Abstract
Purpose
We estimate trends in the prevalence of urinary incontinence in the adult population of the United States from 2001 through 2008 before and after adjusting for other potential associated factors.
Materials and Methods
We analyzed data on 17,850 adults 20 years old or older who participated in the 2001 to 2008 cycles of the National Health and Nutrition Examination Survey. Any urinary incontinence was defined as a positive response to questions on urine leakage during physical activity, before reaching the toilet and during nonphysical activity. During this period changes in demographic and clinical factors associated with urinary incontinence included age, race/ethnicity, obesity, diabetes and chronic medical conditions (prostate disease in men). Age standardized prevalence estimates and prevalence ORs of urinary incontinence trends were determined using adjusted multivariate models with appropriate sampling weights.
Results
The age standardized prevalence of urinary incontinence in the combined surveys was 51.1% in women and 13.9% in men. Prevalence in women increased from 49.5% in 2001 to 2002, to 53.4% in 2007 to 2008 (Ptrend = 0.01) and in men from 11.5% to 15.1%, respectively (Ptrend = 0.01). In women increased prevalence was partially explained by differences in age, race/ethnicity, obesity, diabetes and select chronic diseases across the survey periods. After adjustment the prevalence OR for 2007 to 2008 vs 2001 to 2002 decreased from 1.22 (95% CI 1.03–1.45) to 1.16 (95% CI 0.99–1.37). in men adjustment for potentially associated factors did not explain the increasing prevalence of urinary incontinence.
Conclusions
The age standardized prevalence of urinary incontinence increased in men and women from 2001 through 2008. Decreasing obesity and diabetes may lessen the burden of urinary incontinence, especially in women.
Keywords: urinary incontinence, risk factors, epidemiology, female, male
Urinary incontinence is common and often impairs social, physical and psychological well-being.1 UI is a risk factor for falls and fractures in the elderly population.2 The impact of UI on quality of life in men and women is well documented.3,4 Also, medical and out-of-pocket costs associated with UI are substantial and will increase in the United States as the population ages.4,5
UI is especially burdensome in women.3,6 The prevalence rate is high and varies widely from 15% to 69%, reflecting the study population examined and the definition of incontinence used.6–8 Several risk factors have been established for UI in women7–11 but few have been studied in a population of women with time. The prevalence of UI in men is less than in women with the rate varying from 5% to 24% and with less known about attributable risk factors.1,7,11,12
To our knowledge national estimates of the change in UI prevalence with time are lacking. We report the prevalence of UI in women and men from 2001 to 2002, to 2007 and 2008 in NHANES. We also examined changes in associated demographic and clinical factors as potentially contributing to the change in UI prevalence during this period.
METHODS
Study Population
NHANES is cross-sectional surveys of a nationally representative sample of the noninstitutionalized population that is sampled using a complex, stratified, multistage, probability cluster design. The National Center for Health Statistics ethics review board approved the protocol and all participants provided written informed consent.
The NHANES 2001 to 2002, 2003 to 2004, 2005 to 2006 and 2007 to 2008 (NHANES 2001 to 2008) cycles were combined to provide an overall description and characterization of the population. We identified a subsample of 19,352 men and nonpregnant women 20 years old or older who underwent physical and laboratory examination in an MEC. A total of 1,502 participants (7.8%) who were missing questionnaire items for UI were excluded from analysis. The amount of missing data on incontinence questions from the 4 NHANES cycles did not statistically differ (p = 0.11). Participants were interviewed at home and then underwent standardized physical examination and further questioning in an MEC. Responses to questions on UI symptoms were ascertained in a private home interview for participants 60 years old or older and in the MEC interview room using a computer assisted personal interview system for participants 20 to 59 years old for the 2001 to 2004 cycles. Such questions were asked of all participants 20 years old or older in the MEC using a computer assisted personal interview system for the 2005 to 2008 cycles.
Urinary Incontinence
separate questions with a yes or no response were used to assess urine leakage. SUI was defined as a positive response to the question, “During the past 12 months, have you leaked or lost control of even a small amount of urine with activity like coughing, lifting, or exercise?” UUI was defined based on the question, “During the past 12 months, have you leaked or lost control of even a small amount of urine with an urge or pressure to urinate and you could not get to the toilet fast enough?” Other incontinence was ascertained with the question, “During the past 12 months, have you leaked or lost control of even a small amount of urine during nonphysical activities?” A positive response to any of the 3 questions was used to define any UI. Women and men who responded in the affirmative to questions on SUI and UUI were defined as having mixed UI.
