterbalanced within groups). At the start of each trial, the rat was placed in the starting box, facing the wall opposite the crosspiece, and allowed to swim. Once it had entered an arm, the guillotine door blocking that arm was lowered preventing the rat from retracing. If the rat chose the correct arm, it was allowed to remain on the platform for 5 s after which it was removed from the maze to a holding cage for the 10-s intertrial interval. If the wrong arm was chosen, the rat was confined to the arm for approximately 5 s and then removed from the maze to a holding cage for the duration of the intertrial interval. Each rat was trained until it reached a criterion of 5 consecutive correct trials. On the second day (reversal), reinforcement contingencies were switched so that the choice of the opposite arm was reinforced. Each rat was first retrained until criterion on the position discrimination of day 1 and then trained until criterion on the reversal of this discrimination, ie, with the platform located in the opposite arm. Number of trials to reach the criterion were recorded.
Amphetamine-Induced Activity
AIA was measured in dark gray boxes (45 cm wide × 65 cm long × 40 cm high) illuminated with red 36 W fluorescent light lamps (Philips, Andover, MA). Cameras were mounted above each box and centered approximately 75 cm above the box floor. The cameras were connected to a 16-channel multiplexer (Sony model YS-DX216CE) connected to a computer running image analysis software based on an NIH Image Analysis script (custom-written Visual Basic Program; P. Schmid, Laboratory of Behavioral Neurobiology, ETH Zurich). The software “grabbed” the image from each box every 1 s and compared it, pixel by pixel, with the image obtained in the previous second. The percentage of pixels that went from dark to light or from light to dark from 1 s to the next provided the measure of the magnitude of animal's displacement or “activity.” One-second activity values ranged from 0% (no movement) to approximately 7.5%. Rats were weighed and put into the boxes for 30 min at the end of which they were taken out, injected with amphetamine (1.0 mg/kg), and placed back in the boxes for 90 min.
Experimental Design
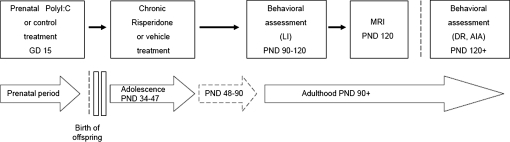
The experimental design is depicted in figure 1. The effects of adolescent RIS treatment were assessed in adulthood from PND 90 onwards. One cohort of adult rats was tested in LI and then imaged, and a second cohort was tested in DR and 10 days later in psychostimulant activity. In all the experiments, each experimental group consisted of subjects derived from multiple independent litters (16 poly I:C litters and 12 saline litters), with no more than 1–2 rats from the same litter in any of the experimental groups.
Fig. 1.
Experimental Design Used to Study the Effects of Preventive RIS Treatment in the Offspring of Poly I:C– or Saline (CON)-Treated Dams. Pregnant rats were exposed to poly I:C (4 mg/kg) or saline (CON) treatment on gestation day 15. The resulting offspring from both treatment conditions (poly I:C offspring and CON offspring) were then subjected to chronic treatment with vehicle, 0.045 mg/kg RIS (RIS-low), or 1.2 mg/kg (RIS-high) during the peri-adolescent stage of development between postnatal days (PND) 34 and 47. MRI and behavioral testing of the offspring were conducted in adulthood, from PND 90, in a drug-free state. LI, latent inhibition; DR, discrimination reversal; AIA, amphetamine-induced activity.
Data Analysis
Data from MRI, DR, and amphetamine-induced hyperactivity were analyzed with 2-way ANOVAs (prenatal treatment × preventive treatment) with repeated measurement factors for DR and AIA experiments. LI data were analyzed with a 3-way ANOVA (prenatal treatment × preventive treatment × preexposure). Times to complete licks 76–100 were logarithmically transformed to allow ANOVA. Significant interactions were followed by Fisher's least significant difference post hoc comparisons.
Results
RIS Treatment Did Not Affect Animals' Weight
Table 1 presents the weight of the animals on PND 34 (first day of injection), PND 40, and PND 47 (last day of injection). It can be seen that weight increased with age, but there was no difference between the 6 groups of offspring at any of the 3 PNDs. ANOVA yielded only main effect of age (F1,174 = 2590.5, P < .0001).
Table 1.
Body Weight of the Offspring of Poly I:C– or Saline-Injected Dams Treated With RIS or Vehicle on PND 34, 40, and 47
| Prenatal Treatment | Preventive Treatment | PND 34 (Before First Injection) | PND 40 (During Injection) | PND 47 (Before Last Injection) |
| Mean ± SEM Body Weight (g) | ||||
| Saline | Vehicle | 131.5 ± 2.9 | 181.4 ± 4.7 | 234.5 ± 4.9 |
| RIS-low | 135.6 ± 3.0 | 182.3 ± 3.4 | 235.6 ± 3.0 | |
| RIS-high | 133.9 ± 4.8 | 179.5 ± 4.7 | 229.9 ± 5.5 | |
| Poly I:C | Vehicle | 145.9 ± 5.0 | 197.4 ± 5.8 | 252.7 ± 6.4 |
| RIS-low | 127.4 ± 2.3 | 175.2 ± 3.4 | 212.6 ± 7.0 | |
| RIS-high | 137.2 ± 5.0 | 185.1 ± 5.9 | 234.5 ± 6.9 |
Note: Body weight (g) of the offspring of saline (CON)- or poly I:C–injected dams treated with vehicle, 0.045 mg/kg RIS (RIS-low), or 1.2 mg/kg RIS (RIS-high) on PND 34 (before commencement of preventive treatment), PND 40, and PND 47 (termination of treatment). All values are means ± standard error of the mean. ANOVA yielded no significant effects for prenatal or preventive treatment.
