Abstract
In isolated myocytes, hypertrophy induced by norepinephrine is mediated via α1-adrenergic receptors (ARs) and not β-ARs. However, mice with deletions of both major cardiac α1-ARs still develop hypertrophy in response to pressure overload. Our purpose was to better define the role of β-AR subtypes in regulating cardiac hypertrophy in vivo, important given the widespread clinical use of β-AR antagonists and the likelihood that patients treated with these agents could develop conditions of further afterload stress. Mice with deletions of β1, β2, or both β1- and β2-ARs were subjected to transverse aortic constriction (TAC). After 3 wk, β1−/− showed a 21% increase in heart to body weight vs. sham controls, similar to wild type, whereas β2−/− developed exaggerated (49% increase) hypertrophy. Only when both β-ARs were ablated (β1β2−/−) was hypertrophy totally abolished. Cardiac function was preserved in all genotypes. Several known inhibitors of cardiac hypertrophy (FK506 binding protein 5, thioredoxin interacting protein, and S100A9) were upregulated in β1β2−/− compared with the other genotypes, whereas transforming growth factor-β2, a positive mediator of hypertrophy was upregulated in all genotypes except the β1β2−/−. In contrast to recent reports suggesting that angiogenesis plays a critical role in regulating cardiac hypertrophy-induced heart failure, we found no evidence that angiogenesis or its regulators (VEGF, Hif1α, and p53) play a role in compensated cardiac hypertrophy. Pressure overload hypertrophy in vivo is dependent on a coordination of signaling through both β1- and β2-ARs, mediated through several key cardiac remodeling pathways. Angiogenesis is not a prerequisite for compensated cardiac hypertrophy.
Keywords: angiogenesis, genes, G protein-coupled receptors
chronic left ventricular (lv) pressure overload is present in patients with aortic stenosis, coarctation of the aorta, and systemic hypertension, and although it is one of the leading causes of heart failure (18), moderate levels of pressure overload can be tolerated for decades. The mechanisms by which the heart adapts to pressure overload, producing either an adaptive (compensated hypertrophy) or a maladaptive (heart failure) phenotype, have been the subject of considerable investigation. One of the signaling pathways implicated in the hypertrophic response is that mediated by catecholamines acting via α- and β-adrenergic receptors (ARs; Refs. 5, 15, 18, 33). In isolated myocytes, hypertrophy induced by norepinephrine is mediated via α1-ARs; however, in vivo, mice with deletions of both of the major cardiac α1-ARs still develop hypertrophy when exposed to pressure overload (29, 30). This suggests that the other target for catecholamine stimulation, the β-ARs, must contribute to the development of cardiac hypertrophy in vivo.
There is considerable heterogeneity between β1- and β2-ARs in their regulation of adaptive and maladaptive cardiac remodeling. In vitro, β1-AR activation is predominantly cardiotoxic (55), whereas β2 activation can switch between cardiotoxic and cardioprotective signaling (56). In vivo, overexpression of β1-ARs results in an early increase in contractility, followed by hypertrophy and fibrosis with age (5, 15). Overexpression of β2-ARs also transiently increases contractility; however, as these mice age, they develop thinning of the ventricular wall and fibrosis (14, 24). Previous studies (20, 31) have produced conflicting data on the role of β-ARs in response to cardiac afterload stress.
Given the widespread clinical use of β-AR antagonists in patients who might also be subject to conditions of altered afterload, we sought to better define the role of β-AR subtypes in mediating cardiac hypertrophy. We subjected β1, β2, and β1β2-AR knockout mice to transverse aortic constriction (TAC) at a level that induces stable, compensated LV hypertrophy and not cardiac failure (53). These conditions were chosen to specifically study the separate mechanisms of hypertrophy as distinct from hypertrophy-induced heart failure. Three weeks after surgery, both wild-type (WT) and β1−/− mice showed significant hypertrophy. The β2−/− showed markedly exaggerated hypertrophy, and only when both β-ARs were absent was the hypertrophic response totally ablated.
MATERIALS AND METHODS
Animal model.
Mice with targeted deletions of β1 and β2 and both β1- and β2-ARs were generated on a congenic FVB background (8, 35, 36). Three-month-old male mice (body weight: 28–33 g) were used in all studies to reduce variability in TAC response and gene expression due to age or gender. For physiologic studies, 10 mice were used of each genotype and compared with 10 sham-operated WT controls. For gene expression studies, an additional nine mice were used in each genotype. To compare the effects of β-ARs on hypertrophy in mice from a mixed strain background, we also performed TAC in β1β2−/− mice from a mixed background (129X1/SvJ, C57BL/6J, DBA/2, and FVB/N), the strain combination that we had previously reported (35) and the one that that is commercially available through the Jackson Laboratory (Bar Harbor, ME). These mice were compared with mixed strain-identical WT mice derived from matched littermates. All protocols were approved by the Stanford Administrative Panel for Laboratory Animal Care and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Surgery and tissue sample preparation.
TAC was performed as previously described (53). Briefly, anesthesia was induced with 3% isoflurane and maintained with 1.5% isoflurane. The aortic arch was isolated by entering the extrapleural space above the second rib. The 7–0 silk suture was tightened around a 27-gauge needle placed adjacent to the aorta, which was then removed, yielding a reproducible degree of constriction. We chose a degree and duration of TAC that induce stable LV hypertrophy and not cardiac failure in both FVB and mixed strain mice (53), as we were interested in isolating the response to afterload stress from the signaling alterations that occur with the transition to heart failure. Sham-operated controls consisted of age-matched mice from each genotype that underwent an identical surgical procedure including isolation of the aortic arch, but without banding, and were studied at an identical time point post-TAC. These mice were not failed TAC mice but a totally separate control group. This rigorous use of sham controls was mandated by our previous studies (53), which have shown dramatic alterations in gene expression associated with sham surgery in the mouse, lasting for as long as 3 wk postsurgery. Three weeks after surgery, at which point FVB mice have reached a stable hypertrophic stage (28), echocardiography was performed and the animals were then killed. Hearts were quickly removed and weighed, and heart weights and body weights were recorded. Half of the heart was quickly placed in RNALater solution (Qiagen) to prevent RNA degradation. Another half was placed in 4% paraformaldehyde for quantitative histopathology.
Echocardiography.
