Abstract
Poly(ethylene glycol) (PEG) and poly(2-methyl-2-oxazoline) (PMOx) are water-soluble, biocompatible polymers with stealth hemolytic activities. Poly(amino acid) (PAA) end-capped PEG and PMOx were prepared using amino-terminated derivatives of PEG and PMOx as macroinitiators for the ring-opening polymerization of γ-benzyl protected L-glutamate N-carboxyanhydride and S-benzyloxycarbonyl protected L-cysteine N-carboxyanhydride, respectively, in the presence of urea, at room temperature. The molecular weight of the PAA moiety was kept between Mn = 2,200 and 3,000 g mol−1, PMOx was polymerized by cationic ring-opening polymerization resulting in molecular weights of Mn = 5,000 and 10,000 g mol−1, PEG was a commercial product with Mn = 5,000 g mol−1. Here, we investigate the self-assembly of the resulting amphiphilic block copolymers in water and the effect of the chemical structure of the block copolymers on the solution properties of self-assembled nanostructures. The PEG-block-poly(amino acid), PEG-b-PAA, and PMOx-block-poly(amino acid), PMOx-b-PAA, block copolymers have a narrow and monomodal molecular weight distribution (PDI < 1.3). Their self-assembly in water was studied by dynamic light scattering and fluorescence spectroscopy. In aqueous solution, the block copolymers associate into particles with hydrodynamic radii (RH) ranging in size from RH 70 to 130 nm, depending on the block copolymer architecture and the polymer molecular weight. Larger RH and critical association concentration values were obtained for copolymers containing poly(S-benzyloxycarbonyl-L-cysteine) compared to their poly(γ-benzyl-L-glutamate) analog. FTIR investigations revealed that the poly(γ-benzyl-L-glutamate) block adopts a helical conformation, while the poly(S-benzyloxycarbonyl-L-cysteine) block exists as β–sheet.
Introduction
The elaboration of biocompatible materials will continue to be of great interest, due to their role as biomimetic analogues and their potential for commercial applications. Poly(ethylene glycol) (PEG) is a well-known biomedical polymer and holds a superior position when protein and cell repulsion are of primary importance.1,2 However, chronic intravenous administration of PEGylated proteins has shown some unintended consequences such as vacuolation of the renal cortical tubular epithelium in laboratory animals.3 Current research focuses on a new class of water-soluble polymers, poly(2-oxazolines), in which the main chain is a pseudopeptide chain. The biocompatibility and interfacial characteristics of poly(2-oxazolines) have been assessed in various biotechnological applications,4 and the polymer has been reported as an alternative to PEG-conjugates for polymeric drug conjugation and delivery.5
The combination of synthetic biocompatible polymers such as PEG or poly(2-methyl-2-oxazoline) (PMOx) with poly(amino acid)s (PAA) generates considerable attention for use in medicine and biotechnology due to their low toxicity and high hydrophilicity6,7 that make them available to applications such as drug-delivery systems,8 tissue engineering scaffolds9 and surface coatings for biomedical devices.10 Hence, new macromolecular designs and controlled syntheses of amino acid-containing polymers are of great interest. During the past decades, many studies have been devoted toward the development of drug carriers based on polymeric micelles. In most cases, these delivery systems consist of a water-soluble poly(ethylene glycol)-based block linked to a hydrophobic moiety. There have been only a few reports so far on the preparation of hydrophobically end-modified poly(oxazolines).11,12
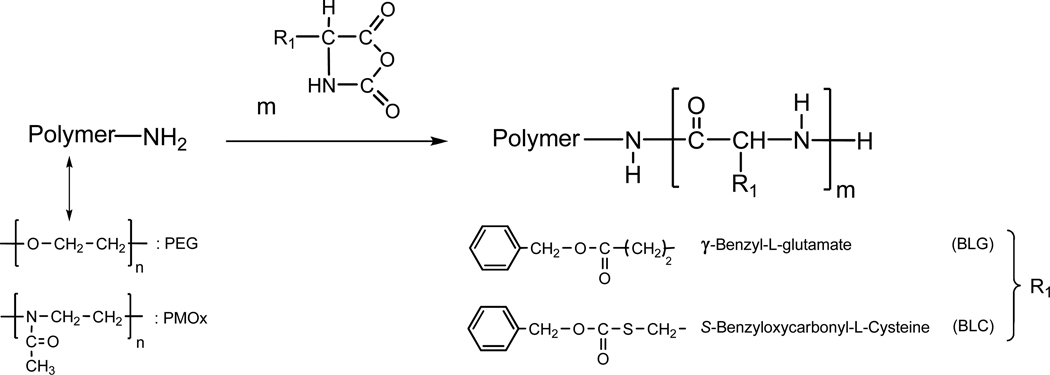
The goal of this work is to present a new strategy for the synthesis of self-assembling polymeric systems. We established a novel synthetic route for amphiphilic block copolymers composed of protected poly(amino acids), i.e, γ-benzyl protected poly(L-glutamate) (PBLG) and S-benzyloxycarbonyl protected poly(L-cysteine) (PBLC), as hydrophobic moieties (Mn = 2,200 to 3,000 g mol−1, with 10 to 13 repeating units) with either PEG or PMOx as water soluble moieties (Mn = 5,000 and 10,000 g mol−1, with 60 to 120 repeating units, depending on the polymer). Polymers bearing poly(amino acid) blocks of narrow polydispersity were synthesized by ring-opening polymerization (ROP) of N-carboxyanhydrides of protected α-amino acid NCA’s using amino-terminated PEG or PMOx, as macroinitiators (Scheme 1), and without using an organo-metal catalyst. Dynamic light scattering (DLS) and static fluorescence measurements were employed on PMOx–block-PAA and PEG–block-PAA block copolymers to determine the onset of aggregation of the polymeric assemblies and the size of the aggregates that formed in aqueous media.
Scheme 1.
Reaction scheme for the ring opening polymerization (ROP) of α-amino acids N-carboxyanhydride (NCAs) initiated with amino-terminated PEG or PMOx
Experimental Section
Materials
All chemicals were purchased from Sigma-Aldrich Chemicals Co. and used as received, unless otherwise stated. 2-Methyl-2-oxazoline was purified by vacuum distillation over calcium hydride (CaH2) and α-hydroxy-ω-amino poly(2-methyl-2-oxazolines) (NH2-PMOxn-OH) was obtained by cationic ring-opening polymerization initiated with N-[2-(p-toluenesulfonyloxy)-ethyl]-phthalimide, following a known procedure.13 Alpha-amino-ω-carboxymethyl poly(ethylene glycol), COOH-PEG-NH2 (Mw = 5, 000 g mol−1) was a generous gift from LaysanBio, Huntsville, AL. Gamma-benzyl-L-glutamate and S-benzyloxycarbonyl-L-cysteine were purchased from Bachem and N-carboxyanhydrides were synthesized according to a new procedure (see below). Acetonitrile was dried by reflux over CaH2 under a dry nitrogen atmosphere and subsequently distilled prior to use. Dimethylformamide (DMF) was distilled under vacuum and over CaH2. Methanolic potassium hydroxide was prepared by the addition of potassium hydroxide (KOH) pellets into methanol. Water was deionized with a Millipore Milli-Q system.
