Abstract
Exercise can prevent endothelial cell (EC) dysfunction and atherosclerosis even in the absence of improvements in plasma lipids. However, the mechanisms responsible for these effects are incompletely understood. In this study we examined in mice whether an acute bout of exercise activates enzymes that could prevent EC dysfunction, such as AMP-activated protein kinase (AMPK) and endothelial nitric oxide synthase (eNOS). We also examined whether exercise alters known regulators of these enzymes. C57BL/6 mice underwent a single bout of exhaustive treadmill exercise after which their aortas were analyzed for activation of AMPK, AMPK regulatory proteins, eNOS, and various enzymes that, like AMPK, activate eNOS. We found that such exercise acutely activates both AMPK and eNOS in the whole aorta and that the magnitude of these effects correlated with both the distance run and activation of the AMPK regulatory proteins silent information regulator-1 (SIRT1)-LKB1 and CaMKKβ. In contrast, Akt, PKA, PKG, and Src, other kinases known to activate eNOS, were unaffected. Immunohistochemical analysis revealed that AMPK and eNOS were both activated in the ECs of the aorta. This study provides the first evidence that an acute bout of exercise activates AMPK and eNOS in the endothelium of the aorta. The results also suggest that AMPK likely is the principal activator of eNOS in this setting and that its own activation may be mediated by both SIRT1-LKB1 and CaMKKβ.
Keywords: adenosine 5′-monophosphate-activated protein kinase, endothelial nitric oxide synthase
the efficacy of regular exercise for the prevention and therapy of type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) is well established (46). In patients with or at risk for type 2 diabetes, it appears to act on skeletal muscle predominantly by increasing insulin sensitivity after each individual exercise bout (25, 41, 45) and to a lesser extent by enhancing physical fitness (20). With respect to ASCVD prevention, exercise-induced decreases in plasma triglycerides and blood pressure and increases in HDL cholesterol have been implicated (46). On the other hand, in both experimental animal models and humans, exercise can exert beneficial effects on both ASCVD and endothelial cell dysfunction even in the absence of overt changes in serum cholesterol and other lipids, suggesting that it could have more direct effects on the endothelium (21, 52).
In this study, we focus on two enzymes that could mediate such vascular benefit: AMP-activated protein kinase (AMPK) and endothelial nitric oxide (NO) synthase (eNOS). AMPK is a fuel and stress sensing enzyme that can be activated in cultured endothelial cells (ECs) in response to shear stress (15), oxidative stress (50), and such pharmacological agents as metformin (57), rosiglitazone (5), and the statins (48). Once activated it phosphorylates key metabolic enzymes resulting in an increase in processes that generate ATP, such as fatty acid oxidation (FAox), and a decrease in others that consume ATP, but are not acutely necessary for survival, such as fatty acid and triglyceride synthesis (17, 23, 44). Due to these effects as well as its actions on transcription factors and coactivators that modulate metabolism, it has been hypothesized that AMPK may be an ideal target for the treatment of EC dysfunction associated with metabolic disorders (42, 43). eNOS is an EC-specific enzyme that produces NO, a molecule key to the regulation of vascular tone and antithrombotic and procoagulant processes (37). A critical role for eNOS in atherogenesis has been established by studies in apoE−/− mice fed a high-fat diet, in which it was demonstrated that concurrent knockout of eNOS−/− greatly accelerated atherosclerosis (26, 30). In addition, eNOS activity is downregulated in diabetes, suggesting that it may contribute to the increased prevalence of cardiovascular disease associated with this disorder (51). Intriguingly, it has been shown that AMPK phosphorylates eNOS at Ser1177 leading to its activation in both cell free preparations (9) and cultured ECs (40).
As alluded to earlier, exercise has been shown to prevent the EC dysfunction caused by metabolic disorders, yet the signals involved in this response are ill defined. We and others have demonstrated previously that acute physical activity activates AMPK in multiple tissues including skeletal muscle, adipose tissue, and the liver of the rat (44), but whether this physiological stimulus can activate AMPK in vascular endothelium in vivo is unknown. The present study was undertaken to determine whether AMPK and eNOS are concurrently activated by an acute bout of treadmill exercise and, if so, whether such activation occurs in the endothelial and/or smooth muscle layers of the mouse aorta. Additionally, we explored the effects of exercise on known upstream regulators of AMPK including LKB1, silent information regulator-1 (SIRT1), and Ca2+/calmodulin kinase kinase-β (CAMKKβ) and on other kinases that have been shown to activate eNOS such as Akt/PKB, PKA, PKG, and Src.
METHODS AND MATERIALS
Mice.
Thirty 9- to 18-wk-old male C57BL/6 mice and one eNOS knock-out mouse were purchased from Jackson Laboratory (Bar Harbor, ME). At the time of the experiment all of the animals were of comparable mean body weights: 32.1 ± 2.5 g (means ± SD). Mice were housed under controlled temperature (22°C), humidity (40%), and light (12-h:12-h light/dark cycle) conditions in a specific pathogen-free vivarium and provided food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Performance outcomes.