Prevention of Brain Structural Abnormalities Produced by Prenatal Poly I:C Exposure in the Adult Offspring by RIS Treatment in Peri-adolescence
The imaging experiment included 6 experimental groups (n/group = 7) with main factors of prenatal treatment (saline and poly I:C) and preventive treatment (vehicle, low RIS, and high RIS).
RIS Prevents Enlargement of Ventricular Volume in Poly I:C Offspring.
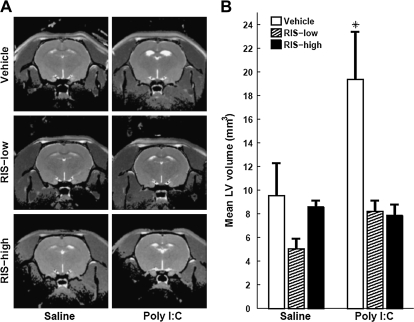
In the 2 groups of adult offspring that received vehicle on PNDs 34–47, the offspring of poly I:C–treated dams had larger LV volume than offspring of saline-treated dams. In stark contrast, no differences in LV volumes were seen between the 4 groups of adult offspring that received either low RIS or high RIS on PNDs 34–47 (figures 2A and 2B). ANOVA yielded main effects of prenatal treatment (F1,36=5.79, P < .02) and preventive treatment (F2,36 = 8.00, P < .001) and prenatal treatment × preventive treatment interaction (F2,36 = 3.3, P < .05). Post hoc comparisons yielded significant differences between the poly I:C-vehicle condition and the other 5 conditions (all P values < .002), which did not differ among themselves. Thus, RIS administration at both doses prevented the development of enlarged ventricles in the offspring of poly I:C–injected dams while having no effect in the offspring of control dams.
Fig. 2.
RIS Treatment in Peri-adolescence Prevents the Enlargement of Lateral Ventricular Volume in Adult Offspring of Poly I:C–Treated Dams. (A) Representative T2-weighted images at the level of the lateral ventricles (LV) of an adult (4 months) offspring of saline- or poly I:C–injected dams treated with vehicle, 0.045 mg/kg RIS (RIS-low), or 1.2 mg/kg rispridone (RIS-high) in peri-adolescence. (B) LV volume of adult offspring of saline- or poly I:C–injected dams treated with vehicle, RIS-low, or RIS-high. All values are means ± standard error of the mean. *, Significant difference between poly I:C-vehicle and the other 5 conditions (all P values < .002).
RIS Prevents Reduction of Hippocampal Volume in Poly I:C Offspring.
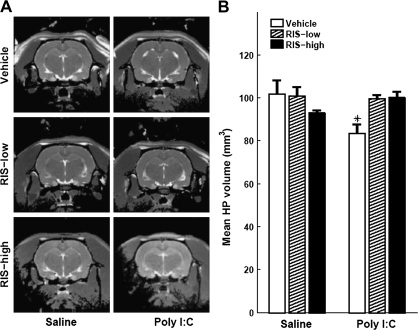
The same pattern of results was obtained with hippocampal volume. In the offspring that received vehicle in peri-adolescence, there were smaller hippocampi in the offspring of poly I:C dams compared with offspring of control dams. In contrast, no differences in hippocampal volume were seen between the offspring of poly I:C dams injected with low RIS or high RIS and the offspring of control dams injected with vehicle in peri-adolescence. Low RIS had no observable effects in control offspring injected with saline, but there was a trend toward volume reduction with high RIS (figures 3A and 3B). ANOVA yielded a significant prenatal treatment × preventive treatment interaction (F2,36 = 5.94, P < .006). Post hoc comparisons yielded significant differences between the poly I:C-vehicle condition and saline-vehicle, and poly I:C-low RIS and poly I:C-high RIS conditions (all P values < .005). In addition, there was a trend toward significant difference between saline-vehicle and saline-high RIS conditions (P = 0.095). No significant differences were found between the other conditions.
Fig. 3.
RIS Treatment in Peri-adolescence Prevents the Reduction of Hippocampal Volume in Adult Offspring of Poly I:C–Treated Dams. (A) Representative T2-weighted images at the level of the hippocampus (HP) of an adult (4 months) offspring of saline- or poly I:C–injected dams treated with vehicle, 0.045 mg/kg RIS (RIS-low), or 1.2 mg/kg rispridone (RIS-high) in peri-adolescence. (B) HP volume of adult offspring of saline- or poly I:C–injected dams treated with vehicle, RIS-low, or RIS-high. All values are means ± standard error of the mean. *, Significant difference between poly I:C-vehicle and saline-vehicle, poly I:C-low RIS, and poly I:C-high RIS conditions (all P values < .005).