Synthesis of protected N-carboxyanhydrides, BAA-NCAs
N-carboxyanhydride of protected amino acids, were synthesized in dry ethyl acetate (EtOAc) in the presence of triphosgene. 13 mmol of γ-benzyl-L-glutamic acid (BLG) or S-benzyloxycarbonyl-L-cysteine (BLC), 1.95 g (6.6 mmol) of triphosgene and 3.7 g (27 mmol) of α-pinene were dissolved in 60 mL of EtOAc. The reaction mixture was heated at reflux (105°C) under stirring until the solution became transparent. The mixture was cooled to room temperature, filtered and the filtrate was concentrated under reduced pressure, then crystallized in EtOAc/Hexane. The obtained NCAs were recrystallized from a mixture of EtOAc/Hexane cooled to 0°C and then vacuum-dried. Yield : 10 mmol (80%). 1H NMR (DMSO-d6, 500 MHz): δ/ppm = 1.90–2.05 (m, 2H, -O-CO-CH2-CH2-), 2.55 (t, 2H, -O-CO-CH2-CH2-), 3.35 (m, 2H, -O-CO-S-CH2-), 4.45–4.75 (t, 1H, -CH-), 5.10–5.28 (s, 2H, -OCH2-), 7.36 (m, 5H, aromatic H), 9.1–9.24 (s, 1H, -NH-).
Preparation of α-hydroxy-ω-amino poly(2-methyl-2-oxazolines) (NH2-PMOxn-OH) macrointiators
The procedure, exemplified here in the case of PMOx 10,000 g mol−1, was followed to prepare all polymers using the monomer/initiator ratios and reaction times listed in Table 1. A solution of 2-methyl-2-oxazoline (3.0 mL, 36 mmol) and N-[2-(p-toluenesulfonyloxy)-ethyl]-phthalimide (105 mg, 0.3 mmol) in acetonitrile (10 mL) was stirred at reflux for 48 h under nitrogen. After cooling to room temperature, 1N methanolic KOH was added to introduce hydroxyl groups at the end of the PMOx chain. The raw product was isolated by precipitation into anhydrous diethyl ether and then vacuum-dried. Water was added to dissolve the raw product, followed by dialysis against distilled water for 2 days using a Spectra/Por CE membrane (molecular weight cutoff of 3,500 g mol−1), the resulting polymer was isolated by freeze-drying. Yield: 2.12 g (71%). 1H NMR (D2O, 500 MHz): δ/ppm = 2.06 (m, 3H, -CO-CH3), 3.4–3.6 (br, 4H, -N-CH2CH2-), 7.50 (d, 2H, aromatic H), 7.26 (d, 2H, aromatic H). The phthalimide-terminated poly(2-methyl-2-oxazoline) (PhtN-PMOxn-OH) (1 g) was dissolved in ethanol (12 mL) and treated with hydrazine monohydrate (6.0 mL). The mixture was refluxed for 6 h and left at room temperature over night. After filtration, the polymer was purified by dialysis against distilled water for 2 days using a Spectra/Por membrane (MWCO of 3,500 g mol−1), the resulting polymer was isolated by freeze-drying. Yield: 0.78 g (78%). 1H NMR (D2O, 500 MHz): δ/ppm = 2.06 (br, 3H, -CO-CH3), 3.4–3.6 (br, 4H, -N-CH2CH2-).
Table 1.
Polymerization Conditions and Properties of the Polymers Investigated
| Polymer | Reaction time (h) |
[MOx]/[I]a | [NCA]/[P]b |
Mnc (g mol−1) |
Mw/Mnc | nd | DPP(BAA)e |
|---|---|---|---|---|---|---|---|
| HO-PMOx-NH2 10K | 72 | 125 | 9930 | 1.22 | 11 6 |
||
| HO-PMOx-NH2 5K | 48 | 65 | 5300 | 1.17 | 62 | ||
| COOH-PEG-NH2 5K | 5100 | 1.25 | 11 4 |
||||
| PMOx5K-b-P(BLG)10 | 48 | 12 | 7800 | 1.26 | 10 | ||
| PMOx5K-b-P(BLC)11 | 72 | 16 | 8200 | 1.25 | 11 | ||
| PMOx10K-b-P(BLG)11 | 48 | 12 | 12600 | 1.24 | 11 | ||
| PMOx10K-b-P(BLC)13 | 72 | 16 | 13300 | 1.23 | 13 | ||
| PEG5K-b-P(BLG)10 | 48 | 12 | 7730 | 1.24 | 10 | ||
| PEG5K-b-P(BLC)13 | 72 | 16 | 8500 | 1.26 | 13 |
I: initiator (N-[2-(p-toluenesulfonyloxy)-ethyl]-phthalimide),
P: amino terminated PMOx or PEG (macroinitiator),
Mn and Mw: number- and weight-average molecular weight from GPC analysis,
number of monomer units,
degree of polymerization of γ–benzyl or S-benzyloxycarbonyl-L-amino acids from 1H NMR.
Synthesis of poly(2-methyl-2-oxazolines-b-(γ-benzyl-L-glutamate or S-benzyloxycarbonyl-L-cysteine)), OH-PMOxn-b-P(BAA)m
The copolymers, OH-PMOxn-b-poly(γ-benzyl-L-glutamate) (OH-PMOxn-b-P(BLG)m) and OH-PMOxn-b-poly(S-benzyloxycarbonyl-L-cysteine) (OH-PMOxn-b-P(BLC)m), were obtained by ROP of NCA in the presence of PMOx-NH2 as macroinitiator. OH-PMOx-NH2 (2 × 10−5 mol) was dissolved in 5 mL of 0.2 M urea/DMF and added with a syringe to a solution of BAA-NCA (24 × 10−5 mol) in 0.2 M urea/DMF (5 mL) kept under dry argon. The polymerization was conducted at room temperature for several days (reaction times see Table 1). The PMOx-b-P(BAA) copolymers were isolated by precipitation into diethyl ether and purified by dialysis against distilled water for 3 days using a Spectra/Por CE membrane (MWCO: 3,500 g mol−1) and isolated by freeze-drying. Yield: 71%. 1H NMR (DMSO-d6, 500 MHz): δ/ppm = 1.9–1.94 (br, 3H, -CO-CH3), 1.9–2.15 (br, 2H, β-CH2-), 2.29 (br, 2H, γ-CH2-), 3.27–3.4 (br, 4H, -N-CH2CH2-), 4.0–4.50 (br, 1H, -CO-CH-), 4.95–5.2 (m, 2H, C6H5-CH2-), 7.25–7.35 (m, 5H, C6H5-), 8.2–8.3 (br, 1H, -CH-NH-).