Transthoracic echocardiography was used to evaluate pressure gradient and cardiac function using a GE Vivid 7 ultrasound platform (GE Health Care, Milwaukee, WI) equipped with both 13- and 10-MHz transducers, while the animals were under light anesthesia (avertin: 0.25 mg/g ip). Two-dimensional short-axis views of the LV were obtained for guided M-mode measurements of the LV posterior wall thickness, LV end-diastolic diameter (LVEDd), and LV end-systolic diameter (LVEDs). For each study, average measurements were made from three beats. The peak gradient across the aortic constriction was calculated from the maximal Doppler blood flow velocity by means of the modified Bernoulli equation: P = 4v2, where P is the peak pressure gradient (mmHg) and v is the maximal velocity across the constriction (m/s). The echocardiographer was blinded to genotype.
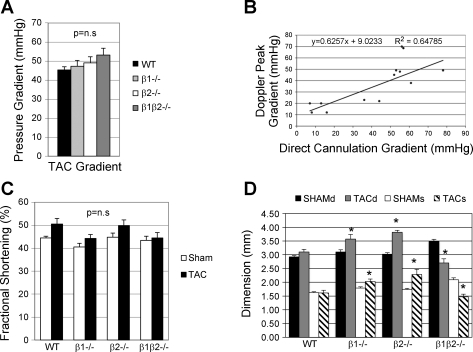
We evaluated the transaortic systolic pressure gradient in all subjects by echocardiography to eliminate the possibility that differences in banding technique could have influenced the degree of hypertrophy. Although double aortic cannulation has been regarded as the gold standard for this measurement, we (53) have previously shown that the stress of the double cannulation procedure triggers the alteration of a large set of genes when compared with nonoperated controls, even though heart samples were obtained within a time frame as short as 30 min after the initiation of anesthesia. Thus, to accurately control for gene expression changes associated solely with the hypertrophic process, we used echo alone to evaluate pressure gradients after TAC. To validate that peak gradient measured by pulsed-wave Doppler was an accurate reflection of the invasively measured pressure gradient, an additional group of TAC mice (n = 12) underwent both echo and catheterization studies at 3 wk to measure the blood pressure gradient across the band by direct cannulation of both carotid arteries. The right (upstream of the constriction) and left (downstream of the constriction) carotid arteries were cannulated with polyethylene-10 catheters connected to calibrated saline-filled Spectramed DTX Plus pressure transducers. Left and right carotid pressures were measured and recorded. None of the cannulated mice were used for any further analyses. We did not perform angle correction on pulsed Doppler measurements, given the high degree of subjectivity of these corrections. However, we demonstrated a good correlation between Doppler gradient and gradient measured by direct aortic cannulation (y = 0.63x + 9.02; r2 = 0.65) (see Fig. 3B). As well, previous data suggests that these gradients underestimate the angle-corrected gradient by ∼20 mmHg (28). Since LV fractional shortening was similar across all genotypes, differences in cardiac function could not have influenced these Doppler pressure gradients.
Fig. 3.
A: Doppler-derived pressure gradient was not different between genotypes (n = 10 in each group). B: validation of echo Doppler measurement of transaortic gradient compared with the double cannulation method in a separate group of mice with a wide variation in TAC gradients (see materials and methods for details, n = 12). C: left ventricular fractional shortening was also not different between genotypes and was unchanged between TAC and sham-operated controls, demonstrating the absence of heart failure in this model. D: left ventricular dimensions (d, diastole; s, systole) comparing sham vs. TAC for each genotype. *P < 0.05, TAC vs. sham.
Morphometric analysis.
Immersion fixed hearts were cut in half transversely and embedded in paraffin and sectioned for 0.5-μm thickness. Slides were stained with wheat-germ agglutinin and DAPI (Molecular Probes, Eugene, OR) to define myocyte boundary for morphometric analysis. After samples were imaged using fluorescent microscopy (×40), NIH image was used to trace individual myocyte cross-sectional areas within a profile margin of 60 myocytes. The average cross-sectional area of the LV free wall was then assessed for each of the three samples within each genotype (180 myocytes for each genotype). Fibrosis was assessed by examining 15 fields each from 3 different sections of the LV free wall using trichrome staining.
For counting capillary and cardiomyocyte numbers, sections were stained for CD31 with a kit (Biocare Medical, Concord CA) that includes trypsin-based antigen retrieval, rat anti-mouse CD31 primary antibody (clone MEC13.3), and diaminobenzidine detection kit. Slides were counterstained with hematoxylin and then assigned new random code numbers. Digital photomicrographs were taken with a ×20 objective capturing the inner half of the LV wall in four standard quadrants: anterior, posterior, lateral, and septal. Two independent investigators, blinded to genotype or condition, counted capillary and cardiomyocyte numbers in representative portions of each photograph. On average, 160 cells were counted per quadrant, including cardiomyocytes and CD-31-positive vessels, and the number of vessels per cardiomyocyte was calculated from these numbers. Comparisons were performed between sham and TAC mice at 2 and 3 wk after surgery, with four mice per genotype evaluated. Data from the two independent investigators were averaged.
RNA preparation and microarray analysis.
LV myocardium from nine TAC and nine sham mice of each genotype were divided into three independent pools (3 mice per pool). Six cDNA arrays, which represent total of 18 hearts, were analyzed for each genotype. Previous studies (19, 47) on the effects of pooling samples in microarray experiments validate this technique when tissue samples are small. An alternative method, using gene amplification, has the potential for introducing significant additional variability in the data (49), and thus we elected to use pooled samples. Total RNA was isolated from 45–60 mg heart tissue using the RNeasy mini kit from Qiagen and quantified via spectrophotometry. A universal mouse reference RNA was prepared from whole embryonic day 17.5 mouse embryos and used for all hybridizations so that all arrays could be compared with each other and with historical data from our laboratory. Three biological replicate hybridizations, using pools of 30-μg input RNA labeled with Cy3 (heart RNA) and Cy5 (30 μg common reference), were performed as previously described (47).
The Mouse transcriptome microarray used in this study was developed in conjunction with the Stanford Functional Genomics Facility (http://microarray.org). The array is composed of 43,200 mouse cDNA probes representing ∼25,000 unique genes and expressed sequence tags (44). It is composed of the National Institutes of Aging 15K developmental gene set, the Riken 22K gene set, and ∼5,000 other unique clones chosen for their biological interest. Microarrays were scanned using an Agilent G2565AA scanner, and features were extracted using SpotReader software (Niles Scientific). Microarray data was analyzed using Significance Analysis of Microarrays (SAM; Ref. 45), Database for Annotation, Visualization, and Integrated Discovery (DAVID; Refs. 12, 17), Hi-Throughput GOMiner, and TIGR TM4 software (39). Array hybridization parameters, and validation are as discussed previously (46) and on the Stanford Functional Genomics Facility website at http://www.microarray.org/sfgf/. Microarray data have been submitted to the NCBI Gene Expression Omnibus (GEO) database in MIAME format.