Physical function was characterized by measuring running time, velocity, and work using a motorized treadmill (Columbus Instruments, Columbus, OH). In brief, all mice (n = 9 to 10 per group) were acclimated to the treadmill for 3 consecutive days for a duration of 10 min, a speed of 10 m/min, and a 10° incline. On the fourth day, animals were randomly divided into two groups: one sedentary and one to be exercised to exhaustion. The exercised group ran on the treadmill at an initial speed and incline of 10 m/min and 10° for 10 min. The speed was increased incrementally by 2 m/min every 2 min until the mice reached their maximum velocity. Empirically, we observed that like humans mice are not all able to run at the same maximum velocities. Thus we defined maximum velocity as the highest velocity at which the mice would continue to run, without falling off the treadmill and being unable to hop back on, despite continued efforts and lack of clear signs of exhaustion. The mice were then run to exhaustion (i.e., run at maximum velocity until they would not remain on the treadmill despite gentle tapping on the hindquarter for 10 s with a plastic pipette). An electric grid was not used to decrease the pain-induced catecholamine production as a confounding variable (27). Likewise, the sedentary group remained in the same room as the treadmill throughout the experiment to reduce the possibility that the treadmill noise/vibrations influenced the results. (The rationale for this exercise procedure is further detailed in the Supplemental Data.) The average run time for the group of exercised animals was 31.1 ± 9.1 min (means ± SD; n = 10). The average velocity was 14.7 ± 1.5 m/min, the average max velocity was 19.1 ± 2.9 m/min, and the average total distance run was 512 ± 196 m.
Tissue isolation.
After completing the exercise, the mice were rested for 10 min in separate cages to prevent fighting. They were then anesthetized with inhaled isoflurane (Aerrane; Baxter, Deerfield, IL) for ∼10 s (until the mouse was immobile) and immediately euthanized by cervical dislocation. The thoracic aorta, including adventitial tissues, was rapidly harvested followed by removal of the tunica adventitia (periaortic fat) with the use of a dissecting microscope, on ice. The aortas were snap-frozen in liquid nitrogen and stored at −80°C until used for Western blot analysis. The adventitia was left on the aortas intended for immunohistochemistry. For this purpose they were fixed immediately in 10% buffered formalin (Fisher, Pittsburgh, PA). The same procedures were performed on the aortas obtained from the sedentary group.
Western blotting.
The aortas were homogenized using a Con-Torque Power Unit with Kontes glass homogenization tubes in 100 μl of buffer containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Nonidet P-40, 2.5 mM NaPPi, 1 mM β-glycerophosphate, 40 mM NaF, 1 mM Na3VO4, 5 mM phenylmethylsulfonyl fluoride (PMSF), and 1 μg/ml leupeptin. The lysates were clarified by centrifugation at 14,000 rpm for 15 min at 4°C. The supernatants were transferred to chilled Eppendorf tubes and stored at −80°C for future analysis. After thawing, the protein quantity of the lysates was assessed by the bicinchoninic acid assay and 8 μg of protein per sample was loaded per lane of a NuPAGE 4–20% gradient gel (Invitrogen, Carlsbad, CA). The samples were run and then transferred to a polyvinylidene fluoride (PVDF) membrane as per manufacturer's instructions (Invitrogen). Immunoblots were carried out with the antibodies, concentrations, and conditions listed in Supplemental Table S1. All primary antibodies were diluted in a buffer consisting of 3% BSA/Tris-buffered saline-Tween (TBS-T) with 0.05% NaN3. The primary antibody solutions were incubated with the PVDFs at 4°C, rocking overnight. A donkey anti-rabbit- horseradish peroxidase (HRP) or sheep anti-mouse-HRP conjugated secondary antibody was used against the appropriate species of primary antibody. The concentrations of each secondary antibody are listed in Supplemental Table S1. The secondary antibodies, diluted in TBS-T only, were incubated for 1 h at room temperature. ECL from Pierce (Rockford, IL) was used as the HRP substrate. The blots were exposed on HyBlot CL X-ray film (Denville Scientific, Metuchen, NJ), and the resulting bands were scanned and quantified using an AGFA Studiostar scanner (Köln, Germany) and Scion Image, respectively. All data are presented as means ± SE.
Immunohistochemistry of aortas and quantitation.
The formalin-fixed aortas were paraffin embedded, cut in 5-μm slices, and mounted on slides by the Experimental Pathology Laboratory Services Core at Boston University School of Medicine. The core also performed the hematoxylin and eosin and Mason's Tri-chrome stains. For immunohistochemistry (IHC) analyses, the slides were deparafinized and hydrated through washes that took the slides from xylene, 100% ethanol, 95% ethanol, 85% ethanol, 75% ethanol, 50% ethanol, and then water. Antigens were unmasked by flash boiling the slides in 10 mM Tris (pH 10) and then cooling for 30 min. Native peroxides were quenched with 3% H2O2 (10 min). The reagents from the UltraTech HRP Streptavidin-Biotin Universal Detection System (Beckman Coulter, Fullerton, CA) were used to block, amplify, and detect the primary antibodies according to manufacturer's guidelines. All primary antibodies, at the concentrations noted in the respective figures and Supplemental Table S1, were incubated overnight at 4°C in a humidified chamber. Detection was based on secondary biotinylated antibody, followed by the addition of streptavidin-HRP ABC complex and 3,3′-diaminobenzidine. Postdetection, the slides were dehydrated and Permount (Fisher) was used to seal the coverslip. For negative controls, the primary antibody was omitted. For quantitative IHC for active phosphorylated AMPK or eNOS all the slides, including negative controls, were developed/stained simultaneously and for the same amount of time to avoid batch-to-batch variability that would preclude accurate quantitation. Postdetection/development, the slides were dehydrated and fixed with Permount. ImageJ for microscopy software was used to quantify the images by first converting them to grayscale and then inverting. The integrated pixel density of the media or intima was then taken and divided by the surface area measured. The media and intima from the negative controls (secondary antibody only) were also measured and their respective average integrated pixel density per squared micrometer (background) were subtracted out of the appropriate phospho-protein measurements. Measurements from 11–13 aortic fields were used per quantitation.