Synthesis of poly(ethylene glycol-b-(γ-benzyl-L-glutamate or S-benzyloxycarbonyl-L-cysteine)), CM-PEGn-b-P(BAA)m
The copolymers, carboxylmethyl-PEG-b-poly(β-benzyl-L-glutamate) (CM-PEGn-b-P(BLG)m) and CM-PEGn-b-poly(S-benzyloxycarbonyl-L-cysteine) (CM-PEGn-b-P(BLC)m), were obtained by ROP of NCA in the presence of CM-PEG-NH2 as macroinitiator. CM-PEG-NH2 (3.5 × 10−5 mol) was dissolved in 5 mL of 0.2 M urea/DMF and added with a syringe to a solution of BAA-NCA (5.5 × 10−4 mol) in 0.2 M urea/DMF (5 mL) kept under dry argon. The polymerization was conducted at room temperature for several days (reaction times see Table 1). The PEG-b-P(BAA) copolymers were isolated by precipitation into diethyl ether and purified by dialysis against distilled water for 3 days using a Spectra/Por CE membrane (MWCO: 3,500 g mol−1) and isolated by freeze-drying. Yield: 78%. 1H NMR (DMSO-d6, 500 MHz): δ/ppm = 1.8–2.2 (br, 2H, β-CH2-), 2.35 (br, 2H, γ-CH2-), 3.5 (s, 4H, -O-CH2CH2-), 3.9–4.5 (br, 1H, -CO-CH-), 5.0–5.2 (m, 2H, C6H5-CH2-), 7.27–7.34 (m, 5H, C6H5-), 8.1–8.3 (br, 1H, -CH-NH-)
Instrumentation
All 1H NMR spectra were recorded on a Varian Unity Inova 500 (500 MHz) spectrometer equipped with a 5 mm triple resonance inverse detectable probe. DMSO-d6, CDCl3, and D2O were used as solvents. All spectra were recorded at 25°C, unless stated otherwise. Attenuated Total Reflectance (ATR) Fourier transform infrared (FTIR) spectra were recorded on a Perkin-Elmer Spectrum. A total of 126 scans were signal-averaged in the range from 4000 to 400 cm−1 at a resolution of 4 cm−1. A background measurement was taken before the sample was loaded onto the ATR unit for measurements. Gel permeation chromatography (GPC) was performed on a Waters Breeze GPC system consisting of a 1515 isocratic pump, a TSK-gel α-M (particle size 13 µm, exclusion limit 1 × 107 g mol−1 for polystyrene in DMF) and a TSK-gel α-3000 (particle size 7 µm, exclusion limit 1 × 105 g mol−1 for polystyrene in DMF) (Tosoh Bioscience) column, and a 2414 refractive index detector under the following conditions: injection volume, 100 µL; flow rate, 0.5 mL min−1; eluent, DMF; temperature, 50 °C, PS (purchased from Shodex) as the calibration standards.
Fluorescence Measurements
Steady-state fluorescence spectra were recorded on a FluoroMax-3 spectrometer (Horiba, Jobin Yvon) equipped with a GRAMS/32 data analysis system. Temperature control of the samples was achieved using a water-jacketed cell holder connected to a Cary circulating water bath. The temperature of the sample fluid was measured with a thermocouple immersed in a water-filled cell placed in one of the four cell holders in the sample compartment. All measurements were carried out at 24 °C. Slit widths were set at 2 and 1.5 nm for the excitation and emission monochromators, respectively. Pyrene fluorescence spectra were recorded from 360 to 600 nm using an excitation wavelength of 334 nm. To determine the onset of association (cass), solutions of polymer concentration ranging from 3.0 to 1.0 × 10−4 g L−1 were prepared by dilution of a polymer stock solution (3.0 g L−1) in water containing pyrene (Py ~ 10−6 M). The aqueous pyrene solution was prepared as follows: A solution of pyrene in ethanol (10 µL, 6.5 × 10−3 M) was added to an empty flask. The ethanol was evaporated with a stream of argon to form a thin film on the bottom of the flask, followed by addition of water. Solutions were stirred at room temperature for 48 h prior to measurement. The emission intensities measured at 373 nm (I1) and 383 nm (I3), the first and third vibronic peaks in the fluorescence emission spectrum of pyrene, were used to calculate the ratio I1/I3.
Dynamic Light Scattering Measurements (DLS)
Particle size measurements were performed using 90Plus particle size analyzer (Brookhaven Instruments), which is a dynamic light scattering instrument at scattering angles ranging between 15° and 90°, equipped with a solid state laser (λ = 532 nm) and a Peltier temperature control. In DLS experiments, the normalized time autocorrelation function of the scattered intensity is measured, which can be expressed in terms of the autocorrelation function of the concentration fluctuations. In our experiments, the relaxations had always a diffusive character with a characteristic time (τ) inversely proportional to q2. A cumulant analysis was applied to obtain the diffusion coefficient (D) of the scattering objects in solution. Extrapolation of the first reduced cumulant (τq2)−1 to q = 0 yields the value of D, which is related to the average hydrodynamic radius RH of the scattering objects by equation 1:
| (1) |
where ηs the viscosity of the solvent, kB is the Boltzmann constant and T is the absolute temperature. The polydispersity index (PDI) was used to describe the width of the particle size distribution, and calculated from a Cumulant analysis of the DLS measured intensity autocorrelation function and is related to the standard deviation of the hypothetical Gaussian distribution (i.e., PDI= σ2/D2, where σ is the standard deviation and D is the average mean size). All measurements were analyzed by BIC software (Brookhaven Instruments). The results are the mean radius of lognormal size distribution (LSD), being calculated by the software based on the non-negatively constrained least-squares (NNLS) algorithm.
Solutions for DLS were obtained by direct dissolution of the polymer in water (stock solution = 5.0 g L−1) and gentle stirring at room temperature for at least 12 h. The stock solution was used to prepare solutions ranging in concentration from 0.25 to 5.0 g L−1. They were filtered through a 0.45 µm Millex Millipore PVDF filter directly into the sample cell. Five measurements of 1 min each were carried out and averaged.