Confirmation of microarray data using quantitative RT-PCR.
Validation of altered gene expression was performed using SYBR Green quantitative real-time PCR (QRT-PCR) using the same total RNA used for the microarray analyses. Four genes previously well described as up- or downregulated by the hypertrophic process were selected for QRT-PCR: atrial natriuretic peptide (ANP), α-skeletal muscle actin, sarcoplasmic reticulum calcium ATPase (SERCA-2), and calcium/calmodulin-dependent serine protein kinase (CaM kinase). Three additional genes that showed differential expression in β1β2−/− mice vs. (WT, β1−/−, and β2−/−) after TAC were also validated with QRT-PCR: FK506 binding protein 5 (Fkbp5), thioredoxin interacting protein (Txnip), and transforming growth factor-β2 (TGF-β2). The QRT-PCR reaction was performed in 384-well plates using QuantiTect SYBR Green RT PCR kit (Qiagen). Assays were measured in an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Each sample was run in triplicate and averaged for final RNA quantitation. The results were compared with microarray data by Student's t-test.
Angiogenic factor assays.
For these studies, heart tissues were harvested and homogenized in lysis buffer and tissue lysates (30 μg) were separated by SDS-PAGE (n = 4 for each genotype). Proteins were transferred onto nitrocellulose membrane (BioTrace NT; Pall, Dreieich, Germany). Blots were sequentially probed with the primary antibody: anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, California), and anti-phosphorylated p53 antibody (Santa Cruz Biotechnology). Nuclear extracts (20 μg) were used for detecting the expression of Hif-1α. Nuclei were pelleted by centrifugation for 10 min at 12,000 g, resuspended, and rotated for 30 min in the cold room. The suspension was centrifuged for 30 min at 4°C at 20,000 g. Anti-Hif-1α antibody and positive control (nuclear extracts from cobalt chloride treated COS-7 cells) were from Novus Biologicals (Littleton, CO). Secondary antibodies were then detected by LumiGLO chemiluminescence and then quantified using a Bio-Rad Imaging Densitometer GS 710 (Bio-Rad, Hercules, CA). Specific protein expression was normalized to GAPDH expression for all experiments.
Statistical analysis.
All data are expressed as means ± SE. Statistical analyses were performed with commercially available software (StatView version 5.0, SAS Institute, NC) except for the microarray data for which custom software was used, as mentioned above. Data for sham-operated and TAC animals within the same genotype were compared using an unpaired Student's t-test. Statistical comparisons of different genotypes and treatments were performed by a two-way ANOVA with post hoc testing by Fisher's protected least significant difference test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Hypertrophic growth is increased in β2−/− and attenuated in β1/β2−/−.
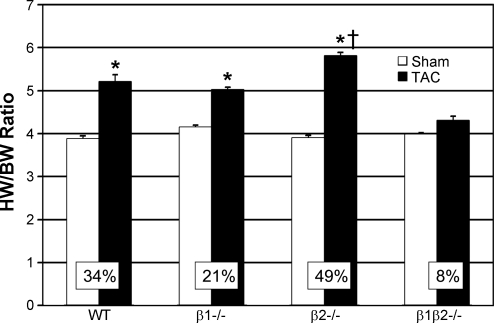
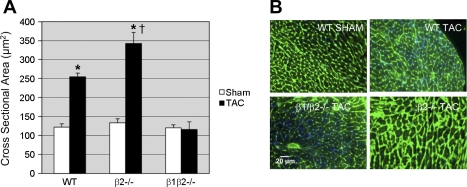
After 3 wk of TAC, heart mass increased for all groups except the β1β2−/− (Fig. 1). WT showed a 34% increase in heart weight to body weight ratio (HW/BW) vs. sham-operated controls (TAC 5.20 ± 0.57; sham 3.87 ± 0.24; P < 0.001). β1−/− TAC showed a 21% increase in HW/BW vs. β1−/− sham (5.02 ± 0.72 vs. 4.14 ± 0.32; P < 0.01), not significantly different than WT. β2−/− mice showed an exaggerated (49%) hypertrophic response after TAC vs. β2−/− sham (5.81 ± 0.53 vs. 3.90 ± 0.19; P < 0.001). In contrast, β1β2−/− TAC did not develop hypertrophy (4.30 ± 0.31 vs. 3.98 ± 0.32; P = NS). Lung weight to body weight and liver weight to body weight were unchanged for all TAC groups compared with sham (data not shown), an indication that our model was one of compensated hypertrophy rather than hypertrophy-induced heart failure. Morphometric analysis confirmed the results of HW/BW data, showing no increase in cardiomyocyte cross-sectional area in β1β2−/− TAC compared with β1β2−/− sham and a greater increase in myocyte cross-sectional area in β2−/− TAC vs. β2−/− WT (Fig. 2). There was no histologic evidence of fibrosis in any genotype as assessed by trichrome staining. Even after 12 wk of TAC, WT FVB mice did not show signs of heart failure and β1β2−/− mice did not show signs of heart failure or hypertrophy.
Fig. 1.
Heart weight-to-body weight ratios (HW/BW) comparing transverse aortic constriction (TAC) with sham-operated controls for each genotype (n = 10 in each group). The β1−/− mice showed no significant difference in the degree of hypertrophy [NS vs. wild type (WT)]; in contrast, β2−/− mice showed exaggerated hypertrophy (P < 0.05 vs. WT). Only in the absence of both β-adrenergic receptor (AR) subtypes was hypertrophy ablated. *P < 0.05 vs. sham. †P < 0.05 vs. WT TAC.
Fig. 2.
A: myocyte cross-sectional area in TAC vs. sham-operated controls for WT, β2−/−, and β1β2−/− mice (n = 10 in each group). *P < 0.05 vs. sham. †P < 0.01 vs. WT TAC. B: micrographs comparing WT sham with TAC in WT, β2−/−, and β1β2−/− mice.
Physiology.