Other Measurements
Information on age (20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, 70 to 79, or 80 years or greater), race/ethnicity (nonHispanic white, nonHispanic black, Mexican-American or other), education (high school or less, or more than high school), poverty income ratio (2 or less, or greater than 2), current cigarette smoking (yes or no) and alcohol consumption in the last 12 months (yes or no) was self-reported. Participant weight and height were measured and BMI (less than 25.0, 25.0 to 29.9 and 30 kg/m2 or greater) was calculated. Diabetes was defined by self-report of being told by a physician or health professional, receiving insulin and/or diabetic pills, or hemoglobin A1C 6.5% or greater. Participants with chronic disease were ascertained by self-report to questions on arthritis, emphysema, chronic bronchitis, asthma, congestive heart failure, coronary heart disease, angina, heart attack, stroke, any liver condition, thyroid problem or cancer.
For nonpregnant women reproductive health status was characterized as current oral contraceptive use for premenopausal women younger than 50 years (yes or no), current hormone replacement therapy for postmenopausal women 50 years old or older (yes or no), parity (none or live birth) and previous hysterectomy. For men prostate disease included any prostate disease (yes or no to the question, “Have you ever been told by a doctor or health professional that you have any disease of the prostate?”) and prostate cancer (yes or no). Information on benign prostatic enlargement (yes or no) was only available for men 40 years old or older.
Statistical Methods
All estimates, SEs and association measures were derived using the sampling weights provided by the National Center for Health Statistics. These weights consider unequal probabilities of selection resulting from sample design, nonresponse and planned over sampling of the elderly, nonHispanic black and Mexican-American populations.
Separate analysis was done for men and women. Estimates of UI prevalence by survey waves were age standardized by the direct method to 2000 Census population using the age groups 20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, 70 to 79 and 80 years old or older. Linear trends during the 4, 2-year survey waves were tested using logistic regression models with adjustment for age group, race, ethnicity group and survey period (continuous variable). We used the 2-sample t test to test differences in means and proportions. Variables with significant differences between NHANES 2001 to 2002 and NHANES 2007 to 2008 were considered potential contributors to differences in UI