Results and Discussion
Polymer synthesis and characterization
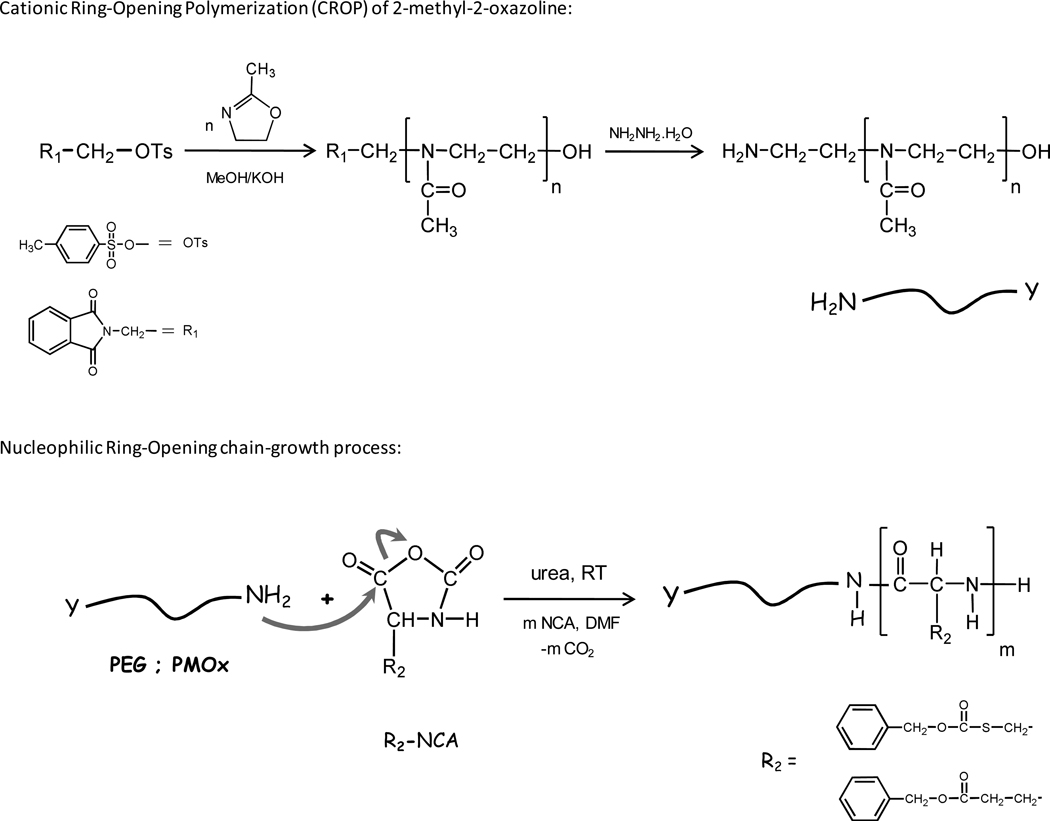
Water-soluble poly(2-methyl-2-oxazolines) and poly(ethylene glycol) end-capped with hydrophobic protected poly(amino acids) blocks were prepared in one or two steps as depicted in Scheme 2. Amphiphilic block copolymers were obtained by combining a long hydrophilic PMOx or PEG segment and a short polypeptide segment of (γ–benzyl-L-glutamate or S-benzyloxycarbonyl-L-cysteine), (P(BLG) or P(BLC)) as summarized in Table 1. Well defined block copolymers of OH-PMOxn-b-P(BAA)m and CM-PEGn-b-P(BAA)m were prepared (i) by ROP of protected L-amino acids NCAs (BAA-NCAs) using an α-amino terminated PEG (PEG-NH2) as a macroinitiator, or (ii) in two steps by a sequence, through a combination of (1) a cationic ROP of a 2-methyl-2-oxazoline according to the procedure reported by Yang, Y. et al13 which generates an ω-amino terminated PMOx (NH2-PMOx) and (2) a ROP of BAA-NCAs (Scheme 2).
Scheme 2.
Reaction schemes for the cationic ring opening polymerization of 2-methyl-2-oxazoline initiated by N-[2-(p-toluenesulfonyloxy)-ethyl]-phthalimide (NH2-PMOx-OH) and the preparation of block copolymers of PMOx (or PEG) and protected poly(amino acids) (OH-PMOxn-b-P(BAA)m, and CM-PEGn-b-P(BAA)m)
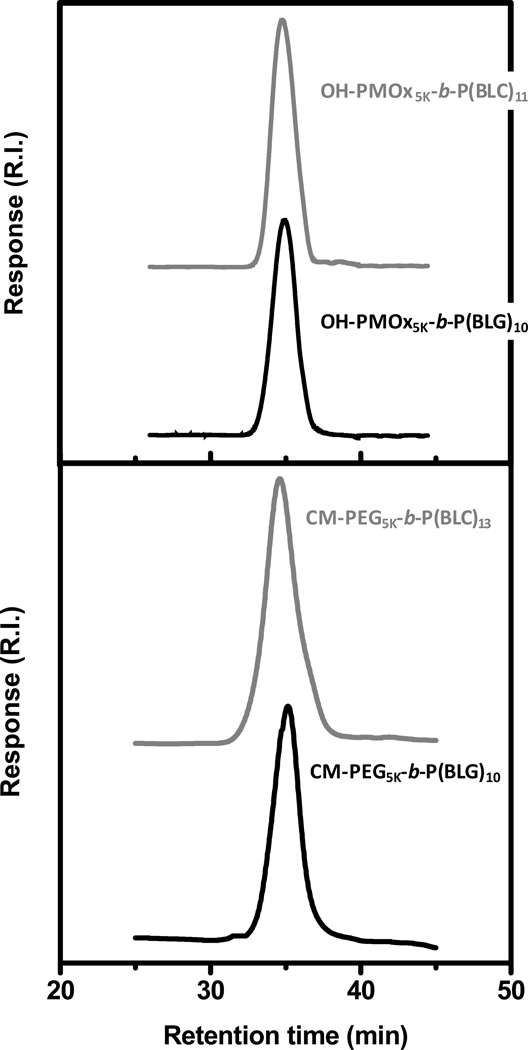
Ring-Opening Polymerization of BAA-NCAs was conducted in the presence of urea at room temperature to avoid spontaneously occurring side reactions during the polymerization initiated by amino-terminated polymers such as termination by formation of hydantoic acid derivatives (dead polymer), or deprotonation of the amine group of the NCA monomer and the resulting NCA anion acts as a nucleophile initiator resulting in wide molecular weight distributions and leading to ill defined block copolymers.14,15,16 Numerous reports indicate that during the early stages of the polymerization, when the degree of polymerization is below 10 ± 1, the oligomers of all amino acids adopt a β-sheet structure that grows more slowly than α-helical chains of poly(amino acid) during the polymerization owing to different steric environments resulting from a more or less pronounced backfolding.17,18 Recently, there has been an argument that urea would interact with the growing polymer-chain by forming hydrogen-bonds, thus preventing the formation of secondary structures, specifically β-sheets, thus, obtaining control over the molecular weight and achieving a narrow and monomodal molecular weight distribution and potentially supporting a living polymerization mechanism.19,20 The GPC traces (Figure 1), exemplified here in the case of OH-PMOx5K-b-P(BAA)10–11 and CM-PEG5K-b-P(BAA)10–13, exhibit a monomodal molecular weight distribution and a narrow polydispersity (PDI < 1.3). These data are comparable with results reported for NCA polymerization via primary amines using organometallic catalysts,21 high vacuum22 or by lowering the polymerization reaction temperature,23 however, these methods require special care because of: (i) the necessary metal catalyst removal, (ii) a time-consuming procedure or (iii) longer reaction times, respectively. Our approach facilitates the synthesis of poly(amino acid) block copolymers with controlled architecture and enhanced control over chain growth by using experimental conditions simple to implement.
Figure 1.
Gel permeation chromatograms of OH-PMOx5K-b-P(BLC)11 (top, gray), OH-PMOx5K-b-P(BLG)10 (top, black) and CM-PEG5K-b-P(BLC)13 (bottom, gray), CM-PEG5K-b-P(BLC)10 (bottom, black): DMF, flow rate: 0.5 mL min−1, temperature: 50°C.