Since the magnitude of resulting afterload elevation is dependent on severity of the aortic constriction (38), we evaluated the pressure gradient in all mice by echocardiography. Peak pressure gradients were similar in all genotypes (Fig. 3A), ranging from 45 to 53 mmHg. There was no difference in LV fractional shortening between TAC and sham for all genotypes, confirming the absence of heart failure (Fig. 3C). However, there were some differences in LV dimensions: LVEDd and LVEDs were greater in the β1−/− and β2−/− after TAC compared with sham; in contrast, in the β1β2−/− LVIDd and LVEDs were both slightly decreased compared with sham (Fig. 3D).
Strain background and hypertrophic response in β1β2−/−.
To evaluate possible strain effect on the development of cardiac hypertrophy, we performed TAC in β1β2−/− from the original mixed background (C57Bl/6, DBA/2, 129/Sv, and BALB-C) originally reported from our laboratory, the strain that is commercially available (Adrb1/Adrb2 null mice; Jackson Laboratory). Comparing TAC vs. sham β1β2−/− on the mixed background and β1β2−/− vs. WT on a similar strain background (derived from the original compound heterozygote crosses), the absence of both β-ARs still markedly attenuated the hypertrophic response (62% increase in HW/BW in WT vs. 21% increase in β1β2−/−), although it did not totally ablate it.
Role of angiogenesis in compensated cardiac hypertrophy.
In a similar TAC model, Sano et al. (40) described an important role for angiogenesis, mediated by an early increase in Hif-1α promoting activation of VEGF. During the transition from hypertrophy to failure, upregulation of p53 inhibited Hif-1α contributing to functional decompensation (40). Our models allowed us to examine the role of angiogenesis in three unique settings: compensated hypertrophy without heart failure (WT), increased transmural pressure without hypertrophic adaptation (β1β2−/−), and in the presence of exaggerated hypertrophy (β2−/−) (13, 34, 40).
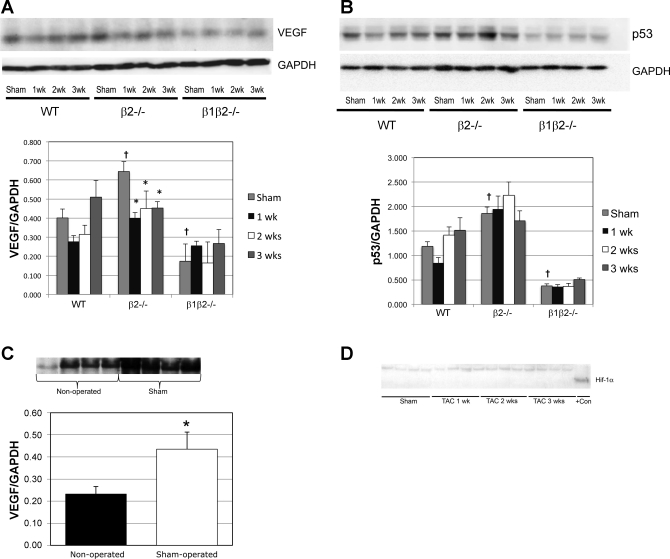
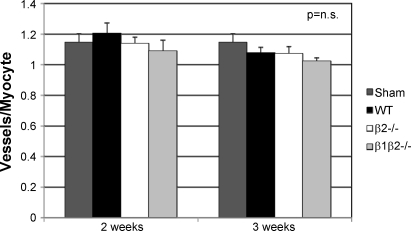
At baseline (sham), VEGF was decreased in β1β2−/− and increased in β2−/− vs. WT (P < 0.005; Fig. 4A). In contrast to Sano et al. (40), we found no increase in VEGF expression in WT, β1β2−/−, or β2−/− at any time point after TAC. We did, however, find a 70% increase in VEGF (P < 0.05) in sham above nonoperated controls (Fig. 4C), emphasizing the importance of using sham-operated controls (53). Hif-1α, which is unstable in normoxic myocytes, was undetectable in any of the genotypes at any time point, although was easily detected in a positive control, suggesting that myocyte oxygenation was not impaired in any genotype (Fig. 4D). Similar to VEGF, there were differences in p53 at baseline but no increases in p53 at any time after TAC (Fig. 4B). In concordance with protein expression, there were also no changes in gene expression of p53, Hif-1α, or VEGF. In further support of these findings, the capillary-to-cardiomyocyte ratio, measured by two observers blinded to experimental condition, was not increased after TAC in WT mice or in any of the knockouts at any time point (Fig. 5).
Fig. 4.
A: VEGF expression in sham-operated controls compared with TAC after 1, 2, and 3 wk (n = 7 in each group). There were no differences in VEGF expression in any of the genotypes at any time point after TAC compared with sham controls. Baseline, i.e., sham-operated, VEGF expression was increased in the β2−/− (†P < 0.005) and decreased in the β1β2−/− (†P < 0.005) compared with WT. VEGF was did not change in WT or β1β2−/− after TAC but fell in the β2−/− (*P < 0.05). B: p53 expression was unchanged with TAC in each genotype (n = 7 in each group). However, similar to VEGF, p53 expression was increased in the sham-operated β2−/− (†P < 0.005) and decreased in the sham-operated β1β2−/− (†P < 0.005) compared with WT. C: sham operation significantly increased VEGF expression at 2 wk compared with nonoperated controls (n = 4 in each group) demonstrating the importance of using sham controls at all time points after TAC (*P < 0.05). D: Hif-1α expression was not increased after TAC at any time point (n = 4 in each group). +Con is positive control (nuclear extract from cobalt chloride-treated COS-7 cells).
Fig. 5.
Vessel-to-myocyte ratios, measured by 2 independent observers blinded to experimental condition, were unchanged at both 2 and 3 wk after TAC in WT, β2−/−, and β1β2−/− mice (n = 4 in each group).
Genome-wide screen reveals β-AR regulation of pathways of hypertrophic remodeling.