prevalence. Survey period was treated as a categorical variable for comparisons in UI prevalence across survey waves. Multivariate logistic regression analysis was used to calculate POR estimates and the corresponding 95% CI for UI prevalence with adjustment for age, race and ethnicity, BMI, diabetes or chronic disease in women and prostate disease in men with p <0.05 considered statistically significant. Statistical analysis was performed using SAS-callable SUDAAN, version 10.0 (Research Triangle Institute, Research Triangle Park, North Carolina).
RESULTS
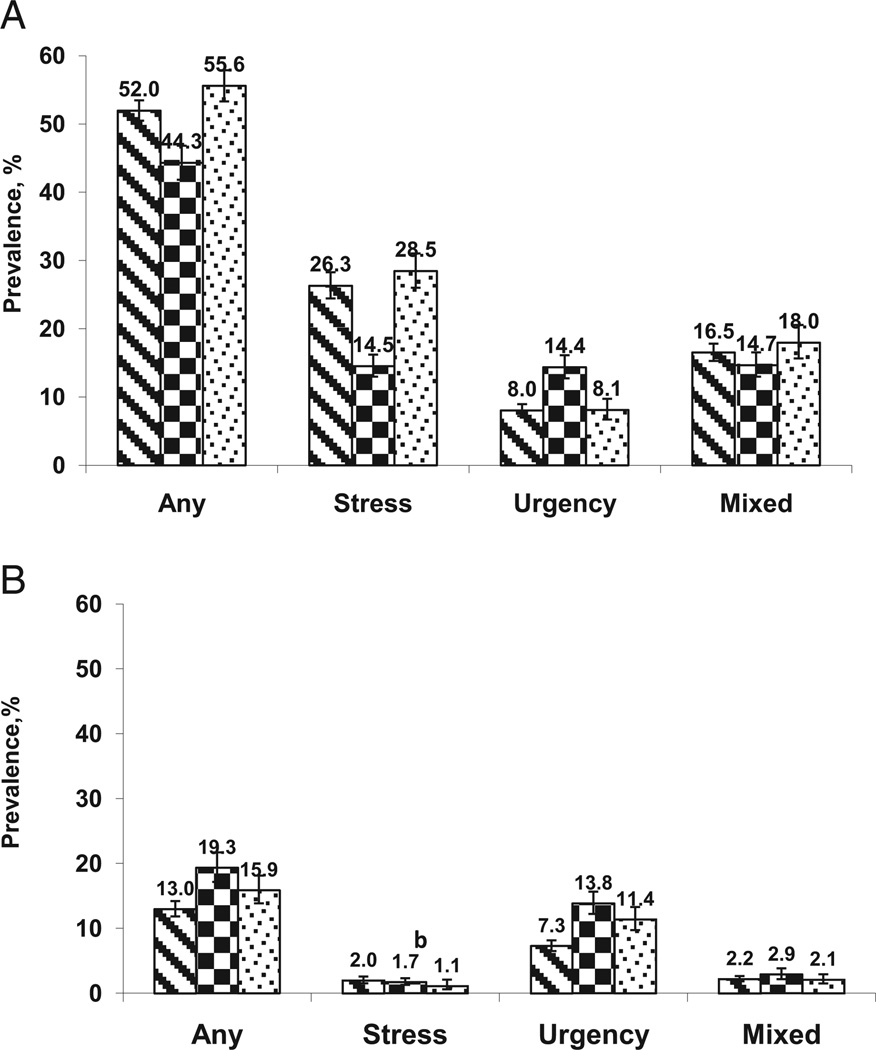
A total of 9,071 men and 8,779 women 20 years old or older provided questionnaire data and completed physical examinations in the MEC in the NHANES 2001 to 2008 cycles. Figure 1 shows the age standardized prevalence of UI and incontinence subtypes (SUI, UUI and mixed) by gender and race/ethnic group using the combined surveys. The age standardized prevalence of UI in the United States was much higher in women than in men (51.1%, 95% CI 49.9–52.4 vs 13.9%, 12.9–15.0). SUI was the most common subtype in women (24.8%, 95% CI 23.4–26.3) while UUI was most common in men (8.3%, 95% CI 7.6 –9.0). NonHispanic black women had the lowest age standardized prevalence rates of UI (44.3%, 95% CI 41.8–46.8) of all women and nonHispanic black men had the highest UI rate (19.3%, 95% CI 17.2–21.7) of all men.
Figure 1.
UI prevalence by race/ethnicity in American women (A) and men (B) standardized by direct method to 2000 Census population using age groups 20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, 70 to 79 and 80 years or greater (relative SE 32%).
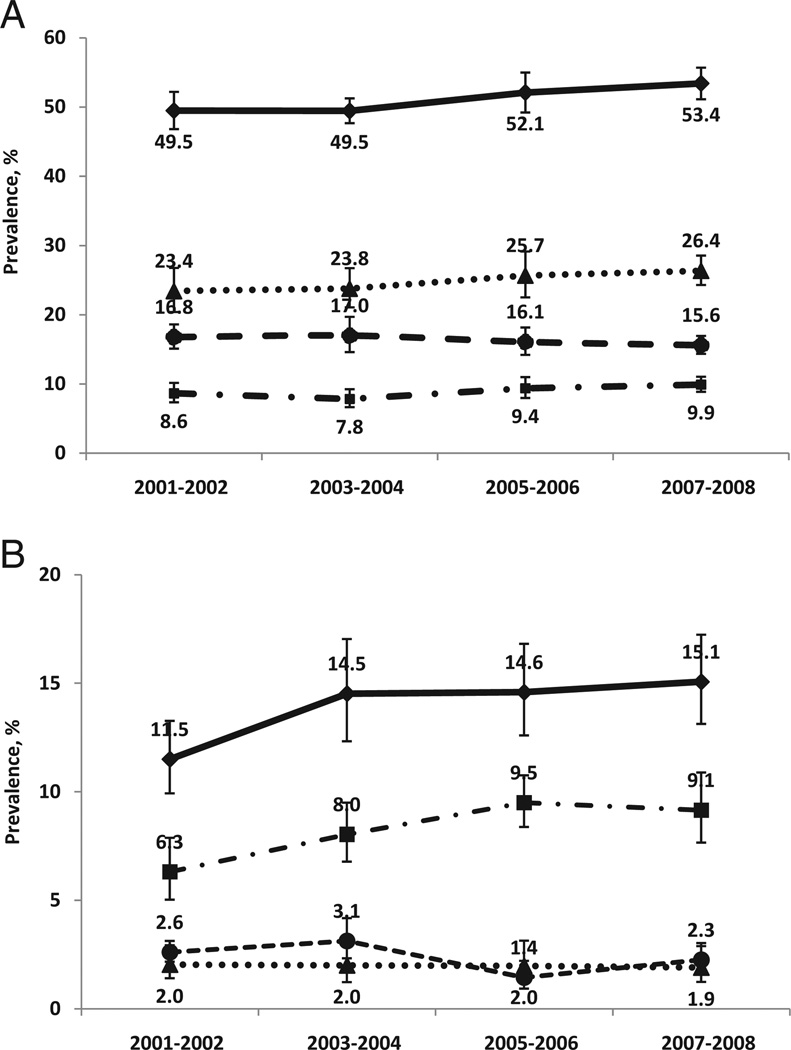
Figure 2 shows linear trends in UI prevalence by NHANES sample year according to gender and incontinence subtype. Overall UI prevalence in women increased significantly from 49.5% in 2001 and 2002 to 53.4% in 2007 and 2008 (Ptrend = 0.01). No statistically significant trends during the 4, 2-year survey cycles were seen in the age standardized prevalence rate in women by UI subtype (fig. 2, A). The age standardized prevalence rate of UI in men also increased significantly from 11.5% in 2001 and 2002 to 15.1% in 2007 and 2008 (Ptrend = 0.01). Of UI subtypes only men with UUI had a significant linear increase in the age standardized prevalence from 2001 to 2008, including 6.3% in 2001 and 2002 to 9.1% in 2007 and 2008 (Ptrend = 0.004, fig. 2, B).
Figure 2.