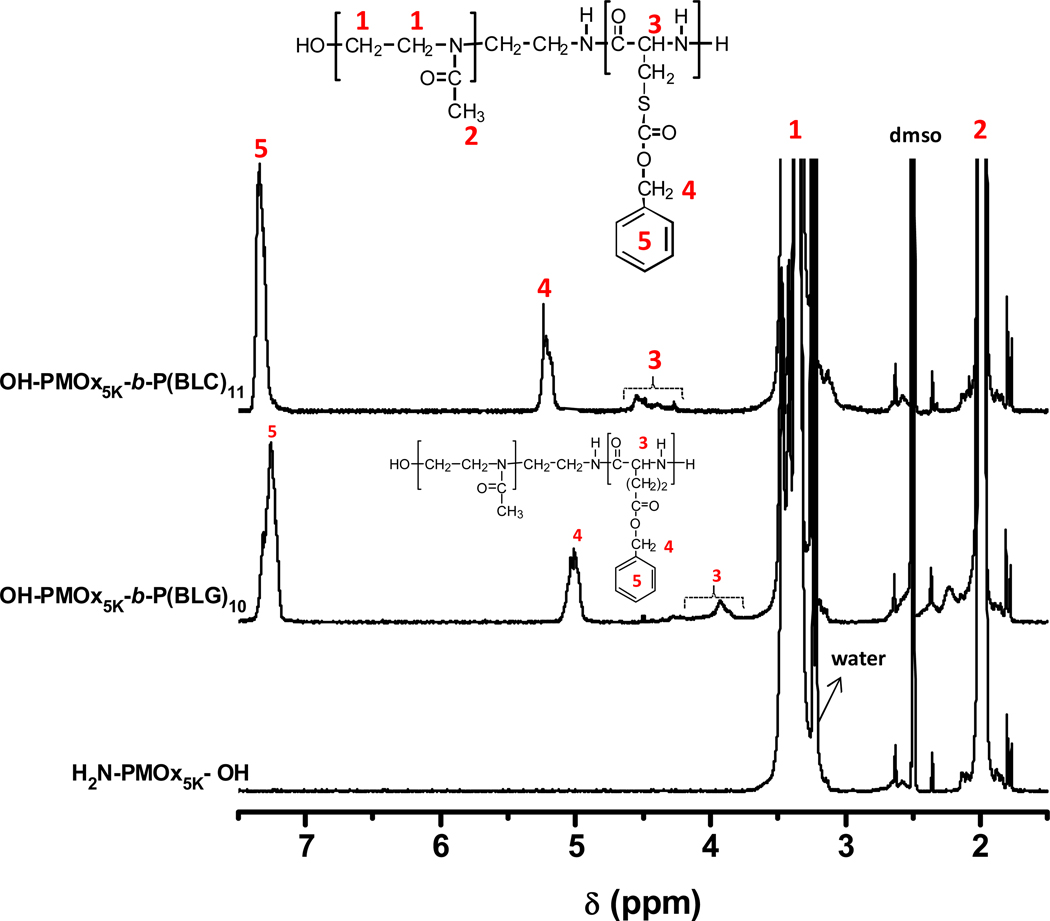
The molecular weight of the amino-terminated PMOx and the block lengths of hydrophobically protected poly(amino acids) P(BAA) can be adjusted by varying the reaction time, the initiator/2-methyl-2-oxazoline and the macroinitiators/BAA-NCAs molar ratios (Table 1). The composition of the block copolymers was determined by 1H NMR spectroscopy in DMSO-d6 at 35 °C. The degree of polymerization of the protected L-amino acids (BLG or BLC), DPP(BAA), was estimated from the peak integration ratios of the signals at δ = 1.9 ppm attributed to the resonance of the methyl protons of the methyl moieties of the PMOx repeat units (see Figure 2) or methylene protons of PEG (O-CH2CH2 at δ = 3.5 ppm) and at δ = 5.0–5.2 and 7.2–7.3 ppm ascribed to the resonance of the benzyl and methylene protons of benzyl ester or benzyloxycarbonyl group of P(BAA) segment as exemplified here in the case of OH-PMOx5K-b-P(BAA)10–11 (Figure 2). The experimental results obtained from both 1H NMR and GPC are in good agreement with the molecular weights calculated from the monomer feed.
Figure 2.
1H NMR spectra of OH-PMOx-NH2 5K (bottom), OH-PMOx5K-b-P(BLG)10 (middle) and OH-PMOx5K-b-P(BLC)11 (top). Solvent: DMSO at 35°C. Polymer concentration = 4.0 g L−1
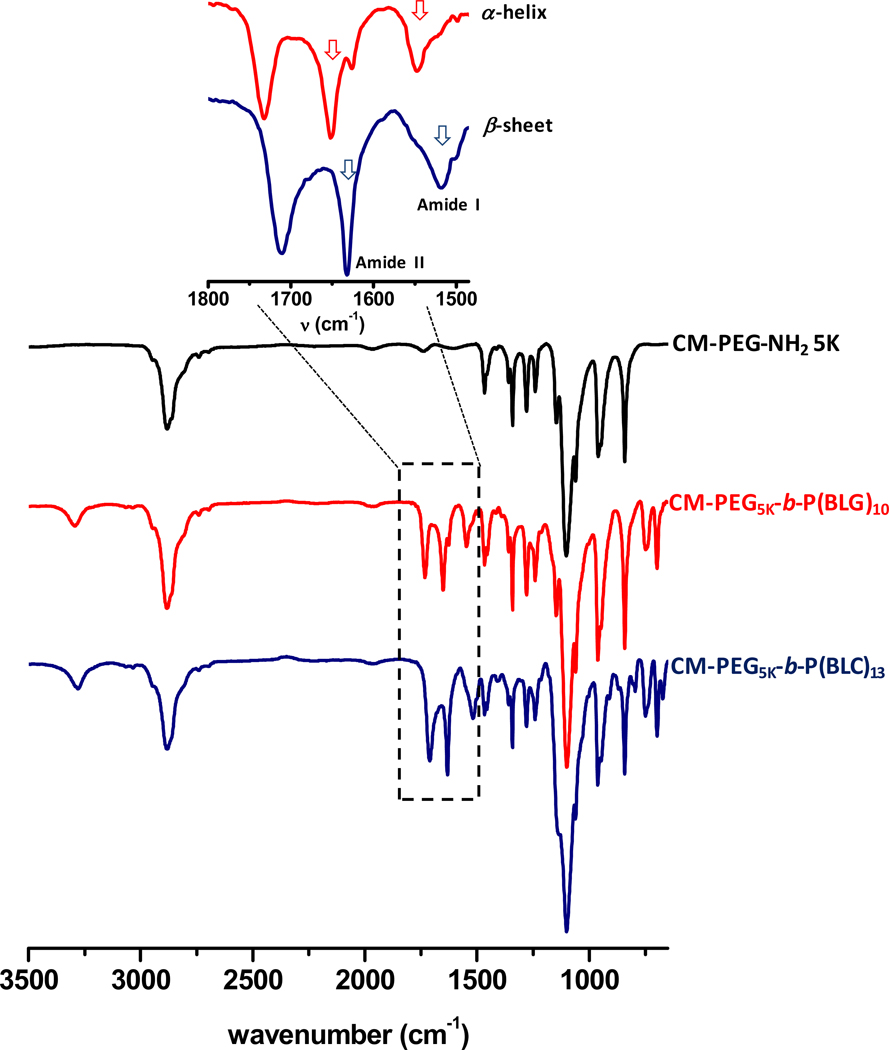
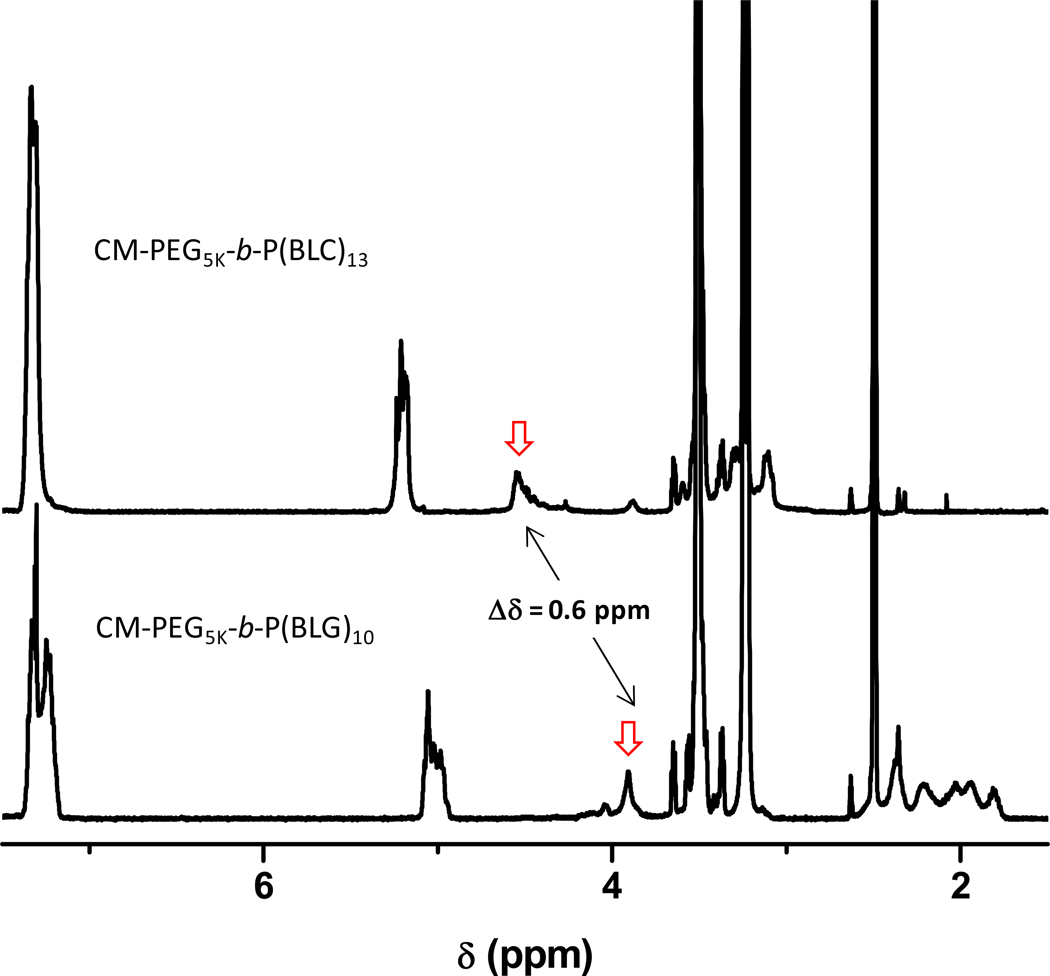
Further proof of the successful preparation of well-defined block copolymers was extracted from the FTIR spectra of the copolymers. Figure 3 presents IR spectra of CM-PEG5K-b-P(BLG)10 and CM-PEG5K-b-P(BLC)13. The absorption bands at 697, 740 and 1730 cm−1 are attributed to the benzyl and carbonyl ester groups, respectively, with wave-numbers characteristic of the protective group in the P(BAA) block. The spectra of