Statistics.
All data were analyzed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). The Western blot and distance analysis were analyzed by Student's t-test and Pearson's correlation, respectively. The IHC comparisons were analyzed by the Mann-Whitney test. A P value less than 0.05 was taken as statistically significant.
RESULTS
An acute bout of treadmill exercise activates AMPK and AMPK regulatory proteins in the mouse aorta.
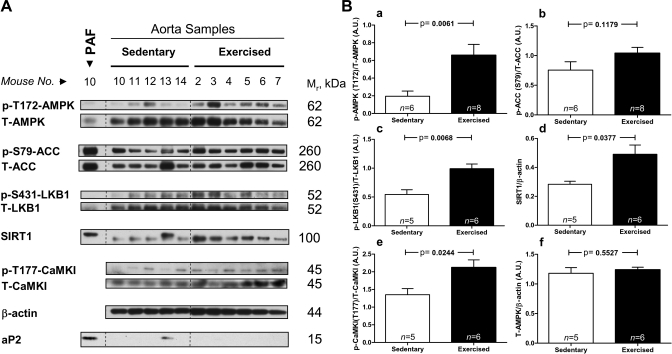
A bout of treadmill running carried out to exhaustion (mean duration of 31 min) increased AMPK (Thr172) phosphorylation (an index of its activation by upstream kinases) by 3.4-fold in the whole aorta (Fig. 1, A and Ba). The phosphorylation of the AMPK substrate, acetyl-CoA carboxylase (ACC) at Ser79, was also increased, but to a lesser degree (P = 0.12, Fig. 1Bb); a possible explanation for this will be discussed in the sections on IHC. Several AMPK regulatory proteins were concurrently activated. Thus the phosphorylation of Ser431 of the AMPK-kinase (AMPKK) LKB1, an event associated with LKB1 activation and mediated potentially by p90RSK or PKA (1), was significantly increased (Fig. 1Bc). The protein abundance of the histone/protein deacetylase SIRT1, an enzyme recently shown to deacetylate LKB1 leading to its activation, and secondarily that of AMPK (32) (Fig. 1Bd) was also increased. Taken together these findings suggest that the SIRT1-LKB1-AMPK cascade is activated by exercise. Another known AMPKK, CaMKKβ, was also activated by exercise as evidenced by a 1.6-fold increase in the phosphorylation of its substrate CaMKI at Thr177 (Fig. 1Be) (2). As an additional check of the data of Fig. 1, all of the phospho-protein data were normalized by β-actin instead of their own total levels, similar results were obtained with this method (data not shown). Finally, there was no change in total AMPK abundance due to exercise (Fig. 1Bf), an observation that could have accounted for its increased phosphorylation levels.
Fig. 1.
AMPK and AMPK regulatory proteins are activated in the aorta of mice exercised to exhaustion on a treadmill. Protein lysates from aortas of sedentary and exercised mice were analyzed by Western blot (A) and quantified in B (a–f). Due to the observation that exercise activates AMPK in fat (27), the peri-aortic fat (PAF; or tunica adventitia) that surrounds the aorta was removed before protein isolation. Thus aortic protein lysates were made only from the tunica media and intima (see Fig. 6B for definitions). To exclude the possibility that the results obtained may have come from incompletely removed PAF, an immunoblot was performed against the fat cell-specific marker aP2 and a positive control of PAF was included in the first lane. As is evident, mouse aortic sample number 13 was contaminated by PAF and was thus excluded from the analysis. SIRT1, silent information regulator-1; ACC, acetyl-CoA carboxylase; p, Phospho; T-, total; AU, arbitrary units; CamK1, Ca2+/calmodulin kinase I.
Exercise activates eNOS in the mouse aorta, an effect likely mediated by AMPK.