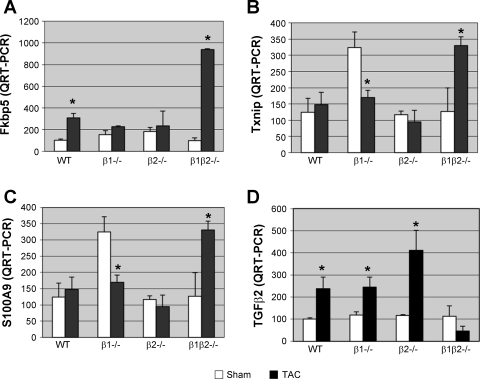
To elucidate the molecular mechanisms underlying β-AR regulation of hypertrophy, we performed a genome-wide expression analysis on TAC and sham after 3 wk. Given the absence of hypertrophy in the β1β2−/− vs. the other genotypes, a comparison was performed of β1β2−/− vs. combination (WT, β1−/−, and β2−/−). With the use of a P value of <0.01, there were 92 transcripts differentially upregulated in the β1β2−/−, each of which represents a candidate for suppression of cardiac hypertrophy (Table 1A). Among these are several high probability candidates based on prior evidence linking them to hypertrophic remodeling; all were confirmed by QRT-PCR. One is Fkbp5 (Fig. 6A), an inhibitor of calcineurin phosphatase (4). Upregulation of Fkbp5 may attenuate cardiac hypertrophy through inhibition of calcineurin (27). A second candidate is Txnip (Fig. 6B). Overexpression of Txnip inhibits hypertrophy in response to pressure overload (52). A third candidate is S100 calcium binding protein A9/calgranulin B (S100A9; Fig. 6C), a secreted protein that acts as a strong inflammatory chemoattractant for monocytes and neutrophils (26). Another member of the calgranulin family, S100A4, is upregulated in both hypertrophy and in the border zone after myocardial infarction (41). With the use of significance analysis of microarrays to compare β1β2−/− TAC vs. WT TAC, 58 genes were upregulated in β1β2−/− vs. WT. The most highly represented gene ontology categories included “metabolic process” with 25 genes, “localization” with 11 genes, “transport” with 9 genes, and “cell development” and “cell differentiation” each with 8 genes.
Table 1.
List of differentially upregulated genes after 3 wk of TAC
| Gene Description | Gene Symbol | Fold Up |
|---|---|---|
| A. Upregulated Genes in β1/β2−/− but not in WT, β1−/−, or β2−/− | ||
| S100 calcium binding protein A9 (calgranulin B) | S100a9 | 4.50 |
| FK506 binding protein 5 | Fkbp5 | 3.17 |
| Metallothionein 2 | Mt2 | 3.06 |
| Tissue inhibitor of metalloproteinase 3 | Timp3 | 2.66 |
| Myb-like, SWIRM, and MPN domains 1 | Mysm1 | 2.56 |
| Stomatin | Epb4 | 2.46 |
| Eukaryotic elongation factor-2 kinase | Eef2k | 2.44 |
| Thioredoxin interacting protein | Txnip | 2.41 |
| Mbt domain containing 1 | Mbtd1 | 2.35 |
| Keratin complex 1, acidic, gene 13 | Krt1-13 | 2.31 |
| Myosin, light polypeptide 2, regulatory, cardiac, slow | Myl2 | 2.28 |
| Flavin containing monooxygenase 2 | Fmo2 | 2.18 |
| Fumarylacetoacetate hydrolase | Fah | 2.18 |
| Zinc finger protein 410 | Zfp410 | 2.13 |
| Homeo box C4 | Hoxc4 | 2.07 |
| Nipped-B homolog (Drosophila) | Nipbl | 2.04 |
| Adipose differentiation related protein | Adfp | 2.01 |
| B. Upregulated Genes in All Other Genotypes (WT, β1−/−, and β2−/−) but not in β1/β2−/− | ||
| Procollagen, type VI, alpha 1 | Col6a1 | 3.08 |
| Transforming growth factor, beta 2 | TGFβ2 | 2.76 |
| 3-hydroxybutyrate dehydrogenase, type 1 | Bdh1 | 2.71 |
| Karyopherin (importin) alpha 2 | Kpna2 | 2.69 |
| CCR4 carbon catabolite repression 4-like | Ccrn4l | 2.54 |
| Phenylalanine hydroxylase | Pah | 2.53 |
| Dynein light chain LC8-type 1 | Dynll1 | 2.42 |
| Lumican | Lum | 2.41 |
| Chemokine (C-X-C motif) ligand 12 | Cxcl12 | 2.39 |
| CDNA sequence BC031181 | BC031181 | 2.35 |
| GTPase, IMAP family member 4 | Gimap4 | 2.31 |
| Tubulin, alpha 4 | Tuba4 | 2.29 |
| Anterior pharynx defective 1a homolog (C. elegans) | Aph1a | 2.28 |
| Dynein light chain LC8-type 1 | Dynll1 | 2.28 |
| Cyclin D2 | Ccnd2 | 2.27 |
| Splicing factor, arginine/serine-rich 3 (SRp20) | Sfrs3 | 2.26 |
| Solute carrier family 33 (acetyl-CoA transporter) | Slc33a1 | 2.20 |
| Apolipoprotein C-II | Apoc2 | 2.17 |
| Jun oncogene | Jun | 2.11 |
| Tubulin, alpha 6 | Tuba6 | 2.06 |
| CAP, adenylate cyclase-associated protein 1 (yeast) | Cap1 | 2.05 |
| Cysteine-rich protein 1 (intestinal) | Crip1 | 2.02 |
Genes are ranked by fold change (only showing those with fold change >2.0; P < 0.01). TAC, transverse aortic constriction.
Fig. 6.
Expression of known regulators of cardiac hypertrophy after TAC in each genotype. Each candidate gene was initially identified as significantly differentially regulated by gene microarray analysis. Data shown are results of confirmatory quantitative (Q)RT-PCR (n = 9 in each group). Transcripts which were differentially increased with TAC in β1β2−/− vs. all other genotypes include FK506 binding protein 5 (Fkbp5; A), thioredoxin interacting protein (Txnip; B), and S100A9 (C). In contrast, transforming growth factor-β2 (TGF-β2; D) expression was increased in all genotypes after TAC except for the β1β2−/−. *P < 0.05 vs. sham.
Comparing β1β2−/− TAC vs. β1β2−/− sham, there were only 2 genes upregulated with TAC and 70 downregulated, dramatically less than reported for WT (53), of which 31 represented gene ontology categories for metabolic pathway genes. Similarly, KEGG pathway analysis showed 14 genes associated with oxidative phosphorylation, representing 28% of regulated genes, and 2 genes associated with glycolysis/gluconeogenesis. There were 110 transcripts differentially upregulated in all other genotypes but not the β1β2−/− (Table 1B), most notably TGF-β2 (Fig. 6D), confirmed by RT-PCR. TGF-β signaling has been shown to play a role in regulating hypertrophy (3). Another differentially regulated transcript is karyopherin alpha 2 (Kpna2), a component of the nuclear localization signal of the nuclear protein import receptor, which transports Ca2+/calmodulin-dependent protein kinase IV (CaMKIV) into the nucleus (21). CaM kinase signaling has been implicated in β-AR-mediated remodeling and apoptosis (55). SAM analysis did not identify any additional genes upregulated in the WT but not in the β1β2−/−.
