Abstract
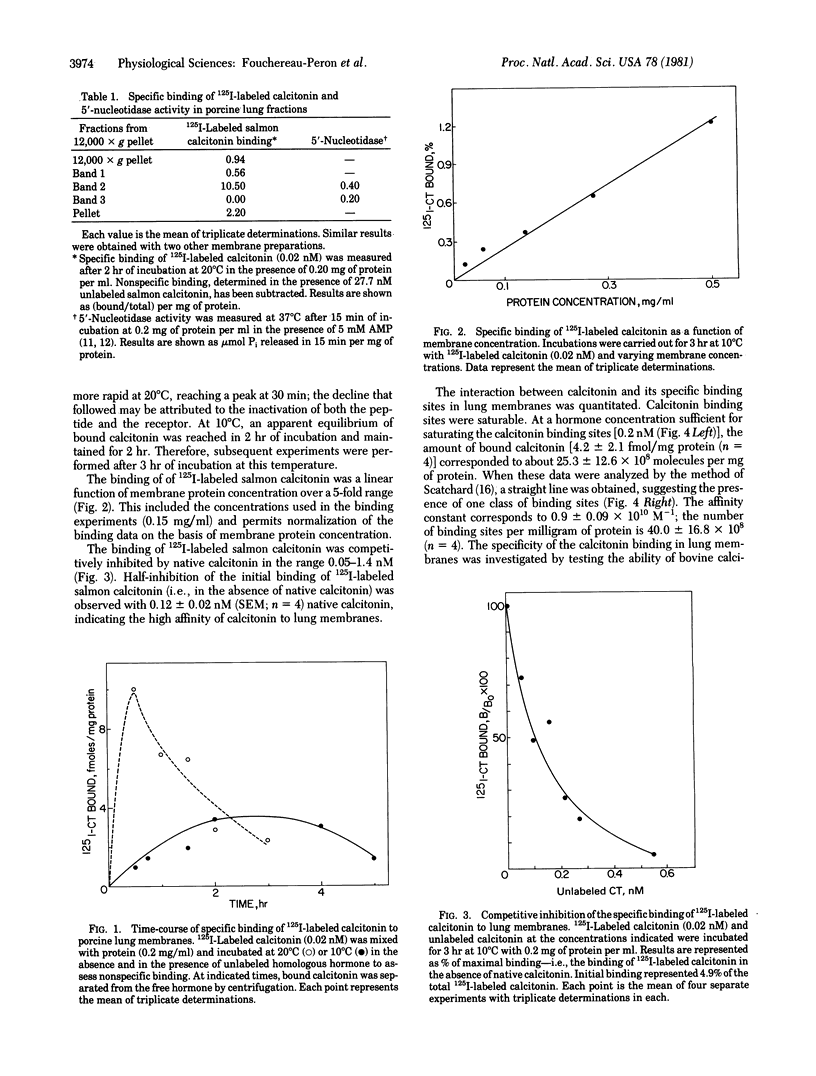
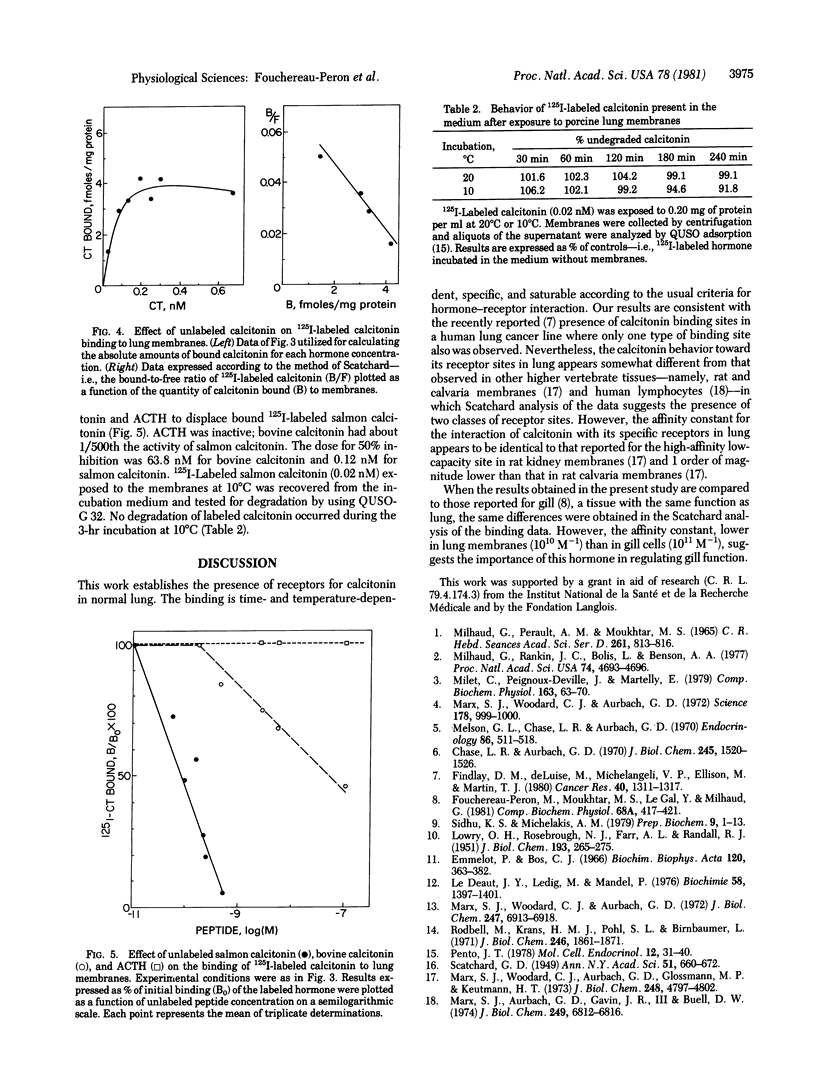
The binding of salmon calcitonin was investigated in subcellular fractions obtained from normal porcine lung. Only the membrane fraction (density, 1.14 g/cm3) showed specific binding for calcitonin. Specific binding of 125I-labeled salmon calcitonin was competitively inhibited by concentrations of unlabeled homologous hormone in the range 0.01-1 nM. Half-maximal inhibition of binding was observed with 0.12 nM salmon calcitonin. Scatchard analysis of the data suggested the presence of one class of binding sites with a mean affinity constant of 0.9 X 10(10) M-1 and a mean receptor number of 40 X 10(8)/mg of protein. The binding of salmon calcitonin was highly specific; half-maximal inhibition of binding was observed with 63.8 nM bovine calcitonin, the hormone corticotropin having no effect in this system.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase L. R., Aurbach G. D. The effect of parathyroid hormone on the concentration of adenosine 3',5'-monophosphate in skeletal tissue in vitro. J Biol Chem. 1970 Apr 10;245(7):1520–1526. [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Deaut J. Y., Ledig M., Mandel P. Etude comparative du dosage du phosphate inorganique libéré après action d'une ATPase. Biochimie. 1976;58(11-12):1397–1399. [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Marx S. J., Fedak S. A., Aurbach G. D. Preparation and characterization of a hormone-responsive renal plasma membrane fraction. J Biol Chem. 1972 Nov 10;247(21):6913–6918. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Woodward C., Aurbach G. D., Glossmann H., Keutmann H. T. Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem. 1973 Jul 10;248(13):4797–4802. [PubMed] [Google Scholar]
- Melson G. L., Chase L. R., Aurbach G. D. Parathyroid hormone-sensitive adenyl cyclase in isolated renal tubules. Endocrinology. 1970 Mar;86(3):511–518. doi: 10.1210/endo-86-3-511. [DOI] [PubMed] [Google Scholar]
- Milhaud G., Perault A. M., Moukhtar M. S. Etude du mécanisme de l'action hypocalcémiante de la thyrocalcitonine. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jul 19;261(3):813–816. [PubMed] [Google Scholar]
- Milhaud G., Rankin J. C., Bolis L., Benson A. A. Calcitonin: Its hormonal action on the gill. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4693–4696. doi: 10.1073/pnas.74.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pento J. T. Characteristics of calcitonin degradation in vitro produced by incubation with procine thyroid tissue. Mol Cell Endocrinol. 1978 Oct;12(1):31–40. doi: 10.1016/0303-7207(78)90099-0. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Sidhu K. S., Michelakis A. M. Isolation and partial characterization of plasma membranes from rat lung alveoli. Prep Biochem. 1979;9(1):1–13. doi: 10.1080/00327487908061668. [DOI] [PubMed] [Google Scholar]