Brain Volume of the Offspring of Poly I:C– or Saline-Injected Dams Treated With RIS or Vehicle During Peri-adolescence
There were no differences in total brain volume between the 3 groups of poly I:C offspring and control offspring injected with vehicle or low RIS. However, high RIS decreased total brain volume in control offspring (table 2). Two-way ANOVA yielded significant prenatal treatment × preventive treatment interaction (F2,36 = 10.55, P < .0002). Post hoc comparisons confirmed significant difference only between offspring of saline-injected dams that received high RIS and offspring of saline-injected dams that received vehicle (P < .0001).
Table 2.
Brain Volume of the Offspring of Poly I:C– or Saline-Injected Dams Treated With RIS or Vehicle During Peri-adolescence
| Prenatal Treatment | Preventive Treatment | Brain Volume (mm3; Mean ± SEM) |
| Saline | Vehicle | 1548.1 ± 16.5 |
| RIS-low | 1511.8 ± 17.4 | |
| RIS-high | 1428.4 ± 29.3* | |
| Poly I:C | Vehicle | 1508.1 ± 22.0 |
| RIS-low | 1516.4 ± 16.1 | |
| RIS-high | 1559.3 ± 07.7 |
Note: Brain volume (mm3) of the offspring of saline (CON)- or poly I:C–injected dams treated with vehicle, 0.045 mg/kg RIS (RIS-low), or 1.2 mg/kg RIS (RIS-high) in peri-adolescence and imaged at adulthood. All values are means ± standard error of the mean (SEM).
*Significant difference between offspring of saline-injected dams treated with high RIS and offspring of saline-injected dams treated with vehicle (P < .0001).
Prevention of Behavioral Abnormalities Produced by Prenatal Poly I:C Exposure in the Adult Offspring by RIS Treatment in Peri-adolescence
RIS Prevents Loss of LI in Poly I:C Offspring.
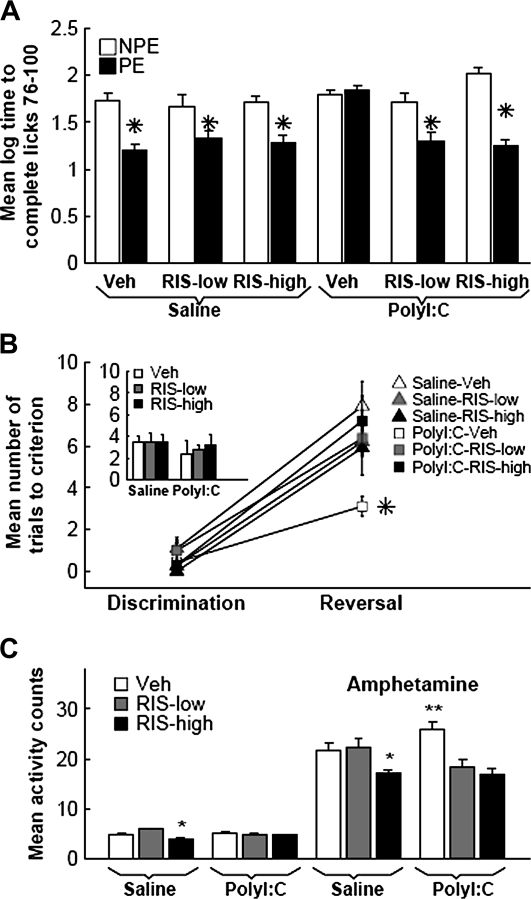
The experiment included 12 experimental groups (n = 7–9) in a 2 × 2 × 3 factorial design with main factors of preexposure (NPE and PE), prenatal treatment (saline and poly I:C), and preventive treatment (vehicle, low RIS, and high RIS). The groups did not differ in their times to complete licks 51–75 prior to tone onset (P values > .05). LI, namely, lower suppression of drinking of the PE compared with NPE rats, was present in the offspring of saline dams treated with vehicle as well as with both RIS doses. The offspring of poly I:C–injected dams injected with vehicle on PND 35–47 failed to show LI, but those injected with either low RIS or high RIS showed intact LI (figure 4A). ANOVA yielded main effects of preexposure (F1,77 = 70.5, P < .0001) and prenatal treatment (F1,77 = 10.95, P < .001), as well as preexposure × prenatal treatment × preventive treatment interaction (F2,77 = 7.77, P < .0008). Post hoc comparisons confirmed the presence of LI in poly I:C-low RIS, poly I:C-high RIS, saline-low RIS, saline-high RIS, and saline-vehicle conditions (P values < .003) but not in the poly I:C-vehicle condition.
Fig. 4.