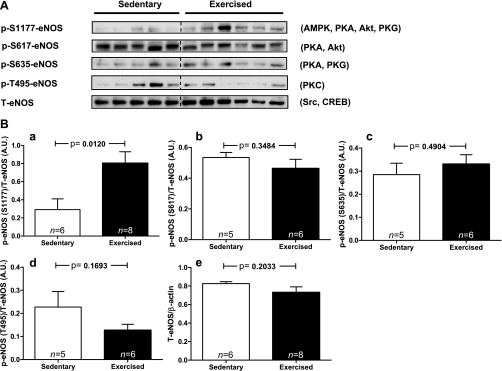
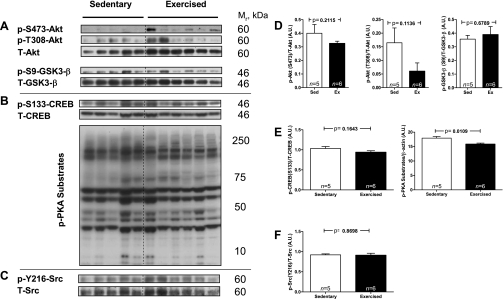
The treadmill exercise also activated eNOS as evidenced by a 2.8-fold increase in phosphorylation at its most critical activation site Ser1177 (Fig. 2, A and B). Because shear stress represents a likely modality whereby exercise would activate AMPK and eNOS in the aorta (55), kinases discovered to phosphorylate and activate eNOS in in vitro (cell culture) models of shear stress were studied. Of the four known shear stress-induced kinases (AMPK, Akt/PKB, PKA, and PKG) that phosphorylate eNOS at Ser1177, two of them also phosphorylate it at Ser617 (PKA and Akt) and two at Ser635 (PKA and PKG; Fig. 2A) (35). Although, phosphorylation at Ser1177 was increased 2.8-fold (Fig. 2Ba) after the treadmill run, phosphorylation at Ser617 (Fig. 2Bb) and Ser635 (Fig. 2Bc) was unaltered suggesting that PKA, Akt, and PKG were not activated. In keeping with this conclusion, the phosphorylation of Akt at both of its activating sites (Ser473 and Thr308) and of its substrate GSK-3β at Ser9 were not increased, nor was the phosphorylation of the PKA substrate cAMP response element-binding transcription factor (CREB; Ser133) or general PKA substrates (Figs. 3, A, B, D, and E). Without their activation, the possibility that PKG was activated by exercise is also unlikely, since the phosphorylation of eNOS at Ser635 was unaltered. Intriguingly, whereas the phosphorylation of eNOS at Ser1177 increased, the phosphorylation of Thr495, which is inhibitory, trended to decrease (Fig. 2Bd), suggesting another reason for eNOS activation. Finally, another kinase known to activate eNOS, although not by Ser1177 phosphorylation, is Src. However, immunoblots of the activating phosphorylation of Src on Y216 revealed that it was not affected by this short bout of exercise (Fig. 3, C and F). Finally, the abundance of eNOS protein (Fig. 2Be) was not altered, suggesting that the two known chronic exercise-induced pathways responsible for increasing eNOS protein (CREB and Src) (28, 47) were not sufficiently activated.
Fig. 2.
Endothelial nitric oxide synthase (eNOS) phosphorylation sites, other than Ser1177, are not significantly altered by treadmill exercise. A: Western blots of the 4 major phosphorylation sites on eNOS and total eNOS abundance (T-eNOS) are presented. The kinases currently known to phosphorylate each site (35) are listed to the right of the blots. B, a–e: densitometric quantitation of the blots in A.
Fig. 3.
Lack of evidence for treadmill exercise-induced activation of Akt, PKA, and Src. Immunoblots for activated/phosphorylated Akt and its substrate glycogen GSK-3β (A), the PKA substrate cAMP response element-binding transcription factor (CREB) and general PKA substrates (high exposure blots are shown to highlight all substrate phosphorylation levels; B), and active Src (C) were performed. The phosphorylation of PKA substrates was quantified by taking the densitometric result from each lane and normalizing it to β-actin. D–F: quantitation of the blots from A through C. Sed, sedentary; Ex, exercised.
AMPK (Thr172) phosphorylation positively correlates with eNOS (Ser1177) phosphorylation and run distance.
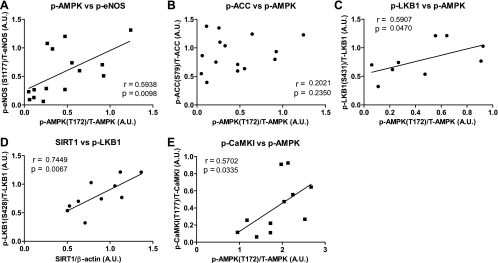
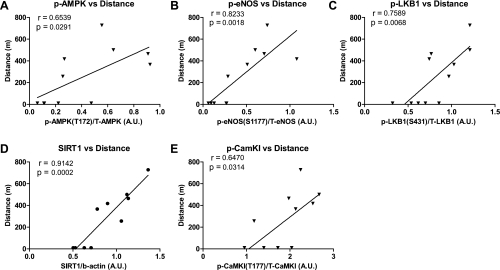
To assess more quantitatively whether a relationship exists between the activation of AMPK and eNOS, a correlation analysis was performed (Fig. 4). Phosphorylation of AMPK (Thr172) positively correlated both with the phosphorylation of its substrate eNOS (Ser1177) (Fig. 4A) and with the activation of two of its upstream kinases, LKB1 as reflected by its phosphorylation at Ser431 (Fig. 4C) and CaMKKβ, as evidenced by Thr177 phosphorylation of its substrate CaMKI (Fig. 4E). Another positive correlation was between SIRT1 abundance and LKB1 phosphorylation (Fig. 4D), suggesting that the SIRT1-LKB1-AMPK cascade may have been activated. Also of note, the distance run by each animal correlated positively with the phospho-status/activation of all of these molecules (Fig. 5), suggesting a causal relationship between these events. No significant correlation was found between the phosphorylation of AMPK and another of its substrates ACC (Fig. 4B).