American trends in UI prevalence and trends by subtype from 2001 to 2008, age standardized by direct method to 2000 Census population using age groups 20 to 29, 30 to 39, 40 to 49, 50 to 59 years, 60 to 69, 70 to 79 and 80 years or greater. There was significant linear trend in prevalence of any UI in women (A) 20 years old or older, and of any and urinary UI in men (B) 20 years old or older during survey cycle (each p <0.05). Y axis differs in 2 figures. Error bars indicate 95% CI.
Significantly different clinical factors in survey years 2001 and 2002 vs 2007 and 2008 were BMI (mean 28.1 to 28.7 kg/m2, p = 0.03), diabetes (prevalence 8.2% to 11.7%, p = 0.01) and any chronic disease (prevalence 49.0% to 54.4%, p = 0.04) in women, and BMI (27.9 to 28.6 kg/m2, p = 0.004), diabetes (9.7% to 11.8%, p = 0.048) and any prostate disease (9.8% to 12.9%, p = 0.02) in men.
Compared with NHANES 2001 to 2002 the prevalence of UI in 2007 to 2008 increased in women who were nonHispanic black (p <0.001) or obese (p = 0.04), or who had diabetes (p <0.001). During the same survey periods the prevalence of UI increased in men who were obese (p = 0.03) and those who did not have diabetes (p = 0.003) or prostate disease (p = 0.003).
In women the unadjusted model revealed a significantly increased POR of 1.22 (95% CI 1.03–1.45, p = 0.02) for 2007 to 2008 and a marginally increased POR of 1.17 (95% CI 0.99–1.38, p = 0.06) for 2005 to 2006 but no significant difference for 2003 to 2004 compared to 2001 to 2002 (referent). In the adjusted multivariate model including age and race/ethnicity the POR for UI decreased to 1.20 (95% CI 1.02–1.40) for NHANES 2007 to 2008. In the multivariate model including age, race/ethnicity, BMI, diabetes and chronic disease the POR for UI further decreased to 1.16 (95% CI 0.99–1.37) for NHANES 2007 to 2008. Further analysis including education, smoking, drinking, hysterectomy and live births, which are variables without a significant distribution change with time, did not significantly change our results.
In men the unadjusted model showed a significantly increased POR of 1.45 (95% CI 1.16–1.81, p = 0.002) for 2007 to 2008, 1.39 (95% CI 1.07–1.80, p = 0.02) for 2005 to 2006 and 1.38 (95% CI 1.09–1.75, p = 0.01) for 2003 to 2004, compared to survey year 2001 to 2002 (referent). In the multivariate model including age, race/ethnicity, BMI, diabetes and prostate disease the POR for UI decreased to 1.41 (95% CI 1.08–1.84) for NHANES 2007 to 2008. However, the significant trends in UI prevalence remained relatively unchanged after any combinations of adjustment for age, race/ethnicity, BMI, diabetes and any prostate disease. Further analysis evaluating education, smoking, drinking or prostate cancer, which were variables without significant change during survey cycles, did not significantly impact UI trends with time.