RIS Treatment in Peri-adolescence Prevents Behavioral Abnormalities in Adult Offspring of Poly I:C–Treated Dams. (A) RIS prevents LI loss. Times (logarithmically transformed) to complete 25 licks in the presence of a tone that was previously paired with shock in 6 experimental conditions denoted according to prenatal treatment received by the pregnant dams (saline and poly I:C) and preventive treatment received by the offspring (vehicle, Veh; 0.045 mg/kg RIS [RIS-low]; and 1.2 mg/kg RIS [RIS-high]). In each condition, preexposed (PE) rats received 40 nonreinforced tone presentations prior to tone-shock conditioning, whereas non-preexposed (NPE) rats did not receive any tones. LI is manifested as shorter log times to complete 25 licks after tone onset of the PE compared with the NPE group. *, Significant difference (P values < .003) between the PE and NPE groups. (B) RIS prevents rapid reversal learning. Number of trials to criterion on day 1 discrimination (inset) and on day 2 discrimination and reversal in the 6 experimental conditions denoted according to prenatal treatment received by the pregnant dams (saline and poly I:C) and preventive treatment received by the offspring (vehicle, Veh; 0.045 mg/kg RIS [RIS-low]; and 1.2 mg/kg RIS [RIS-high). *, Significant difference between poly I:C-vehicle and the other 5 conditions (all P values < .03). (C) RIS prevents increased locomotor response to amphetamine induced by prenatal poly I:C treatment. Total activity counts in the 6 experimental conditions before (6 bars on the left) and after amphetamine injection. **, Significant difference in amphetamine-induced activity between poly I:C-vehicle and saline-vehicle, poly I:C-low RIS, and poly I:C-high RIS (all P values < .0009). *, Significant difference in spontaneous and amphetamine-induced activity between saline-vehicle and saline-high RIS, and saline-low RIS and saline-high RIS conditions (all P values < .03). All values are means ± standard error of the mean.
RIS Prevents Abnormally Rapid Reversal in Poly I:C Offspring.
The experiment included 6 experimental groups (n = 7–9) in a 2 × 3 factorial design with main factors of prenatal treatment (saline and poly I:C) and preventive treatment (vehicle, low RIS, and high RIS). The 6 groups did not differ on day 1 discrimination performance (figure 4B inset). Reversal slowed down performance in all groups, but poly I:C offspring treated with vehicle reversed more rapidly than all other groups (figure 4B). Thus, abnormally rapid reversal caused by prenatal poly I:C exposure was normalized by both RIS doses to levels seen in the control offspring. ANOVA with a repeated measurements factor of stage (discrimination and reversal) yielded main effect of stage (F1,57 = 189.7, P < .0001) and prenatal treatment × preventive treatment × stage interaction (F1,57 = 5.80, P < .005). Post hoc comparisons yielded significant differences between the poly I:C-vehicle condition and the other 5 conditions (all P values < .03), which did not differ from one another.
RIS Prevents Excessive Locomotor Response to Amphetamine in Poly I:C Offspring.
The experiment included 6 experimental groups (n = 7–8) in a 2 × 3 factorial design with main factors of prenatal treatment (saline and poly I:C) and preventive treatment (vehicle, low RIS, and high RIS). Two-way ANOVAs with repeated measurements factor of 6 blocks before amphetamine injection and 18 blocks after amphetamine injection yielded interactions of prenatal treatment × preventive treatment (F2,38 = 9.0, P < .0006 and F2,38 = 10.82, P < .0002, respectively) with no interaction between these factors and blocks. Total activity scores before and after amphetamine injection are presented in figure 4C. Post hoc comparisons for amphetamine-induced activity yielded significant differences between the poly I:C-vehicle condition and the other 5 conditions (all P values < .03), which did not differ from one another.
Discussion
As shown by us and others previously,23,24,26,29,31 adult offspring of dams exposed to poly I:C on GD 15 exhibited selective attention deficit as manifested in loss of LI and abnormally rapid reversal as well as increased sensitivity to the activating effects of amphetamine while normally acquiring fear conditioning (in the NPE condition of LI experiment) and discrimination learning. In addition, we replicated our recent neuroimaging findings23 that adult offspring of dams exposed in pregnancy to poly I:C exhibit the hallmark structural abnormalities associated with schizophrenia, namely, enlarged LV and smaller hippocampus, albeit in the absence of changes in total brain volume. Finally, replicating our previous outcomes using peri-adolescent treatment with clozapine, here all 3 behavioral abnormalities as well as brain structural abnormalities were absent in poly I:C offspring that received treatment with 0.045 or 1.2 mg/kg RIS in peri-adolescence (PND 35–47).
The fact that behavioral and brain structural abnormalities following prenatal poly I:C exposure emerge postpubertally23 and are prevented by clozapine23 and RIS (here) administered during prepuberty implies that there is a critical period between peri-adolescence and early adulthood during which aberrant neurodevelopment may lead to the emergence of psychotic-like behaviors. Given that prenatal exposure to infection is a well-documented risk factor in schizophrenia, this constellation is consistent with the notion that schizophrenia involves both early (prenatal) neurodevelopmental insult and aberrant late (particularly postpubertal) neurodevelopmental processes.2–4,51 It is of interest to note in this context that children are resistant to drugs producing psychosis in adults such as amphetamine and phencyclidine52,53, and this is paralleled by lower sensitivity to psychostimulants in peri-adolescent rats.54,55 These observations complement our findings in suggesting that brain mechanisms/systems whose dysfunction mediates the emergence of psychosis and psychotic-like behaviors in humans and animals, respectively, mature postpubertally.54 The efficacy of peri-adolescent clozapine22,23 and RIS to block the emergence of prenatal poly I:C–induced abnormalities is likely to reflect these drugs' capacity to arrest the development of neuropathological processes prior to this critical postpubertal stage.