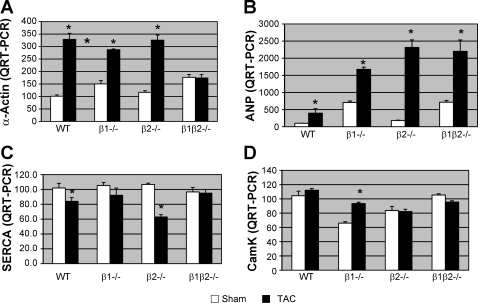
Four genes that have been well characterized in pathologic hypertrophy were also measured by QRT-PCR: α-actin, ANP, CaM kinase, and SERCA-2. After 3 wk TAC, α-actin was increased in all genotypes except the β1/β2−/− (Fig. 7A). ANP was increased in all groups, although the greatest increase was in the β2−/− (13-fold increase over sham). SERCA was decreased in both WT and β2−/− but not in the β1−/− or β1β2−/− and CaM Kinase was increased only in the β1−/−.
Fig. 7.
Expression of hypertrophy genes after TAC in each genotype by QRT-PCR (n = 9 in each group). A: expression of α-skeletal actin was significantly increased in all TAC groups except for the β1/β2−/−. B: atrial natriuretic peptide (ANP) expression was increased in all TAC groups, although the greatest increase was in the excessively hypertrophic β2−/− (13-fold increase over sham). C: sarcoplasmic reticulum calcium ATPase (SERCA) decreased in both WT and β2−/− but was unchanged in the β1−/− and β1β2−/−. D: calcium/calmodulin-dependent serine protein kinase (CaM kinase) was increased significantly only in the β1−/−. *P < 0.05 vs. sham.
To elucidate the mechanisms by which β2-AR signaling may serve to limit the extent of cardiac hypertrophy, we compared β2−/− vs. WT TAC (Table 2). At a P value <0.01, there were 49 transcripts differentially upregulated in the β2−/−, including calcineurin B type I (Ppp3r1), which has been shown to mediate the hypertrophic response of cardiomyocytes to Ca2+ (27) and could be one mechanism for the exaggerated hypertrophic response in the β2−/−. Among 418 differentially downregulated transcripts in the β2−/− vs. WT, there were several apoptosis-related genes, including peptidyl-tRNA hydrolase 2 (Ptrh2), a BCL2 inhibitor of transcription that induces cell death with characteristics of caspase-independent apoptosis (10).
Table 2.
List of upregulated genes in β2−/− vs. WT after 3 wk of TAC
| Gene Description | Gene Symbol | Fold Change |
|---|---|---|
| Protein phosphatase 3 regulatory subunit B, alpha | Ppp3r1 | 10.9 |
| Dickkopf 3 | Dkk3 | 7.9 |
| Myosin, light ploypeptide 4 | Myl4 | 5.6 |
| Leukotriene A4 hydrolase | Lta4 h | 5.4 |
| Mesothelin | Msln | 4.4 |
| Strawberry notch homolog 2 | Stno | 3.8 |
| Elastin | Eln | 3.6 |
| Heat shock protein 110 | Hsp110 | 3.5 |
| StAR-related lipid transfer (START) domain | Stard10 | 3.4 |
| Cadherin 1, type 1, E-cadherin | Cdh1 | 2.9 |
| Solute carrier family 4, anion exchanger, member 1 | Slc4a1 | 2.6 |
| BAI1-associated protein 2 | Baiap2 | 2.5 |
| Lectin, galactose binding, soluble 7 | Lgals7 | 2.2 |
Genes are ranked by fold change.
DISCUSSION
Coordinated signaling through both β-AR subtypes is required for a normal hypertrophic response to pressure overload. Normal levels of β2-AR signaling appear to limit the hypertrophic response and put a brake on potentially deleterious remodeling. In support of this hypothesis, previous studies (14) using transgenic mice overexpressing the β2-AR show more severe cardiac dysfunction and increased fibrosis when subjected to TAC. In contrast, only β1/β2 double knockout mice fail to develop pressure overload hypertrophy, suggesting that both β-ARs are required for a normal hypertrophic response in vivo. Our conclusions are supported by both gross and microscopic data with careful controls for genetic strain, sham operation, and the degree of afterload stress.
Since wall thickness did not increase in β1/β2−/− and chamber size was unchanged, wall stress was highest in these mice, yet cardiac function was maintained despite failure to adapt to the increased wall stress with hypertrophy. These data suggest that hypertrophy is not a requirement per se for the maintenance of normal systolic function during moderate degrees of afterload stress.
Our results, using knockout mice, can be placed into context with pharmacologic studies using β1- or β2-specific antagonists to evaluate the roles of these receptors in cardiac remodeling, although most of these studies examine hypertrophic remodeling after myocardial ischemia. In studies using TAC, the β1-antagonist celiprolol (23) or the nonspecific β-antagonists propranolol (32) and carvedilol (51) were found to attenuate, but not eliminate, hypertrophy, the latter by suppression of calcineurin/NFAT signaling. Ahmet et al. showed that the β2-agonists fenoterol and zinterol had better efficacy in reducing infarct size and preserving cardiac function after myocardial infarction than the β1-antagonist metoprolol (1) and that a combination of a β2-agonist with a β1-antagonist was the most beneficial (2).