both copolymers exhibit a stretching band at 3290 cm−1, characteristic of the secondary amide stretching vibration mode of the main-chain amide group of the peptide repeat unit. The FTIR spectrum of CM-PEG5K-b-P(BLG)10 presents bands around 1650 and 1550 cm−1, characteristic of the stretching and deformation modes of amide I and amide II, thus indicating that P(BLG)10 segments adopt an α-helix conformation even at low DP of approximately 10.24 In contrast, the FTIR spectrum of CM-PEG5K-b-P(BLC)13 exhibits an absorption band at 1630 cm−1, a wave-number characteristic of the β-sheet conformation (see inset in Figure 3),25 which is in good agreement with previous reports that showed that poly(L-cysteine) and protected P(BLC) exclusively adopt β-sheet structures in bulk or in solvent.26,27 Blout first recognized that in principle all poly(amino acids) can adopt a β-sheet structure, but some prefer to adopt an α-helix structure when the chain length is large enough. It is however, not completely understood yet as to why different poly(amino acids) switch their conformation while others remain in a β-sheet conformation.32 Moreover, a close examination of 1H NMR data gathered for the two copolymers bearing P(BLG)m and P(BLC)m segments unveiled a significant difference of the resonance of the methylene proton (C-H) of the main-chain of the PAA repeat unit (Cα - proton). The signal ascribed to the resonance of the Cα - proton of P(BLG) at δ = 3.9 ppm is shifted to δ = 4.5 ppm (PBLC) as exemplified here in the case of CM-PEG5K-b-P(BLG)10 and CM-PEG5K-b-P(BLC)13, respectively, (see Figure 4) which reveal the impact of the nature of side chains.
Figure 3.
FTIR spectra of CM-PEG-NH2 5K (black), CM-PEG5K-b-P(BLG)10 (red) and CM-PEG5K-b-P(BLG)13 (blue)
Figure 4.