The mechanisms underlying the efficacy of RIS in preventing the development of structural brain abnormalities in poly I:C offspring remain to be investigated. As detailed earlier, at the 0.045 mg/kg dose, RIS has significant 5HT2A receptor antagonism and weak D2 receptor occupancy, while at the 1.2 mg/kg dose, 5HT2A occupancy is high while binding is also increased at receptor populations with lower binding affinity, including DA receptors D2, D3, and D4, and serotonin 5HT2C and 5HT1A receptors.38,39,56 The fact that low RIS dose was as effective as the high dose suggests that 5HT2A antagonism is a critical player in the preventive effects we observed here.
The serotonergic system plays a central role in brain development. The activity of 5HT2 receptors is increased at critical stages of brain development, and they are approximately 10-fold higher in the developing brain compared with mature adult brain.57–61 Pre- and postnatal environmental stressors known as epidemiological risk factors for schizophrenia later in life, including viral infections, can alter brain serotonin levels and the number and function of 5HT2 receptors,57–61 and RIS administration has been shown to change the number of cortical 5HT2A and other 5HT receptors of juvenile animals.62 In fact, the serotonergic system in developing animals is more sensitive than in adults to the long-term effects of RIS.62 Given the recent reports that prenatal poly I:C alters brain serotonin levels,63–65 it is possible that an aberrant serotonin-dependent developmental process contributes to the effects observed following this insult and is targeted by RIS.
The preventive action of RIS could in part stem from its neuroprotective effects because there is increasing in vitro and in vivo evidence that atypical APDs exert such effects, including protection against glutamate excitotoxicity; oxidative stress and apoptosis; and promotion of neurogenesis, connectivity, and neuronal survival.51,66–71 Indeed, it has been suggested that developmental dysfunction of the hippocampus in schizophrenia may be associated with reduced neurogenesis in the DG where normal levels could be reestablished by neuroleptic treatment.71 Prenatal poly I:C exposure suppresses hippocampal neurogenesis and delays myelination and axonal development in the adolescent brain,72,73 and RIS could protect against such processes.70 These actions of RIS could be also related to its 5HT2A antagonism because serotonin influences neurogenesis, apoptotic mechanisms, dendritic refinement, cell migration, and synaptic plasticity, particularly in the hippocampus.58,60 Interestingly, more severe forms of hippocampal neuropathology, including pyramidal cell loss, are seen in adult compared with adolescent brains of poly I:C offspring.26,36 RIS could prevent the development of hippocampal pathology by targeting earlier cellular disease processes.
RIS administration on PNDs 22–42 also alters DA receptors in medial prefrontal cortex, hippocampus, and the nucleus accumbens of juvenile animals.74 Given that prenatal poly I:C exposure leads to numerous perturbations of the DA systems,26,30,64,75,76 RIS is likely to act also via this system. However, the efficacy of low RIS dose suggests that if D2 antagonism does play a role in prevention, weak D2 antagonism suffices.
It should be emphasized that RIS treatment at both low and high doses selectively affected behavioral abnormalities produced by poly I:C, namely, disrupted LI, rapid reversal and excessive amphetamine-induced hyperactivity, without affecting the behaviors that were not affected by prenatal poly I:C, namely, fear conditioning and discrimination learning. This selectivity of RIS protection indicates that the drug specifically targeted the neuropathological mechanisms set in motion by prenatal poly I:C insult without concomitantly interfering with normal brain maturation. The latter possibility was supported by the results obtained in the offspring of saline-injected dams treated with the low RIS dose. This dose had no effect on any of the behaviors assessed compared with vehicle-treated counterparts and no effects on brain morphology. The lack of any apparent long-term detrimental effects of low RIS dose in offspring born to control mothers is in line with the data with RIS of Richtand et al.,19 as well as with our23 findings and of Meyer et al.22 in rats and mice, respectively, that clozapine treatment during adolescence/early adulthood was devoid of any negative effects in control offspring. While such paucity of long-term effects of adolescent APD treatment may seem puzzling, it is well documented that developing and adult brains differ dramatically in their physiology and neurochemistry as well as their response to physiological and pharmacological challenges.52,54,55,77,78 Indeed, RIS effects on the developing brain differ from those produced by identical administration regimes in adult brains62,74,79.
The high RIS dose did have deleterious effects in controls, including a trend toward reduced hippocampal volume and decreased total brain volume, as well as reduced spontaneous and amphetamine-induced activity. There is some evidence in patients that APDs may change brain morphology, with increased and decreased volumes reported,80,81 but in these studies, it is not possible to determine if the changes in brain volume reflected the underlying disease process and/or the effects of antipsychotic medications. To the best of our knowledge to date, only one study, using macaque monkeys, showed that chronic exposure to haloperidol or olanzapine was associated with smaller brain volume82.
Attenuated responsiveness to acute administration of amphetamine can be interpreted as reflecting reduced mesolimbic DA function in high RIS rats. Interestingly, such reduced DA function is characteristic of peri-adolescent rats,37,55,83,84 suggesting that high RIS may interfere with the normal maturation of the mesolimbic DA system. Given the intimate links between the temporolimbic and the DA systems,47 it can be further speculated that subcortical DA hypofunction in high RIS control rats is consequent to the hippocampal damage produced by this dose and subsequent disrupted hippocampal input to the DA system.