Fig. 4.
Phosphorylation of AMPK (Thr172) correlates positively with the phosphorylation or abundance of AMPK regulatory proteins and with eNOS (Ser1177) phosphorylation. A–E: a correlation analysis was performed on the results presented in Figs. 1 and 2 from both sedentary and exercised mice. The correlation coefficient and P value are listed on the graph.
Fig. 5.
Phosphorylation of AMPK (Thr172) and eNOS (Ser1177), and the phosphorylation or abundance of AMPK regulatory proteins, correlates positively with distance run by the mice. To determine whether a relationship exists between the phosphorylated states or quantities of the various proteins measured in Figs. 1 and 2 to the distance run by each mouse, a correlation analysis was performed (A–E). Data from both sedentary and exercised mice are included. The correlation coefficient and P value are listed on the graph.
IHC localization of the enzymes studied in the mouse aorta.
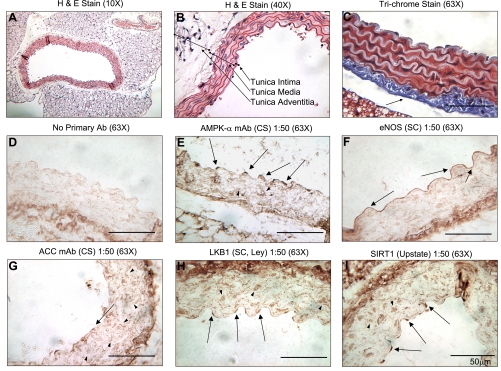
To gain a better understanding of which aortic cells contributed to the Western blot data, an IHC analysis was performed to determine the location within the aorta of the studied enzymes. IHC revealed that total AMPK-α is abundant throughout the tunica media and intima (Fig. 6, A and B) in both smooth muscle cells (SMCs) and ECs (Fig. 6E). The cellular localization is different between the two cell types in that it is both nuclear and cytoplasmic in the EC and cytoplasmic in the SMCs. This observation was confirmed with the use of a second antibody from another manufacturer (Supplemental Fig. S1). As expected, eNOS was found throughout the ECs, but not in the SMCs (Fig. 6F). The specificity of the eNOS antibody used was confirmed by performing the same IHC on an aorta from an eNOS knock-out mouse in which no staining was detectable (Supplemental Fig. S2). ACC has very low abundance in the ECs compared with the SMCs (when quantified ∼4% of the total ACC is in the ECs) and is predominantly localized to the cytoplasm of both cell types (Fig. 6G). This observation and the data from the next section may explain why the phosphorylation of ACC in the whole aorta appears to be only modestly affected by exercise, whereas AMPK phosphorylation was clearly increased (Figs. 1 and 4B). LKB1 and SIRT1 were abundant and predominantly located in the nuclei of the ECs and SMCs (Fig. 6, H and I), although LKB1 was also found in a spotty cytoplasmic pattern in the SMCs (Fig. 6H). Thus, with the exceptions of ACC, which is principally found in the SMCs, and eNOS, which is exclusively in ECs, all of the studied enzymes were abundant in both cells.
Fig. 6.
Hematoxylin and eosin (H&E), Masson's trichrome, and immunohistochemistry (IHC) of aortas from sedentary mice. Both A and B are H&E-stained aortas at different magnifications. The 3 layers of the aorta are defined in B. All Western blots were performed on protein lysates from the tunica intima [endothelial cell (EC) layer] and media [smooth muscle cell (SMC) layer] only. The aorta in C was stained with Masson's trichrome, which makes the nuclei appear black, cytosolic proteins appear red, and collagen appear blue. Note the thick layer of collagen attached to the tunica media (arrow). This collagen layer is immunoreactive with all antibodies, as is evident in D–I. D is a no primary antibody IHC control (i.e., only secondary antibody was used). AMPK-α (E), eNOS (F), ACC (G), LKB1 (H), and SIRT1 (I) were detected by IHC, respectively. Arrows point at positively stained EC, and arrowheads point at positively stained SMC. Above the IHCs, the primary antibody, company, dilution, and magnification of the micrograph are listed. CS, Cell Signaling; SC, Santa Cruz Biotechnology.
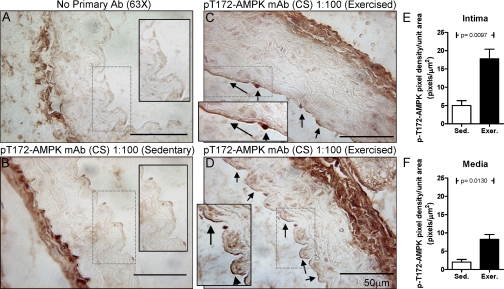
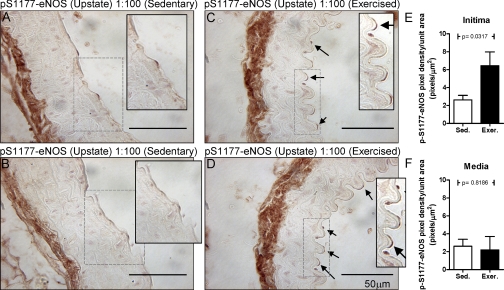
Both AMPK and eNOS are activated in the ECs of aortas from exercised mice.