Our results for the β1/β2 double knockout are similar to those of Kiriazis et al. (20) and extend their results to studies of single receptor knockouts. Furthermore, one of the potential concerns in Kiriazis' study, the substantial differences in body mass and HW/BW between the β1β2−/− and WT controls, was not a factor in our study and may reflect differences in strain background. Because of this difference, Kiriazis et al. used slightly different surgical technique in the knockouts compared with WT controls that could call into question their results. This was not an issue in our study and we confirmed similar transaortic gradients in all of our subjects. Similar to Kiriazis et al. we also found elevated expression of α-actin and ANP at baseline in the β1β2−/−. In contrast, our results differ significantly from those of Palazzesi et al. (31), who concluded that neither β1- nor β2-ARs are necessary for pressure overload hypertrophy. There are several possible reasons for this discrepancy. First, their study used mixed strain β1β2 mice (129X1/SvJ, C57BL/6J, DBA/2, and FVB/N), which are the only β1/β2−/− mice available commercially. Thus it would not have been possible for this group to use strain-matched controls, since mice with the exact mix of these four strains are not commercially available. We generated congenic double knockout FVB mice and used WT littermates from the original heterozygote crosses as controls. Furthermore, to address whether our results were unique to the FVB background, we repeated TAC on the mixed strain background but were able to use littermate WT mice generated from the original compound heterozygote crosses as controls. We did confirm that absence of both β-ARs dramatically attenuated the hypertrophic response, although, in contrast to the FVB strain, it was not totally ablated, suggesting the role of strain-specific modifiers. Second, in the study by Palazzesi only three mice in each group had their aortic gradient measured so there is no way to know whether these gradients were identical in the mice in which cardiac hypertrophy was assessed. In our experience with the TAC procedure, gradients can vary considerably, resulting in the potential for different degrees of afterload stress. We measured both TAC gradient and cardiac output in every one of our subjects.
In a similar TAC model, Sano et al. (40) described an important role for angiogenesis in hypertrophic adaptation, mediated by an early increase in Hif-1α activating VEGF and later upregulation of p53 contributing to functional decompensation. Our models allowed us to examine the role of angiogenesis in three unique settings: compensated hypertrophy without heart failure (WT), increased transmural pressure without hypertrophy (β1β2−/−), and exaggerated hypertrophy (β2−/−) (13, 34, 40). In contrast to Sano, we found no role for angiogenic regulators in mediating compensated pressure overload hypertrophy. We did find a 70% increase in VEGF (P < 0.05) in shams compared with nonoperated controls (Fig. 4C), emphasizing the importance of using sham-operated controls at all time points (53). Increased capillary-to-cardiomyocyte ratio was not present after TAC in WT or any of the knockouts at any time point (Fig. 5).
These data differ dramatically from that of Sano, failing to show a role for neovascularization after TAC. However, our models are quite different. Our data suggest that angiogenesis does not play a role in stable, compensated cardiac hypertrophy and may only be a factor when afterload stress is significant enough to induce cardiac failure. In humans with increased afterload due to hypertension or structural heart disease, it would be unusual for the heart to suddenly experience a sustained increase in afterload of the degree required to induce heart failure in mice (∼70% increase in intracavitary pressure). Of note, in the β1β2−/− mice, where transmural wall stress (11) would be highest, expression of angiogenic regulators and capillary density were not increased, yet these mice did not develop heart failure. Even with the exaggerated hypertrophy of the β2−/−, where oxygen diffusion distance would be greatest, angiogenesis did not play a role in compensated adaptation to pressure overload. Another potential difference between our study and that of Sano et al. (40) is the strain used. We have found that FVB mice are particularly resistant to heart failure after TAC (even after greater degrees of aortic constriction and for periods up to 6 mo) whereas C57 mice readily transition from hypertrophy to heart failure within the first week or two after mild-moderate aortic constriction. FVB mice are similarly resistant to heart failure in other models, e.g., TNF-α overexpression (42).
To elucidate molecular mechanisms underlying β-AR regulation of hypertrophy, we performed a genome-wide expression analysis on TAC and sham-operated mice. Several genes that have previously been implicated in the regulation of cell growth were differentially expressed in the β1β2−/− heart after TAC compared with the other genotypes. Those that were differentially upregulated in the β1β2−/− represent candidate suppressors of cardiac hypertrophy including Fkbp5 (3.2-fold up; Fig. 6A) an inhibitor of calcineurin (48). Sussman et al. (43) showed that FK506 attenuates pressure overload hypertrophy. Another potential candidate is Txnip, an inhibitor of thioredoxin (22). Yoshioka et al. (52) demonstrated that hypertrophy in response to pressure-overload was inhibited by overexpression of Txnip. However, in a contrasting report by Yamamoto et al. (50) overexpression of dominant negative thioredoxin enhanced pressure overload-induced cardiac hypertrophy. Our data support the model suggested by Yoshioka et al. (52) that Txnip, via inhibition of thioredoxin, suppresses cardiac hypertrophy.
A third potential candidate is S100A9, a member of the S100 family of EF-hand calcium binding proteins, implicated in cell growth mediated through NF-κB (16) Another member of the calgranulin family, S100A4, is upregulated in hypertrophy (41).
One-hundred and ten transcripts were differentially upregulated in all genotypes except the β1β2−/−, most notably TGF-β2 (Fig. 6D) (7, 25). TGF-β signaling has been shown to play a role in regulating hypertrophy (3, 37). Another differentially regulated transcript is karyopherin alpha 2 (Kpna2), a component of the nuclear localization signal of the nuclear protein import receptor, which transports CaMKIV into the nucleus (21). CaMKII signaling has been implicated in β-AR-mediated remodeling (55).
To elucidate the mechanisms by which β2-AR signaling limits cardiac hypertrophy, we next compared β2−/− vs. WT TAC. Forty-nine transcripts were differentially upregulated in the β2−/− and 418 differentially downregulated. Downregulated candidates include several apoptosis-related genes, including peptidyl-tRNA hydrolase 2 (Ptrh2), a BCL2 inhibitor of transcription that induces cell death with characteristics of caspase-independent apoptosis (10).
We also examined a panel of well characterized pathologic hypertrophy genes, including α-actin, ANP, CaM kinase, and SERCA-2. After 3 wk of TAC, both α-actin and ANP were increased in all genotypes; the greatest increase in ANP was in the β2−/−, with the greatest degree of hypertrophy. SERCA was decreased in both WT and β2−/− but not in the β1−/− or β1β2−/−. Previous studies (6) have shown that α-actin and ANP upregulation occur in compensated hypertrophy in the absence of heart failure. In mild hypertrophy, sarcomplasmic reticulum calcium handling decreases even before SERCA downregulation is evident (9). These genes have traditionally been used to separate “physiologic hypertrophy,” i.e., that induced by exercise, from “pathologic hypertrophy,” i.e., that which progresses to heart failure. In our model of stable hypertrophy (systolic function remaining normal for up to 3 mo after TAC), these gene changes may be reflective of the hypertrophic process alone or could represent subtle alterations in diastolic function associated with afterload stress.