It should be pointed out that while reduced DA function by high RIS is deleterious in normal offspring, this same capacity should be beneficial in poly I:C–exposed offspring because prenatal poly I:C leads to the development of overactive DA system26,75,76,85. Indeed, strong DA antagonism does not reduce the effectiveness of prevention in poly I:C offspring because here high RIS was fully effective, and Meyer et al.22 reported that the selective D2 blocker haloperidol was effective in preventing LI loss and amphetamine-induced hyperactivity. However, potent D2 antagonism did have deleterious effects in the control offspring here as well as in the study of Meyer et al.22 where haloperidol led to abnormally increased amphetamine-induced activity and impaired prepulse inhibition. Conversely, potent 5HT2A antagonism coupled with weak D2 antagonism apparently underlies the benign actions of low RIS as well as clozapine, which is also characterized by such a profile.40 These data emphasize the need for screening diverse compounds to identify the most effective candidates for primary prevention. However, our data join those of Richtand et al.19,86 to support the use of low-dose RIS in psychosis prevention. Indeed, although dose translation from rats to humans is fraught with problems, using the body surface area normalization method,87,88 the 0.045 mg/kg dose used here translates to human equivalent dose of about 0.5 mg for a 60-kg person, which is lower than average doses used in children and adolescents.8,13,35,89,90 Furthermore, our data suggest that highly selective 5HT2A receptor antagonists may be promising drug development targets for psychosis prevention, in line with the recently rekindled interest in the involvement of the 5HT2A receptor in psychosis vulnerability91.
If active brain changes are occurring as the illness of schizophrenia is emerging and these changes can be prevented, ameliorated, or delayed by early intervention, this would revolutionize the treatment of schizophrenia.3,67 Based on this rationale, there has been a continuous growth in programs evaluating preventive treatments for individuals at high risk of developing psychosis.7,8,10,92–94 While some of the results have been encouraging, human research in this field faces complicated methodological, diagnostic, and practical challenges, limiting conclusions. Given this background, it will clearly take some time before prevention of structural brain pathology is attempted in humans. Valid animal models are indispensible for evaluating the feasibility of prevention and can be effectively utilized at the “proof of concept” level.
In vivo rodent imaging and in particular volumetric changes provide a robust endophenotype in animal models that permits direct comparison with human illness manifestations.95–98 The fact that volumetric changes resulting from in utero insult respond to pharmacotherapy opens up a new venue for assessing the efficacy of prevention in neurodevelopmental animal models. Specifically, brain structural changes may represent a robust and easily identifiable target for developing and screening preventive treatments. Effective drug development requires technologies that allow rapid translation from the preclinical to the clinical stage; in vivo volumetric changes may provide just such a technology. Moreover, because it allows the repeated assessment of the same brain over time as well as correlating ongoing brain changes with behavioral changes within the same subject, in vivo imaging is the only available method to monitor disease “progression” and, consequently, to monitor the efficacy of therapeutic interventions in “preventing disease progression.”
Clearly, in vivo imaging must be supplemented with the assessment of underlying cellular and molecular changes for comprehensive phenotyping. However, results of imaging can direct this search to the critical developmental windows and brain regions and even cellular processes. Caution is also dictated by the fact that volumetric changes, and in particular hippocampal volume changes, are not specific to schizophrenia because they are also seen in other brain diseases such as dementia and depression. However, their developmental trajectory, namely, postpubertal emergence, may be uniquely relevant to schizophrenia.23,32,99 We are now in the process of identifying the longitudinal course of the LV and hippocampal volumetric changes resulting from prenatal poly I:C exposure that may reveal additional, possibly later, time windows for effective prophylaxis.
In summary, taken together with our previous results with clozapine,23 our results (1) support the concept that prenatal insult leads to progressive brain changes involving abnormal postpubertal brain processes that lead to the emergence of symptoms and that can be prevented. (2) Define an easily identifiable neural target for developing and screening preventive treatments in a well-validated animal model that offers rapid translation from the preclinical to the clinical stage. (3) Suggest that highly selective 5HT2A receptor antagonists may be promising drug development targets for psychosis prevention. Given that the prenatal poly I:C model is based on a well-known risk factor that produces long-term schizophrenia-like neuropathological and behavioral abnormalities, thus presumably mimicking both the etiology and the long-term neurodevelopmental processes of schizophrenia, these data may have important implications for the clinic.
Funding
United States-Israel Binational Science Foundation (BSF 2007188).
Acknowledgments
The authors would like to thank Janssen, Belgium, for their generous gift of risperidone. The MRI scanner used in this study was purchased with a grant from the Israel Science Foundation and operated under the Raymond and Beverly Sackler Center for Biophysics, Tel-Aviv University, and The Alfredo Federico Strauss Center for Computational Neuro-Imaging, Tel-Aviv University.