DISCUSSION
The prevalence of UI was high in a nationally representative sample of the adult population in the United States with almost 1/2 women and about 1/6 men reporting symptoms. From 2001 to 2002, to 2007 to 2008 the age standardized prevalence rate of UI in women 20 years old or older significantly increased from 49.5% to 53.4%. During the same time the age standardized prevalence rates of UI in men also increased significantly from 11.5% to 15.1%. The increase in prevalence in women but not in en, was partially explained by increasing BMI and the associated high prevalence rate of diabetes.
Body weight is an important modifiable risk factor for UI and LUTS.13–17 Obesity, defined as BMI 30 kg/m2 or greater, was a factor contributing to the UI trends in women and men. A possible explanation of the effect of body weight on UI is that increased abdominal pressure may contribute to pelvic muscle weakness, resulting in LUTS.13 A recent NHANES report showed that women have a higher obesity rate than men.18 Modest decreases in weight have improved UI severity in obese and overweight women with UI, and may prove to be a useful public health strategy to decrease the burden of UI.19 In women substantial weight loss after bariatric surgery also resulted in significant improvement in UI.17 Less is known about the impact of excess weight on LUTS in men. Obese men may have more benign prostatic enlargement and LUTS than their normal weight counterparts.15,16,20 More studies are needed to evaluate the effects of obesity and weight loss on UI and LUTS in men.
Women and men with diabetes are also at increased risk for LUTS, including incontinence.16,20–23 This may be secondary to the effect of diabetes on nerve function as well as microvascular inflammatory processes.24 Thus, efforts to improve diabetes management and prevent diabetes may serve to decrease the UI risk. Interestingly the UI rate increased significantly in men who did not report having diabetes or prostate disease. Despite increasing trends of obesity, diabetes and prostate disease in men these factors were less likely to be factors contributing to the increased prevalence of UI.
Projected demographic changes in age, race and ethnicity in the Untied States may contribute to a continued increasing trend in UI. Increasing UI prevalence by age decade was more pronounced in men than in women. The UI prevalence rate varies by race and ethnicity with black women consistently reporting a lower UI rate than women of other racial/ethnic groups.25,26 Less is known about racial/ethnic differences in UI prevalence in men.11,27
The UI prevalence in men and women was substantially higher than in previous reports, given our UI definition. The response level and the order used to define UI frequency changed in the 2005 to 2006 and 2007 to 2008 NHANES cycles compared to the 2 previous cycles used in this analysis. This change did not allow us to compare UI frequency or severity across all 4 cycles. Recently published NHANES data on the 2005 to 2006 and 2007 to 2008 cycles using the validated Incontinence Severity Index showed a prevalence rate of moderate to severe UI, defined as a score incorporating frequency and urine loss volume, of 15.7% and 4.5% in women and men, respectively.6,27,28 However, less severe UI may be a risk factor for more severe, frequent UI in older adults as well as an important target in the course of this condition for intervention.29
The strength of this study include a nationally representative sample of the American population, sequential surveys examining trends in UI prevalence during an 8-year period, and a wide range of demographic and clinical factors previously associated with UI. Several limitations deserve mention. NHANES is cross-sectional and causation with associated factors cannot be established. Moreover, incident cases of UI could not be identified from this study design. Since our sample included only non-institutionalized individuals, we may have underestimated the burden of UI, given that residents of long-term care facilities were not represented. The small numbers of men with SUI and mixed UI limited our ability to reliably report UI subtype trends. Chronic diseases except diabetes were analyzed as a composite count rather than as individual disease types. Lastly, hypertension and stroke were associated with UI in other population based studies.11
CONCLUSIONS
Survey data from the last decade suggest that the UI prevalence has increased significantly in women and men in the United States. The burden of UI risk from obesity and diabetes explains much of the increase in UI prevalence in women but not in men. Public health efforts to decrease obesity, and improve diabetes management and prevention may decrease the impact of this burdensome condition in the American population, especially in women.
Acknowledgments
Study received National Center for Health Statistics ethics review board approval.
Abbreviations and Acronyms
- BMI
body mass index
- LUTS
lower urinary tract symptoms
- MEC
Mobile Examination Center
- NHANES
National Health and Nutritional Examination Survey
- POR
prevalence odds ratio
- SUI
stress UI
- UI
urinary incontinence
- UUI
urgency UI
Footnotes
Supplementary material for this article can be obtained at http://www.aging.uab.edu/SubChannel/Research/genito-urinary.aspx.
