Abstract
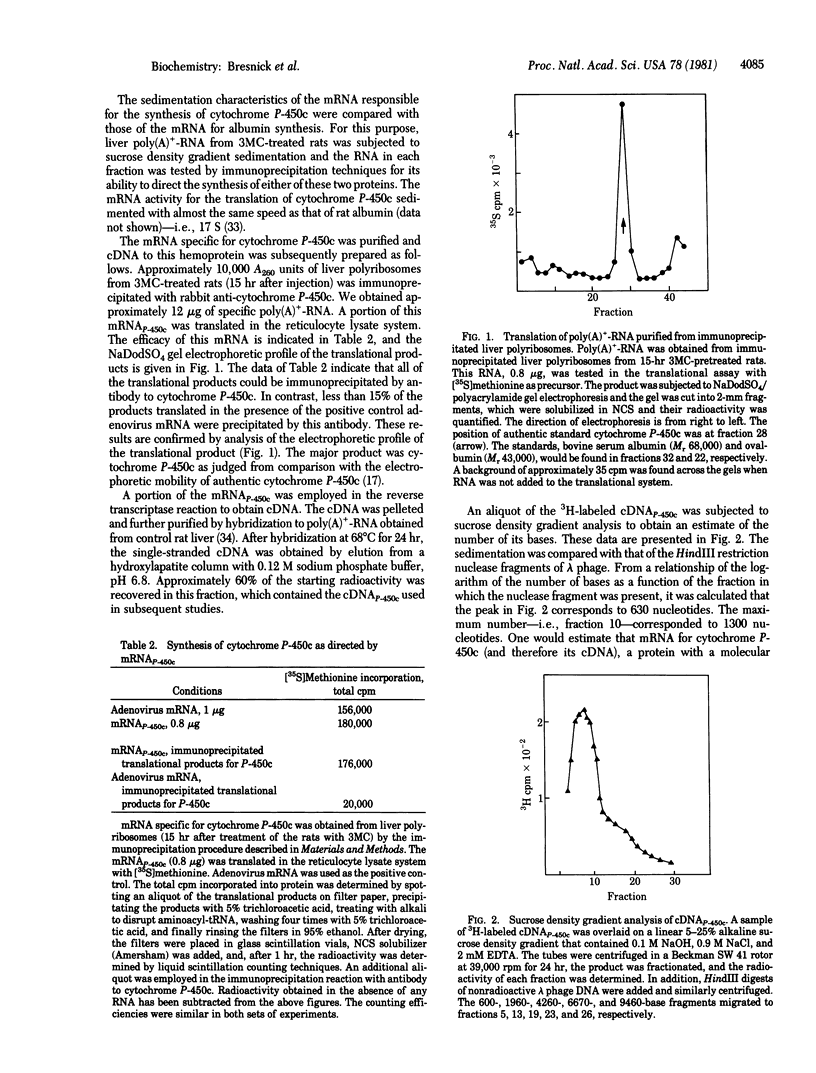
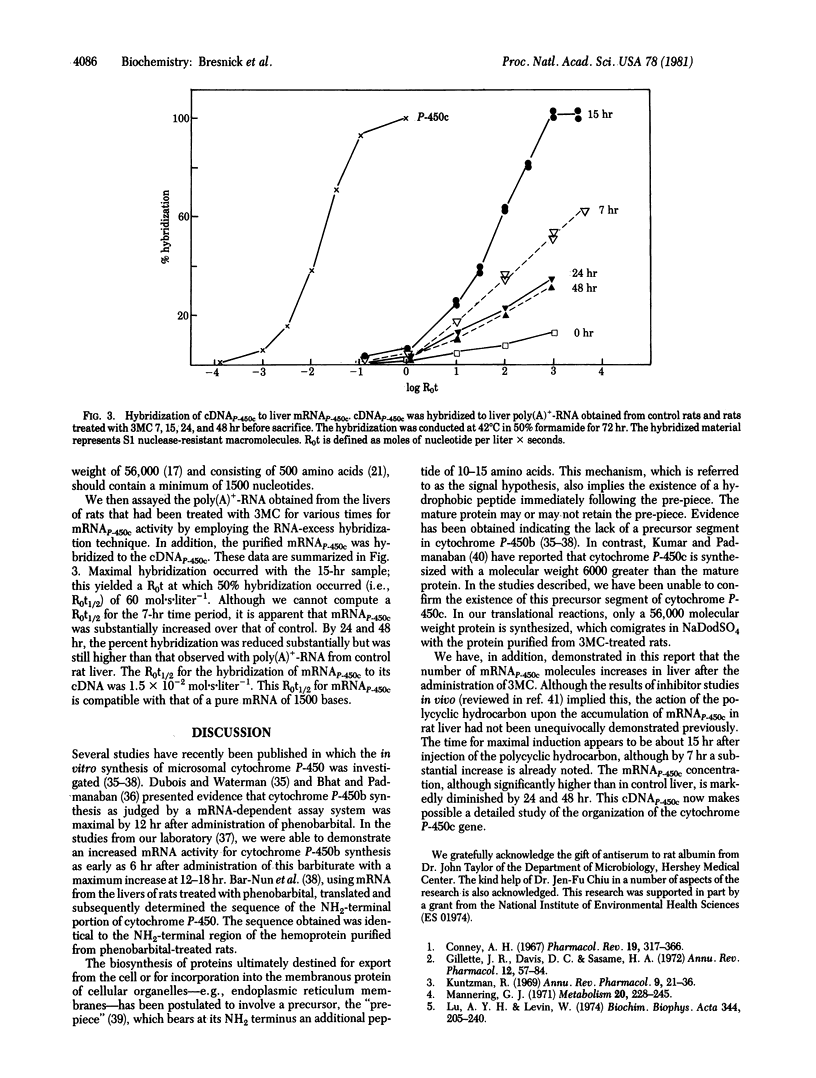
Poly(A)+-RNA obtained from the livers of 3-methylcholanthrene (3MC)-treated rats was translated into cytochrome P-450c in a cell-free reticulocyte system. In this translational system, no precursor cytochrome P-450c was observed. The mRNA responsible for the synthesis of this cytochrome was isolated by immunoprecipitation of liver polyribosomes obtained at 15 hr after 3MC treatment, and a cDNA was constructed by the reverse transcriptase reaction. The cDNA was further purified by hybridizing at a high R0t (product of RNA concentration and incubation time) to poly(A)+-RNA isolated from control rat liver, and the nonhybridized, single-stranded cDNA was isolated by hydroxylapatite chromatography. This cDNAp-450c was employed in hybridization reactions with poly(A)+-RNA isolated from the livers of rats treated with 3MC for various times. These studies indicated a maximal induction of mRNAp-450c at about 15 hr after 3MC injection, although levels of this mRNA were significantly increased by 7 hr. The mRNAp-450c concentration had diminished by 24 hr but remained higher than control levels for at least 48 hr. These studies establish an effect of 3MC upon the accumulation of mRNAp-450c in rat liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alvares A. P., Schilling G., Levin W., Kuntzman R. Studies on the induction of CO-binding pigments in liver microsomes by phenobarbital and 3-methylcholanthrene. Biochem Biophys Res Commun. 1967 Nov 30;29(4):521–526. doi: 10.1016/0006-291x(67)90515-3. [DOI] [PubMed] [Google Scholar]
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat K. S., Padmanaban G. Cytochrome P-450 synthesis in vivo and in a cell-free system from rat liver. FEBS Lett. 1978 May 15;89(2):337–340. doi: 10.1016/0014-5793(78)80250-6. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Ryan D. E., Levin W. Amino acid compositions and partial amino acid sequences of three highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital, or 3-methylcholanthrene. J Biol Chem. 1979 Jul 10;254(13):5635–5640. [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Decha-Umphai W., Commer P. alpha 1-Fetoprotein mRNA of rat yolk sac and hepatoma. Nucleic Acids Res. 1979 Sep 11;7(1):239–249. doi: 10.1093/nar/7.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert R. A., Bresnick E., Levin W., Ryan D. E., Thomas P. E. Synthesis of liver cytochrome P-450b in a cell-free protein synthesizing system. Biochem Biophys Res Commun. 