Financial disclosure: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–321. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 5.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Waddington JL. Neuroimaging and other neurobiological indices in schizophrenia: relationship to measurement of functional outcome. Br J Psychiatry. 2007;50(suppl):s52–s57. doi: 10.1192/bjp.191.50.s52. [DOI] [PubMed] [Google Scholar]
- 7.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 8.McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 9.McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. doi: 10.1002/j.2051-5545.2008.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhrmann S, Schultze-Lutter F, Maier W, Klosterkotter J. Pharmacological intervention in the initial prodromal phase of psychosis. Eur Psychiatry. 2005;20:1–6. doi: 10.1016/j.eurpsy.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee C, McGlashan TH, Woods SW. Prevention of schizophrenia: can it be achieved? CNS Drugs. 2005;19:193–206. doi: 10.2165/00023210-200519030-00002. [DOI] [PubMed] [Google Scholar]
- 12.Salokangas RK, McGlashan TH. Early detection and intervention of psychosis. A review. Nord J Psychiatry. 2008;62:92–105. doi: 10.1080/08039480801984008. [DOI] [PubMed] [Google Scholar]
- 13.Phillips LJ, Nelson B, Yuen HP, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: study design and baseline characteristics. Aust N Z J Psychiatry. 2009;43:818–829. doi: 10.1080/00048670903107625. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman JA, Fenton WS. Delayed detection of psychosis: causes, consequences, and effect on public health. Am J Psychiatry. 2000;157:1727–1730. doi: 10.1176/appi.ajp.157.11.1727. [DOI] [PubMed] [Google Scholar]
- 15.Rosen A. Ethics of early prevention in schizophrenia. Aust N Z J Psychiatry. 2000;34(suppl):S208–S212. doi: 10.1080/000486700247. [DOI] [PubMed] [Google Scholar]
- 16.Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2006;18(4):CD004718. doi: 10.1002/14651858.CD004718.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran C, Malaspina D, Hercher L. Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk”. Schizophr Res. 2005;73:173–184. doi: 10.1016/j.schres.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlashan TH, Addington J, Cannon T, et al. Recruitment and treatment practices for help-seeking “prodromal” patients. Schizophr Bull. 2007;33:715–726. doi: 10.1093/schbul/sbm025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richtand NM, Taylor B, Welge JA, et al. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 21.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 22.Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 27.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 29.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”? Schizophr Bull. 2008;34:774–790. doi: 10.1093/schbul/sbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Psychiatry. DSM-V at a glance. Science. doi: 10.1126/science.327.5967.770-b. 2010;327:770–771. [DOI] [PubMed] [Google Scholar]
- 34.Masi G, Cosenza A, Mucci M, Brovedani P. A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J Clin Psychiatry. 2003;64:1039–1047. doi: 10.4088/jcp.v64n0909. [DOI] [PubMed] [Google Scholar]
- 35.Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–e641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 37.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 38.Leysen JE, Janssen PM, Schotte A, Luyten WH, Megens AA. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl) 1993;112(suppl):S40–S54. doi: 10.1007/BF02245006. [DOI] [PubMed] [Google Scholar]
- 39.Schotte A, Janssen PF, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 40.Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 41.Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J Pharmacol Exp Ther. 1988;244:685–693. [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 43.Wolf OT, Dyakin V, Vadasz C, de Leon MJ, McEwen BS, Bulloch K. Volumetric measurement of the hippocampus, the anterior cingulate cortex, and the retrosplenial granular cortex of the rat using structural MRI. Brain Res Brain Res Protoc. 2002;10:41–46. doi: 10.1016/s1385-299x(02)00181-2. [DOI] [PubMed] [Google Scholar]
- 44.Hajos M. Targeting information-processing deficit in schizophrenia: a novel approach to psychotherapeutic drug discovery. Trends Pharmacol Sci. 2006;27:391–398. doi: 10.1016/j.tips.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 47.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 48.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayalon L, Doron R, Weiner I, Joel D. Amelioration of behavioral deficits in a rat model of Huntington's disease by an excitotoxic lesion to the globus pallidus. Exp Neurol. 2004;186:46–58. doi: 10.1016/S0014-4886(03)00312-1. [DOI] [PubMed] [Google Scholar]
- 50.Joel D, Ayalon L, Tarrasch R, Weiner I. Deficits induced by quinolinic acid lesion to the striatum in a position discrimination and reversal task are ameliorated by permanent and temporary lesion to the globus pallidus: a potential novel treatment in a rat model of Huntington's disease. Mov Disord. 2003;18:1499–1507. doi: 10.1002/mds.10622. [DOI] [PubMed] [Google Scholar]
- 51.Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Annu Rev Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- 52.Barkley RA. A review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry. 1977;18:137–165. doi: 10.1111/j.1469-7610.1977.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 53.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 54.Zuckerman L, Rimmerman N, Weiner I. Latent inhibition in 35-day-old rats is not an “adult” latent inhibition: implications for neurodevelopmental models of schizophrenia. Psychopharmacology (Berl) 2003;169:298–307. doi: 10.1007/s00213-003-1460-8. [DOI] [PubMed] [Google Scholar]
- 55.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 56.Weiner I, Schiller D, Gaisler-Salomon I. Disruption and potentiation of latent inhibition by risperidone: the latent inhibition model of atypical antipsychotic action. Neuropsychopharmacology. 2003;28:499–509. doi: 10.1038/sj.npp.1300069. [DOI] [PubMed] [Google Scholar]