1H NMR spectra of CM-PEG5K-b-P(BLG)10 (bottom) and CM-PEG5K-b-P(BLC)13 (top). Solvent: DMSO at 35°C. Polymer concentration : 4.0 g L−1
Self-assembly of the amphiphilic block copolymers in water
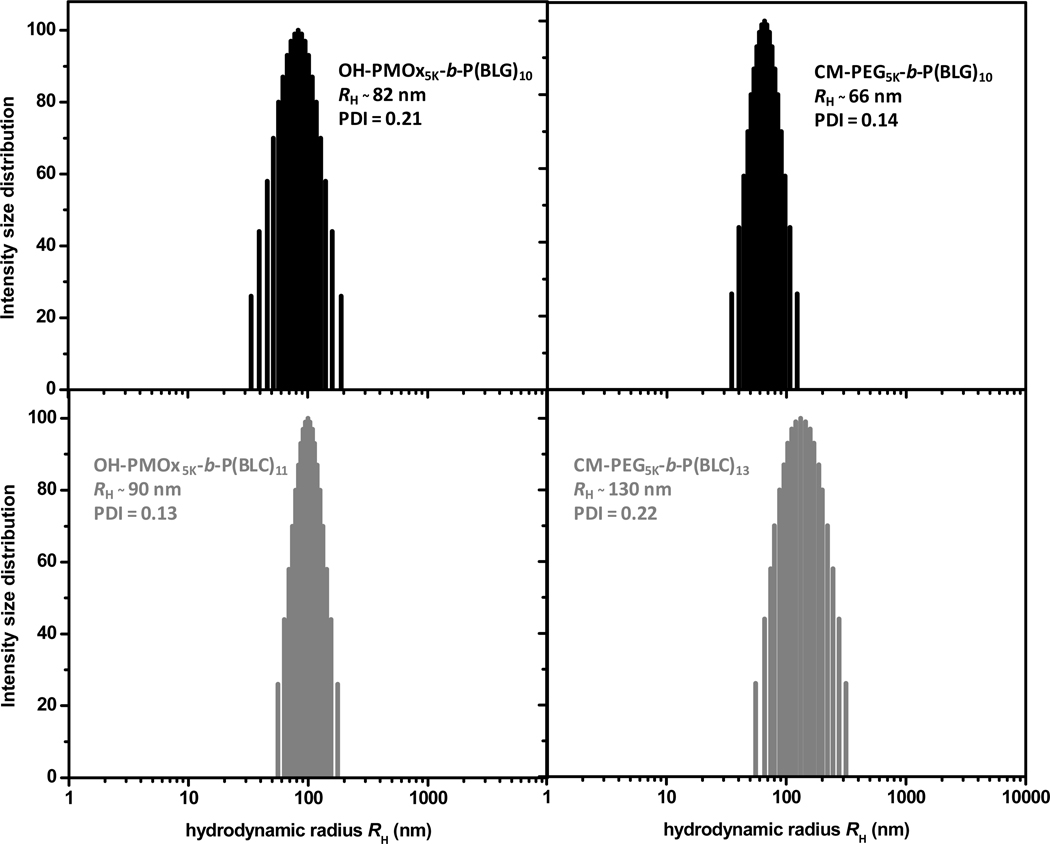
In aqueous media, amphiphilic block copolymers self-assemble following well-established patterns: formation of aggregates or nanoparticles in solutions of low concentration above a critical association concentration (cass).28,31 The experiments described below were aimed at confirming the formation of assemblies in aqueous OH-PMOxn-b-P(BAA)m and CM-PEGn-b-P(BAA)m solutions and at characterizing them in terms of size and (critical) association concentration. PMOx and PEG end-capped with γ-benzyl-L-glutamate blocks formed assemblies in water with hydrodynamic radii, RH, ranging from 66 to 90 nm depending on the polymer chain length and the chemical nature of the hydrophilic block. These self-assembled structures showed a unimodal size distributions with a polydispersity index (PDI) of ~ 0.17 ± 0.04 (Table 2). In contrast, assemblies of the PMOx or PEG samples end-capped with S-benzyloxycarbonyl-L-cysteine segments were significantly larger and less compact with RH ranging from 100 to 130 nm, as depicted in Figure 5, where we present the size distributions recorded for solutions of OH-PMOx5K-b-P(BLG)10 and CM-PEG5K-b-P(BLG)10 (Figure 5, top) and OH-PMOx5K-b-P(BLC)11 and CM-PEG5K-b-P(BLC)13 (Figure 5, bottom). RH values increased slightly with increasing solution concentration, the largest shift, from RH = 90 nm (0.25 g L−1) to RH = 210 nm (5.0 g L−1) being registered in the case of OH-PMOx10-b-P(BLG)11. No angular dependence was found for both PEG or PMOx copolymers, the RH being almost identical at every angle. This suggests that the scattering particles are isotropic objects, such as sphere.
Table 2.
Fluorescence and Light Scattering Data for Solutions of Various Block Copolymers in Dilute Aqueous Solutions At 24°C
| Polymer | Cassa/g L−1 | RH / nm b | PDIc |
|---|---|---|---|
| PMOx5K-b-P(BLG)10 | 0.008 | 82 ± 0.6 | 0.21 |
| PMOx5K-b-P(BLC)11 | 0.012 | 89 ± 0.7 | 0.13 |
| PMOx10K-b-P(BLG)11 | 0.015 | 90 ± 0.3 | 0.22 |
| PMOx10K-b-P(BLC)13 | 0.020 | 103 ± 0.5 | 0.14 |
| PEG5K-b-P(BLG)10 | 0.005 | 66 ± 0.4 | 0.14 |
| PEG5K-b-P(BLC)13 | 0.008 | 130 ± 0.6 | 0.22 |
From fluorescence probe measurements,
Polymer concentration : 1.0 g L−1,
Polydispersity index
Figure 5.
Particles size distributions in an aqueous solution of OH-PMOx5K-b-P(BLG)10 and COOH-PEG5K-b-P(BLG)10 (top) and OH-PMOx5K-b-P(BLC)11 and COOH-PEG5K-b-P(BLC)13 (bottom). Polymer concentration: 1.0 g L−1, temperature 24 °C, Θ : 90°
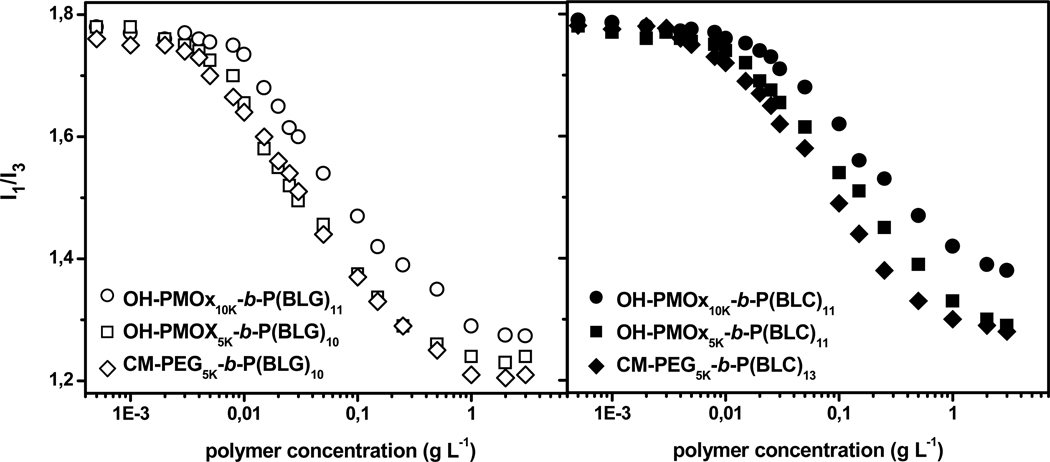
We determined the critical association concentrations of the polymers in water by fluorescence spectroscopy using pyrene (Py) as probe. In aqueous micellar solutions Py, which is poorly soluble in water, is preferentially solubilized within hydrophobic microdomains. The ratio, I1/I3, of the intensities of the first to third bands of the pyrene emission takes a value (~1.8) for Py in water and decreases to ≅ 1.25−1.20, when Py is solubilized in non polar media, such as alkanes or within the hydrophobic core of surfactant micelles.29 Thus, the amphiphile concentration for which a decrease in I1/I3 value occurs, gives an estimate of the amphiphile critical association concentration. Plots of the changes in the ratio I1/I3 as a function of polymer concentration are presented in Figure 6 for solutions of OH-PMOxn-b-P(BLG)m, CM-PEG5K-b-P(BLG)m (Figure 6, left, open symbols) and OH-PMOxn-b-P(BLC)m, CM-PEG5K-b-P(BLC)m (Figure 6, right, closed symbols). The ratio remained constant (~ 1.8) with increasing polymer concentration up to a point beyond which it decreased gradually towards a plateau value of ~ 1.20 in the most concentrated solutions tested (3.0 g L−1). The decrease took place over a very large concentration range unlike low molecular weight surfactants, polymers do not assemble cooperatively.30 We used the polymer concentrations corresponding to the onset of the drop of I1/I3 as an estimate of the lowest polymer concentration, cass, for which hydrophobic domains able to host Py molecules form in solution via assembly of the end groups (Table 2). The cass values of the polymer samples with γ-benzyl-L-glutamate block are ~ 32 % (average) lower than that samples with S-benzyloxycarbonyl-L-cysteine block, indicating the enhanced hydrophobicity of the γ-benzyl-L-glutamate chain compared to S-benzyloxycarbonyl-L-cysteine. For the samples of similar PMOx and PEG molecular weights, Mn ~ 5000 g mol−1, the cass of PMOx block copolymers was higher than that of the PEG block copolymers, i.e, cass values ranging from 0.008 g L−1 to 0.005 g L−1 for γ-benzyl-L-glutamate end-capped polymers. Furthermore, increasing the PMOx chain length by doubling the molecular weight produced, as expected, higher cass values.