IHC analysis using phospho-protein-specific antibodies for active AMPK (p-Thr172) revealed that treadmill exercise induced increases in p-AMPK staining in both ECs and SMCs (Fig. 7) with densitometric data indicating that the increase was greater in the ECs. Exercise-induced increases in eNOS phosphorylation at Ser1177 (Fig. 8) were found only in ECs. To demonstrate that the antibodies used for this analysis are specific for their phospho-targets, a set of aortic slices from exercised mice was subjected to 30 min of protein phosphatase 2A treatment before the addition of the respective primary antibodies. As seen in Supplemental Figs. S3 and S4, the ability of these antibodies to stain their phospho-targets was almost completely lost. Additionally, IHCs were carried out for total AMPK and total eNOS in aorta from sedentary versus exercised mice. No differences in the abundance of either enzyme were found (data not shown).
Fig. 7.
Active AMPK (p-Thr172) is found by IHC in the EC of the aortas of exercised mice. A: no primary antibody control, only secondary antibody was used. Phospho-AMPK was stained in the aortas of sedentary (Sed; B) and exercised (Exer; C and D) mice. Arrows point to positively staining ECs, which are principally found in the samples from the exercised mice. Above the IHCs, the primary antibody, company, dilution, and magnification of the micrograph are listed. A–D, insets: 2× magnification of the areas selected by dashed rectangles. In E and F, 13 random fields, including those in B–D, were analyzed from the aortas of sedentary and exercised mice for AMPK phosphorylation. The quantitation from the intima (E: EC layer) and media (F: SMC layer) is graphed.
Fig. 8.
Active eNOS (p-Ser1177) is found by IHC in the EC of the aortas of exercised mice. Phospho-eNOS was stained in the aortas of sedentary (A and B) and exercised (C and D) mice. Arrows point to positively staining ECs. A–D, insets: 2× magnification of the areas selected by dashed rectangles. In E and F, 11 random fields, including those in A–D, were analyzed from the aortas of sedentary and exercised mice for eNOS phosphorylation. The quantitation from the intima (E: EC layer) and media (F: SMC layer) is graphed.
DISCUSSION
We investigated whether exercise acutely activates AMPK in the mouse aorta, how it might accomplish this, and what are some of its possible ramifications. The major findings were that: 1) Treadmill running to exhaustion activated AMPK and several upstream AMPK regulatory proteins; 2) AMPK activation was associated with the activation of eNOS, whereas the activities of other enzymes known to phosphorylate eNOS at Ser1177 were not concurrently increased; and 3) Exercise-induced activation of AMPK occurred in the ECs and to a lesser extent SMCs and eNOS only in ECs.
To our knowledge, this is the first report to demonstrate the concurrent activation of AMPK, AMPK regulatory proteins, and eNOS by an acute bout of exercise in the mouse aorta (Figs. 1 and 2). Two observations strongly suggest a functional relationship between these molecules. The first is that the levels of phosphorylation/activation of AMPK (Thr172) correlated positively with the phosphorylation/activation of eNOS (Ser1177; Fig. 4A), LKB1 (Ser431; Fig. 4C), and CaMKI (Thr177; Fig. 4E). The second is that the distance (Fig. 5) and time (data not shown) each mouse ran also correlated positively with the increased phosphorylation/activation of all of these molecules. In other words, there was a positive relationship between the degree of activation of these enzymes both with each other and the amount of exercise performed. The extent to which the AMPKKs, LKB1, and CaMKKβ individually contributed to the activation of AMPK remains to be determined, as does the identity of the factor(s) that activated these AMPKKs. One likely factor is shear stress. Cultured ECs exposed to shear stress activate LKB1 very quickly (within 2 min) as measured by the LKB1 kinase activity assay (55). In addition, shear stress could activate CaMKKβ in a similar time frame by its effects on mechanoreceptors that induce intracellular calcium mobilization (3). Hypothetically, AMPK could also have been activated by a shear stress-induced increase in EC reactive oxygen species (ROS) (12, 34). Such a mechanism has been shown to activate AMPK in the ischemic heart (29) and in cultured ECs incubated with H2O2 (50). Presumably these conditions cause a decrease in ATP that increases the AMP-to-ATP ratio. As reviewed elsewhere, such increases in the AMP-to-ATP ratio increase the binding of AMP to CBS domains on the regulatory γ-subunit of AMPK, which allosterically activates AMPK and even more dramatically increases its activity by leading to increased phosphorylation of Thr172 on its catalytic α-subunit (16).
An intriguing observation made in this study was that the protein abundance of the histone/protein deacetylase SIRT1, which has recently been shown to modulate LKB1 activity (32), almost doubled within 1 h (the total time frame of the experiment). Several studies have used increases or decreases in SIRT1 protein abundance as a proxy for its activity, especially from tissues, in which direct measurement of SIRT1 activity is very challenging (24, 49). Here we found that SIRT1 protein abundance correlated positively with both LKB1 (Ser431) phosphorylation (Fig. 4D) and the distance run by the mice (Fig. 5), suggesting that it too may have played a role in the activation of AMPK. We do not know why SIRT1 protein increased in such a short period of time; however, this is not the first report of rapid changes in its protein abundance. Suwa and colleagues (49) observed significant increases in SIRT1 protein abundance after 1 h of treadmill running in rat soleus muscle, and Kemper et al. (24) reported a 50% decrease of SIRT1 in mouse liver after a 24-h fast followed by 1 h of refeeding. It has been suggested that such rapid changes in SIRT1 protein levels are due to post-translational modifications that prevent or accelerate its degradation (24, 49).