1979 Dec 14;91(3):886–891. doi: 10.1016/0006-291x(79)91962-4. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Dubois R. N., Waterman M. R. Effect of phenobarbital administration to rats on the level of the in vitro synthesis of cytochrome P-450 directed by total rat liver RNA. Biochem Biophys Res Commun. 1979 Sep 12;90(1):150–157. doi: 10.1016/0006-291x(79)91602-4. [DOI] [PubMed] [Google Scholar]
- Gillette J. R., Davis D. C., Sasame H. A. Cytochrome P-450 and its role in drug metabolism. Annu Rev Pharmacol. 1972;12:57–84. doi: 10.1146/annurev.pa.12.040172.000421. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Isolation and purification of cytochrome P-450, and the existence of multiple forms. Pharmacol Ther. 1979;6(1):99–121. doi: 10.1016/0163-7258(79)90057-3. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Separation and purification of multiple forms of microsomal cytochrome P-450. Activities of different forms of cytochrome P-450 towards several compounds of environmental interest. J Biol Chem. 1977 Jun 10;252(11):3970–3979. [PubMed] [Google Scholar]
- Guengerich F. P. Separation and purification of multiple forms of microsomal cytochrome P-450. Partial characterization of three apparently homogeneous cytochromes P-450 prepared from livers of phenobarbital- and 3-methylcholanthrene-treated rats. J Biol Chem. 1978 Nov 10;253(21):7931–7939. [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Huang M. T., West S. B., Lu A. Y. Separation, purification, and properties of multiple forms of cytochrome P-450 from the liver microsomes of phenobarbital-treated mice. J Biol Chem. 1976 Aug 10;251(15):4659–4665. [PubMed] [Google Scholar]
- Johnson E. F., Muller-Eberhard U. Resolution of two forms of cytochrome P-450 from liver microsomes of rabbit treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1977 May 10;252(9):2839–2845. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Padmanaban G. Studies on the synthesis of cytochrome P-450 and cytochrome P-448 in rat liver. J Biol Chem. 1980 Jan 25;255(2):522–525. [PubMed] [Google Scholar]
- Kuntzman R. Drugs and enzyme induction. Annu Rev Pharmacol. 1969;9:21–36. doi: 10.1146/annurev.pa.09.040169.000321. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Levin W. The resolution and reconstitution of the liver microsomal hydroxylation system. Biochim Biophys Acta. 1974 Sep 16;344(2):205–240. doi: 10.1016/0304-4157(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Mannering G. J. Properties of cytochrome P-450 as affected by environmental factors: qualitative changes due to administration of polycyclic hydrocarbons. Metabolism. 