Figure 6.
Changes in the ratio I1/I3 of the intensity of the first and third vibronic bands of pyrene (10−6 M) as a function of polymer concentration for solutions of OH-PMOxn-b-p(BLG)m, CM-PEG5K-b-P(BLG)m (left, open symbols) and OH-PMOxn-b-P(BLC)m, CM-PEG5K-b-P(BLC)m (right. closed symbols) samples at 24 °C
Conclusion
We have described a convenient procedure for the synthesis of amphiphilic diblock copolymers via ring-opening polymerizations. Analyses of aqueous solutions of CM-PEGn-b-P(BAA)m and OH-PMOxn-b-P(BAA)m block copolymers by DLS and fluorescence spectroscopy confirm the formation of self-assembled nanostructures in aqueous solutions. Further work is in progress aimed at assessing the structure and morphology of these self-assemblies that exhibit rather large RH values and are associated with specific poly(amino acid) segments by imaging methods, such as AFM and TEM.
Acknowledgement
This work was supported by the National Institute of Health (NIH), National Eye Institute under the American Recovery and Reinvestment Act of 2009, grant # 2R01EY016674-04A1 and is a collaborative effort within the Boston Retinal Implant Project. We would like to thank Egen, Inc. in Huntsville, AL, for their support with light scattering analyses.
References
- 1.Holmberg K, Bergström K, Brink C, Österberg E, Tiberg F, Harris JM. J. Adhes. Sci. Technol. 1993;7:503–517. [Google Scholar]
- 2.K Ista L, Fan H, Baca O, P. López G. FEMS Microbiol. Lett. 1996;142:59–63. doi: 10.1111/j.1574-6968.1996.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 3.Bendele A, Seely J, Richey C, Sennello G, Shopp G. Toxicol. Sci. 1998;42:152–157. doi: 10.1006/toxs.1997.2396. [DOI] [PubMed] [Google Scholar]
- 4.Goddard P, Hutchinson LE, Brown J, Brookman LJ. J. Controlled Release. 1989;10:5–16. [Google Scholar]
- 5.Mero A, Pasut G, Via LD, Fijten MWM, Schubert US, Hoogenboom R, Veronese FM. J. Controlled Release. 2008;125:87–95. doi: 10.1016/j.jconrel.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Zalipsky S, Hansen CB, Oaks JM, Allen TM. J. Pharm. Sci. 1996;85:133–137. doi: 10.1021/js9504043. [DOI] [PubMed] [Google Scholar]
- 7.Kwon G, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. J. Controlled Release. 1994;29:17–23. [Google Scholar]
- 8.Kataoka K, Harada A, Nagasaki Y. Adv. Drug Delivery Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 9.Han DK, Hubbell JA. Macromolecules. 1996;29:5233–5235. [Google Scholar]
- 10.Emoto K, Nagasaki Y, Kataoka K. Langmuir. 1999;15:5212–5218. [Google Scholar]
- 11.Volet G, Chanthavong V, Wintgens V, Amiel C. Macromolecules. 2005;38:5190–5197. [Google Scholar]
- 12.Obeid R, Maltseva E, Thünemann AF, Tanaka F, Winnik FM. Macromolecules. 2009;42:2204–2214. [Google Scholar]
- 13.Yang Y, Kataoka K, Winnik FM. Macromolecules. 2005;38:2043–2046. [Google Scholar]
- 14.Deming TJ. J. Polym. Sci. A Polym. Chem. 2000;38:3011–3018. [Google Scholar]
- 15.Lundberg RD, Doty P. J. Am. Chem. Soc. 1957;79:3961–3972. [Google Scholar]
- 16.Kricheldorf HR. Makromol. Chem. 1977;178:1959–1970. [Google Scholar]
- 17.Doty P, Lundberg RD. J. Am. Chem. Soc. 1956;78:4810–4812. [Google Scholar]
- 18.Mitchell JC, Woodward AE, Doty P. J. Am. Chem. Soc. 1957;79:3955–3960. [Google Scholar]
- 19.Vayaboury W, Giani O, Cottet H, Bonaric S, Schué F. Macromol. Chem. Phys. 2008;209:1628–1637. [Google Scholar]
- 20.Scholz C, Vayaboury W. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2008;49:486–487. [Google Scholar]
- 21.Deming TJ. Nature. 1997;390:386–389. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]
- 22.Aliferis T, Iatrou H, Hadjichristidis N. Biomacromolecules. 2004;5:1653–1656. doi: 10.1021/bm0497217. [DOI] [PubMed] [Google Scholar]
- 23.Vayaboury W, Giani O, Cottet H, Deratani A, Schué F. Macromol. Rapid Commun. 2004;25:1221–1224. [Google Scholar]
- 24.Bloom SM, Fasman GD, de Lozé C, Blout ER. J. Am. Chem. Soc. 1962;84:458–463. [Google Scholar]
- 25.Matsusaki M, Waku T, Kaneko T, Kida T, Akashi M. Langmuir. 2006;22:1396–1399. doi: 10.1021/la053051o. [DOI] [PubMed] [Google Scholar]
- 26.Blout ER, de Lozé C, Bloom SM, Fasman GD. J. Am. Chem. Soc. 1960;82:3787–3789. [Google Scholar]
- 27.Kricheldorf HR. Angew. Chem. Int. Ed. 2006;45:5752–5784. doi: 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- 28.Allen C, Maysinger D, Eisenberg A. Colloids Surf., B. 1999;16:3–27. [Google Scholar]
- 29.Kalyanasundaram K, Thomas JK. J. Am. Chem. Soc. 1977;99:2039–2044. [Google Scholar]
- 30.Alami E, Almgren M, Brown W, François J. Macromolecules. 1996;29:2229–2243. [Google Scholar]
- 31.Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Nat Mater. 2004;3:244–248. doi: 10.1038/nmat1093. [DOI] [PubMed] [Google Scholar]
- 32.Blout ER. Madison, Wis.: University of Wisconsin Press; 1962. p. 283. [Google Scholar]