Of the four known shear stress-induced kinases (35) that have been shown to activate eNOS, we found that AMPK but not Akt, PKA, or PKG (Fig. 2Bc and 3) was activated in the same time frame in which eNOS phosphorylation at Ser1177 was increased. It has been demonstrated that Akt is unnecessary for shear-induced eNOS phosphorylation, whereas PKA is essential (4). Thus the finding that PKA was not activated under the current exercise conditions was surprising, especially because we found an increase in LKB1 phosphorylation at Ser431, which is typically PKA or P90RSK mediated. Possibly activation of PKA (and Akt) is evanescent and was no longer present at the time the aortas were sampled or it was downregulated during the rest period. Future studies in which earlier time points are studied and the rest period eliminated should address this possibility. The kinase c-Src, which is activated by chronic exercise (3 wk) and induces increases in eNOS mRNA and protein (11), was also not activated by the acute bout of exercise (Fig. 3, C and F). Thus by a process of elimination AMPK appears to be the most likely enzyme that mediates exercise-induced eNOS Ser1177 phosphorylation. When this manuscript was in preparation, Zhang and colleagues (54) reported that AMPK (Thr172) and eNOS (Ser1177) phosphorylation and activity are increased in the iliac and femoral arteries of mice after 50 min of treadmill running. The extent of phosphorylation of AMPK and eNOS was similar to that which we observed in the aorta. In contrast with our results, they found that Akt was activated by exercise; however, when they chemically completely inhibited Akt activation, eNOS phosphorylation at Ser1177 was diminished by 50%, suggesting that in addition to AMPK perhaps Akt is also important in this process. More definitive proof that AMPK and not another, possibly yet to be identified kinase is responsible for exercise-induced eNOS Ser1177 phosphorylation will require EC-specific AMPK-α1 & -α2 knock-out mice, which presently do not exist. Finally, NO itself has been implicated in the activation of AMPK in cultured endothelial and other cells possibly by its effects on Ca2+ signaling (55), peroxynitrite formation (56), or mitochondrial respiration (10). Thus a primary increase in eNOS-mediated NO release hypothetically could have contributed to the increase in AMPK phosphorylation in the aortic endothelium during exercise.
To our knowledge this is the first report to demonstrate that AMPK is activated acutely by a physiological stimulus (i.e., exercise) in the endothelium of the aorta (Fig. 7). We have previously shown that a single bout of exercise activates AMPK in skeletal muscle, liver, and adipose tissue (44), suggesting that its activation in multiple tissues is part of a general (or systemic) response to the stresses created by physical activity. We also demonstrated that eNOS (Ser1177) phosphorylation occurs in the ECs of the aorta (Fig. 8), reinforcing the likelihood that there is a functional relationship between these two molecules. Whether AMPK is responsible for eNOS activation, the fact that it is activated is important. For example, both high levels of blood glucose and free fatty acid (FFA) are found in patients with poorly controlled diabetes. Such a fuel surfeit causes EC dysfunction as manifested by diminished EC-dependent relaxation or EC apoptosis (the ultimate dysfunction), which are considered to be early events in atherogenesis (13). In this regard, AMPK has been studied by many groups since various factors that activate it, including adiponectin (38), thiazolidinediones (36), and polyphenols (53) maintain EC function and have antiatherogenic properties. Furthermore, in addition to their systemic benefits, such as glucose and lipid lowering, many of these compounds have been shown to have AMPK-mediated cytoprotective properties in cultured ECs. For example, in ECs rosiglitazone increases NO production (5) and prevents glucose-induced ROS generation (8) and metformin prevents cytokine-induced inflammation (19) and glucose-induced ROS generation (31). We have also demonstrated that activation of AMPK by the chemical activator AICAR or adenoviral overexpression of a constitutively active-AMPK-α prevents high glucose (22) and FFA (Refs. 6 and 43, preliminary data)-induced apoptosis, insulin resistance, and mitochondrial dysfunction in cultured human ECs, possibly due to its ability to increase FAox and decrease lipid synthesis and the accumulation of potentially toxic metabolites, such as diacylglycerol and ceramides (22). In addition, AMPK activation inhibits the proinflammatory effects of the FFA palmitate and such cytokines as TNF-α and IL-1 (reviewed in Ref. 14). Collectively, these data suggest that AMPK activation benefits the EC and that its activation very likely mediates, at least in part, the ability of exercise to prevent macrovascular dysfunction. Because even modest exercise can have cardiovascular benefits in humans, studies with lower intensities of exercise (e.g., 40%, 60%, and 80% VO2 max) are needed to determine over what range exercise activates AMPK in the aorta.