Financial interest and/or other relationship with Pfizer.
Financial interest and/or other relationship with Astellas, IDEO, Pfizer and Uromedica.
REFERENCES
- 1.Landefeld CS, Bowers BJ, Feld AD, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 2.Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 3.Swithinbank LV, Abrams P. The impact of urinary incontinence on the quality of life of women. World J Urol. 1999;17:225. doi: 10.1007/s003450050137. [DOI] [PubMed] [Google Scholar]
- 4.Stothers L, Thom D, Calhoun E. Urologic diseases in America project: urinary incontinence in males—demographics and economic burden. J Urol. 2005;173:1302. doi: 10.1097/01.ju.0000155503.12545.4e. [DOI] [PubMed] [Google Scholar]
- 5.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in women. JAMA. 2008;300:1311. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milsom I, Altman D, Lapitan MC, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP) In: Abrams P, Cardozo L, Khoury S, editors. Incontinence. 4th ed. Paris, France: International Consultation on Incontinence; 2009. [Google Scholar]
- 8.Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data. Study of Women’s Health Across the Nation. Am J Epid. 2007;165:309. doi: 10.1093/aje/kwk018. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SL, Scholes D, Boyko EJ, et al. Predictors of urinary incontinence in a prospective cohort of postmenopausal women. Obstet Gynecol. 2006;108:855. doi: 10.1097/01.AOG.0000236446.17153.21. [DOI] [PubMed] [Google Scholar]
- 10.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women. Variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111:324. doi: 10.1097/01.AOG.0000267220.48987.17. [DOI] [PubMed] [Google Scholar]
- 11.Tennstedt SL, Link CL, Steers WD, et al. Prevalence and risk factors for urine leakage in a racially and ethnically diverse population of adults. The Boston Area Community Health (BACH) Survey. Am J Epidemiol. 2008;167:390. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 12.Malmsten UG, Milsom I, Molander U, et al. Urinary incontinence and lower urinary tract symptoms: an epidemiological study of men aged 45 to 99 years. J Urol. 1997;158:1733. doi: 10.1016/s0022-5347(01)64113-2. [DOI] [PubMed] [Google Scholar]
- 13.Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol, suppl. 2009;182:S2. doi: 10.1016/j.juro.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend MK, Danforth KN, Rosner B, et al. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110:346. doi: 10.1097/01.AOG.0000270121.15510.57. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Smit E, Giovannuci E, et al. Association of obesity with lower urinary tract symptoms and non cancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2004;159:390. doi: 10.1093/aje/kwh060. [DOI] [PubMed] [Google Scholar]
- 16.Seim A, Hoyo C, Ostbye T, et al. The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int. 2005;96:88. doi: 10.1111/j.1464-410X.2005.05573.x. [DOI] [PubMed] [Google Scholar]
- 17.Richter HE, Burgio KL, Clements RH, et al. Urinary and anal incontinence in morbidly obese women considering weight loss surgery. Obstet Gynecol. 2005;106:1272. doi: 10.1097/01.AOG.0000187299.75024.c4. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 19.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol. 2009;182:616. doi: 10.1016/j.juro.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JS, Vittinghoff E, Lin F, et al. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES 2001–2001) Diabetes Care. 2006;29:1307. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lifford KL, Curhan GC, Hu FB, et al. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851. doi: 10.1111/j.1532-5415.2005.53565.x. [DOI] [PubMed] [Google Scholar]
- 23.Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan S, Grodstein F, Brown JS. Clinical research in diabetes and urinary incontinence: what we know and need to know. J Urol. 2009;182:514. doi: 10.1016/j.juro.2009.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thom DH, Van Den Eeden SK, Ragins AI, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend MK, Curhan GC, Resnick NM, et al. The incidence of urinary incontinence across Asian, black and white women in the United States. Am J Obstet Gynecol. 2010;202:378.e1. doi: 10.1016/j.ajog.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markland AD, Goode PS, Redden DT, et al. Prevalence of urinary incontinence in US men: results from the National Health and Nutrition Examination Survey. J Urol. 2010;184:1022. doi: 10.1016/j.juro.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Sandvik H, Seim A, Vanvik A, et al. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Goode PS, Burgio KL, Redden DT, et al. Population based study of incidence and predictors of urinary incontinence in black and white older adults. J Urol. 2008;179:1449. doi: 10.1016/j.juro.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]