1971 Feb;20(2):228–245. doi: 10.1016/0026-0495(71)90094-1. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Sullivan D., Summers N. M., Kiely M. L., Schimke R. T. Purification of ovalbumin messenger ribonucleic acid by specific immunoadsorption of ovalbumin-synthesizing polysomes and millipore partition of ribonucleic acid. J Biol Chem. 1973 Jan 25;248(2):540–548. [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Philpot R. M., Arinç E. Separation and purification of two forms of hepatic cytochrome P-450 from untreated rabbits. Mol Pharmacol. 1976 May;12(3):483–493. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Korzeniowski D., Levin W. Separation and characterization of highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital, and 3-methylcholanthrene. J Biol Chem. 1979 Feb 25;254(4):1365–1374. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Levin W. Properties of purified liver microsomal cytochrome P450 from rats treated with the polychlorinated biphenyl mixture Aroclor 1254. Mol Pharmacol. 1977 May;13(3):521–532. [PubMed] [Google Scholar]
- Ryan D., Lu A. Y., West S., Levin W. Multiple forms of cytochrome P-450 in phenobarbital- and 3-methylcholanthrene-treated rats. Separation and spectral properties. J Biol Chem. 1975 Mar 25;250(6):2157–2163. [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Sladek N. E., Mannering G. J. Evidence for a new P-450 hemoprotein in hepatic microsomes from methylcholanthrene treated rats. Biochem Biophys Res Commun. 1966 Sep 8;24(5):668–674. doi: 10.1016/0006-291x(66)90376-7. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]
- Taylor J. M., Tse T. P. Isolation of rat liver albumin messenger RNA. J Biol Chem. 1976 Dec 10;251(23):7461–7467. [PubMed] [Google Scholar]
- Thomas P. E., Korzeniowski D., Ryan D., Levin W. Preparation of monospecific antibodies against two forms of rat liver cytochrome P-450 and quantitation of these antigens in microsomes. Arch Biochem Biophys. 1979 Feb;192(2):524–532. doi: 10.1016/0003-9861(79)90122-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Lu A. Y., Ryan D., West S. B., Kawalek J., Levin W. Immunochemical evidence for six forms of rat liver cytochrome P450 obtained using antibodies against purified rat liver cytochromes P450 and P448. Mol Pharmacol. 1976 Sep;12(5):746–758. [PubMed] [Google Scholar]