Finally, the results of this study could have a number of additional physiological implications. First, in vivo, tissues that are actively undergoing physical activity require increased blood flow. Such exercise-induced hyperemia likely requires signaling events in the EC that allow it to occur. The observation that AMPK, AMPK regulatory proteins, and eNOS are activated after a single bout of exercise suggests they may be a critical component of these signaling events. Second, aerobic-type exercise can cause a hypercoagulant state associated with increases in platelet count (33), plasma catecholamines, thromboxane A2, and endothelin-1 release (39). The activation of eNOS, and secondarily NO release from the endothelium, could represent a counterbalance that maintains hemostasis during such physical activity. Finally, in humans with type 2 diabetes, exercise is associated with a reduced risk of cardiovascular mortality (21) and in animals it prevents dietary fat- or cholesterol-induced atherosclerosis and endothelial inflammation (18, 52). In both species, the benefit of exercise occurs even when it does not lower serum cholesterol levels (18, 21, 52). An attractive possibility is that exercise-induced AMPK and eNOS activation in the endothelial cell, by leading to anti-inflammatory and possibly anticoagulant effects, played a pivotal role.
GRANTS
This work was supported by United States Public Health Services Grant PO1-HL-68758-01-A6, a Diabetes Complications Center grant from the Juvenile Diabetes Research Foundation (to N. B. Ruderman), a grant from the Kilo Foundation (to Y. Ido), and a postdoctoral research fellowship from Fonds de la Recherche en Santé du Québec (to M.-S. Gauthier). J. M. Cacicedo is the recipient of a fellowship from the National Institute of Diabetes and Digestive and Kidney Diseases Ruth Kirschstein T32 training grant (DK-007201).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Michelle Ganno and Mena Bakhit from the Metabolic Phenotyping Core. Portions of this study were presented in poster form at the American Diabetes Association Scientific Sessions in New Orleans, LA, June 5-9, 2009 (7).
REFERENCES
- 1. Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem 75: 137–163, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem 273: 31880–31889, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol 24: 871–877, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Boyle JG, Logan PJ, Ewart MA, Reihill JA, Ritchie SA, Connell JM, Cleland SJ, Salt IP. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J Biol Chem 283: 11210–11217, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cacicedo J, Keaney J, Jr, Ruderman N, Ido Y. Activation of AMP-activated protein kinase (AMPK) in human umbilical vein endothelial cells (HUVEC) prevents palmitate-induced increases in oxidative stress and apoptosis. Diabetes 54, Suppl 1: A480, 2005 [Google Scholar]
- 7. Cacicedo JM, Gauthier MS, LeBrasseur NK, Jasuja R, Ruderman NB, Ido Y. Acute exercise activates AMPK in the mouse aorta. Diabetes 58, Suppl 1: 1063-P: A280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol 27: 2627–2633, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Cidad P, Almeida A, Bolanos JP. Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5′-AMP-activated protein kinase. Biochem J 384: 629–636, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol 284: H1449–H1453, 2003 [DOI] [PubMed] [Google Scholar]
- 12. De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation 109: 2617–2625, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 118: 4103–4111, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J Physiol 574: 7–15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hasler CM, Rothenbacher H, Mela DJ, Kris-Etherton PM. Exercise attenuates diet-induced arteriosclerosis in the adult rat. J Nutr 117: 986–993, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55: 512–517, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care 28: 799–805, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51: 159–167, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31: 162–168, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab 10: 392–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297: E151–E156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS−/− Apoe−/− mice are ameliorated by enalapril treatment. J Clin Invest 105: 451–458, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J 403: 473–481, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation 103: 2839–2844, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta 1301: 67–75, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104: 448–454, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006 [PubMed] [Google Scholar]
- 32. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283: 27628–27635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee G, Amsterdam EA, DeMaria AN, Davis G, LaFave T, Mason DT. Chapter 8: effect of exercise on hemostatic mechanisms. In: Exercise in Cardiovascular Health and Disease, edited by Amsterdam EA, Wilmore JH, DeMaria AN. New York: Yorke Medical Books, 1977, p. 122 [Google Scholar]
- 34. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Nakaya H, Summers BD, Nicholson AC, Gotto AM, Jr, Hajjar DP, Han J. Atherosclerosis in LDLR-knockout mice is inhibited, but not reversed, by the PPARgamma ligand pioglitazone. Am J Pathol 174: 2007–2014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res 32: 1103–1108, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106: 2767–2770, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Petidis K, Douma S, Doumas M, Basagiannis I, Vogiatzis K, Zamboulis C. The interaction of vasoactive substances during exercise modulates platelet aggregation in hypertension and coronary artery disease. BMC Cardiovasc Disord 8: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun 354: 1084–1088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3: 340–351, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans 31: 202–206, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Ruderman NB, Park H, Kaushik VK, Dean D, Constant S, Prentki M, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand 178: 435–442, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29: 1433–1438, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Skerrett PJ, Horton E. Exercise and diabetes prevention: reduction in risk of coronary heart disease. In: Handbook of Exercise in Diabetes, edited by Ruderman NB, Devlin JT, Schneider SH, and K, riska A. Alexandria: American Diabetes Association, 2002, p. 155–182 [Google Scholar]
- 47. Sun D, Huang A, Koller A, Kaley G. Decreased arteriolar sensitivity to shear stress in adult rats is reversed by chronic exercise activity. Microcirculation 9: 91–97, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 114: 2655–2662, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism 57: 986–998, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs 65: 31–74, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol 95: 1194–1200, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 587: 3911–3920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 26: 1281–1287, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277: 32552–32557, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.