- 57.Pletnikov MV, Rubin SA, Schwartz GJ, Carbone KM, Moran TH. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 58.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 59.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 60.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 61.Whitaker-Azmitia P, Zhou F, Hobin J, Borella A. Isolation-rearing of rats produces deficits as adults in the serotonergic innervation of hippocampus. Peptides. 2000;21:1755–1759. doi: 10.1016/s0196-9781(00)00327-2. [DOI] [PubMed] [Google Scholar]
- 62.Choi YK, Moran-Gates T, Gardner MP, Tarazi FI. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol. 2010;20:187–194. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fatemi SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winter C, Djodari-Irani A, Sohr R, et al. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 65.Winter C, Reutiman TJ, Folsom TD, et al. Dopamine and serotonin levels following prenatal viral infection in mouse—implications for psychiatric disorders such as schizophrenia and autism. Eur Neuropsychopharmacol. 2008;18:712–716. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A. Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci. 2010;260:151–162. doi: 10.1007/s00406-009-0033-1. [DOI] [PubMed] [Google Scholar]
- 67.Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 68.Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 69.Wakade CG, Mahadik SP, Waller JL, Chiu FC. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res. 2002;69:72–79. doi: 10.1002/jnr.10281. [DOI] [PubMed] [Google Scholar]
- 70.Newton SS, Duman RS. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21:715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]
- 71.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 72.Makinodan M, Tatsumi K, Manabe T, et al. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. J Neurosci Res. 2008;86:2190–2200. doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- 73.Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moran-Gates T, Grady C, Shik Park Y, Baldessarini RJ, Tarazi FI. Effects of risperidone on dopamine receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2007;17:448–455. doi: 10.1016/j.euroneuro.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- 76.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Ben-Ari Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008;31:626–636. doi: 10.1016/j.tins.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Wood GK, Lipska BK, Weinberger DR. Behavioral changes in rats with early ventral hippocampal damage vary with age at damage. Brain Res Dev Brain Res. 1997;101:17–25. doi: 10.1016/s0165-3806(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 79.Moran-Gates T, Gan L, Park YS, Zhang K, Baldessarini RJ, Tarazi FI. Repeated antipsychotic drug exposure in developing rats: dopamine receptor effects. Synapse. 2006;59:92–100. doi: 10.1002/syn.20220. [DOI] [PubMed] [Google Scholar]
- 80.Vita A, De Peri L. The effects of antipsychotic treatment on cerebral structure and function in schizophrenia. Int Rev Psychiatry. 2007;19:429–436. doi: 10.1080/09540260701486332. [DOI] [PubMed] [Google Scholar]
- 81.Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 82.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 83.Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 84.Cirulli F, Laviola G. Paradoxical effects of D-amphetamine in infant and adolescent mice: role of gender and environmental risk factors. Neurosci Biobehav Rev. 2000;24:73–84. doi: 10.1016/s0149-7634(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 85.Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 86.Richtand NM, McNamara RK. Serotonin and dopamine interactions in psychosis prevention. Prog Brain Res. 2008;172:141–153. doi: 10.1016/S0079-6123(08)00907-2. [DOI] [PubMed] [Google Scholar]
- 87.Center for Drug Evaluation and Research CfBEaR. Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD: U.S. Food and Drug Administration; 2002. [Google Scholar]
- 88.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 89.Posey DJ, Stigler KA, Erickson CA, McDougle CJ. Antipsychotics in the treatment of autism. J Clin Invest. 2008;118:6–14. doi: 10.1172/JCI32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McConville BJ, Sorter MT. Treatment challenges and safety considerations for antipsychotic use in children and adolescents with psychoses. J Clin Psychiatry. 2004;65(suppl 6):20–29. [PubMed] [Google Scholar]
- 91.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Cornblatt BA. The New York high risk project to the Hillside recognition and prevention (RAP) program. Am J Med Genet. 2002;114:956–966. doi: 10.1002/ajmg.b.10520. [DOI] [PubMed] [Google Scholar]
- 93.Woods SW, Breier A, Zipursky RB, et al. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol Psychiatry. 2003;54:453–464. doi: 10.1016/s0006-3223(03)00321-4. [DOI] [PubMed] [Google Scholar]
- 94.Addington J, Cadenhead KS, Cannon TD, et al. North American prodrome longitudinal study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Natt O, Watanabe T, Boretius S, Radulovic J, Frahm J, Michaelis T. High-resolution 3D MRI of mouse brain reveals small cerebral structures in vivo. J Neurosci Methods. 2002;120:203–209. doi: 10.1016/s0165-0270(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 96.Nieman BJ, Bishop J, Dazai J, et al. MR technology for biological studies in mice. NMR Biomed. 2007;20:291–303. doi: 10.1002/nbm.1142. [DOI] [PubMed] [Google Scholar]
- 97.Nieman BJ, Bock NA, Bishop J, Sled JG, Josette Chen X, Mark Henkelman R. Fast spin-echo for multiple mouse magnetic resonance phenotyping. Magn Reson Med. 2005;54:532–537. doi: 10.1002/mrm.20590. [DOI] [PubMed] [Google Scholar]
- 98.Torres G, Meeder BA, Hallas BH, et al. Ventricular size mapping in a transgenic model of schizophrenia. Brain Res Dev Brain Res. 2005;154:35–44. doi: 10.1016/j.devbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Pantelis C, Yucel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: the search for a moving target. Neuropsychol Rev. 2009;19:385–398. doi: 10.1007/s11065-009-9114-1. [DOI] [PubMed] [Google Scholar]