Our study has several limitations. Our microarray data reflect only one time point in the development of cardiac hypertrophy. As we (53) have previously shown, genome-wide expression changes dynamically during the progress of cardiac hypertrophy. We chose a degree of aortic constriction that is not associated with decompensation to separate the effects of β-AR deletion on hypertrophy vs. heart failure. Although prior studies have reported higher transaortic gradients than ours, these studies have used angle correction, which we have avoided as it adds too objective a factor. We have not seen any heart failure for periods of up to 12 wk, at which point nearly all TAC models that develop heart failure will have shown some decompensation. It should also be emphasized that our findings may not be applicable to all mouse strains. We have studied the role of β-receptor knockouts on both congenic FVB and mixed background strains and found that there are strain-dependent differences in the degree to which hypertrophy is attenuated. Other commonly used strains for murine cardiovascular research, such as C57Bl6/J, may be different. Another limitation to our study is that we have not expanded on which (or which combinations) of the several gene alterations we have described is responsible for the attenuation of hypertrophy in the double knockout. Such studies would require generation of triple and potentially quadruple gene-deleted animals and the introduction of additional strain backgrounds to the mix and are clearly beyond the scope of the present investigation.
In summary, cardiac pressure overload hypertrophy is regulated by a coordination of β1- and β2-AR signaling. Only when both major cardiac β-AR subtypes are ablated is the hypertrophic response abolished, suggesting that β1 and β2-ARs act in concert to produce a normal hypertrophic response. β2-AR signaling, which has been linked to both similar and divergent downstream effectors compared with the β1-AR, may limit deleterious remodeling by putting a brake on the hypertrophic process. Altered expression of genes regulating calcineurin and TGF-β signaling suggest that these two important hypertrophic pathways play a central role, although the mechanisms by which β-ARs mediate alterations in these signaling molecules remain to be determined. The implications of these results for patients receiving β-AR antagonists will also require further study, with the usual caveat of translation of murine data to humans. Are patients receiving nonsubtype specific β-blockers for heart failure less able to respond with physiologically appropriate degrees of hypertrophy to mild-to-moderate degrees of afterload stress such as induced by hypertension or bicuspid aortic valve stenosis? Are there circumstances where subtype-specific β-blockers would be more appropriate? Our finding that β2-ARs limit the extent of hypertrophic remodeling is intriguing in light of evidence that β2-AR agonists may be beneficial in some models of heart failure (54). Finally, in contrast to models of hypertrophy leading to heart failure, we found no evidence that cardiac angiogenesis plays a role in stable, compensated pressure overload hypertrophy.
GRANTS
Supported for this work was provided by National Heart, Lung, and Blood Institute Grant HL-061535 (to D. Bernstein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation 110: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2-adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 10: 289–296, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Azhar M, Schultz Jel J, Grupp I, Dorn GW, 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev 14: 391–407, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol 15: 4395–4402, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci W, Bristow MR, Port DJ. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol 32: 817–830, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res 75: 23–32, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem 274: 16694–16700, 1999 [DOI] [PubMed] [Google Scholar]
- 9. de la Bastie D, Levitsky D, Rappaport L, Mercadier JJ, Marotte F, Wisnewsky C, Brovkovich V, Schwartz K, Lompre AM. Function of the sarcoplasmic reticulum and expression of its Ca2(+)-ATPase gene in pressure overload-induced cardiac hypertrophy in the rat. Circ Res 66: 554–564, 1990 [DOI] [PubMed] [Google Scholar]
- 10. De Pereda JM, Waas WF, Jan Y, Ruoslahti E, Schimmel P, Pascual J. Crystal structure of a human peptidyl-tRNA hydrolase reveals a new fold and suggests basis for a bifunctional activity. J Biol Chem 279: 8111–8115, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Delhaas T, Arts T, Prinzen FW, Reneman RS. Regional fibre stress-fibre strain area as an estimate of regional blood flow and oxygen demand in the canine heart. J Physiol 477: 481–496, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 13. Dorn GW., II Myocardial angiogenesis: its absence makes the growing heart founder. Cell Metab 5: 326–327, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Du XJ, Autelitano DJ, Dilley RJ, Wang B, Dart AM, Woodcock EA. Beta(2)-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation 101: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96: 7059–7064, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghavami S, Rashedi I, Dattilo BM, Eshraghi M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C, Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol 83: 1484–1492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 287: 781–787, 1972 [DOI] [PubMed] [Google Scholar]
- 19. Kendziorski CM, Zhang Y, Lan H, Attie AD. The efficiency of pooling mRNA in microarray experiments. Biostatistics 4: 465–477, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kiriazis H, Wang K, Xu Q, Gao XM, Ming Z, Su Y, Moore XL, Lambert G, Gibbs ME, Dart AM, Du XJ. Knockout of beta(1)- and beta(2)-adrenoceptors attenuates pressure overload-induced cardiac hypertrophy and fibrosis. Br J Pharmacol 153: 684–692, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotera I, Sekimoto T, Miyamoto Y, Saiwaki T, Nagoshi E, Sakagami H, Kondo H, Yoneda Y. Importin alpha transports CaMKIV to the nucleus without utilizing importin beta. EMBO J 24: 942–951, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lemmens K, Segers VF, Demolder M, Michiels M, Van Cauwelaert P, De Keulenaer GW. Endogenous inhibitors of hypertrophy in concentric versus eccentric hypertrophy. Eur J Heart Fail 9: 352–356, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Liao Y, Asakura M, Takashima S, Ogai A, Asano Y, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Kitamura S, Tomoike H, Hori M, Kitakaze M. Celiprolol, a vasodilatory beta-blocker, inhibits pressure overload-induced cardiac hypertrophy and prevents the transition to heart failure via nitric oxide-dependent mechanisms in mice. Circulation 110: 692–699, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW., 2nd Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 101: 1707–1714, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Lu L, Saulis AS, Liu WR, Roy NK, Chao JD, Ledbetter S, Mustoe TA. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg 201: 391–397, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol 23: 1034–1043, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura A, Rokosh DG, Paccanaro M, Yee RR, Simpson PC, Grossman W, Foster E. LV systolic performance improves with development of hypertrophy after transverse aortic constriction in mice. Am J Physiol Heart Circ Physiol 281: H1104–H1112, 2001 [DOI] [PubMed] [Google Scholar]
- 29. O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest 111: 1783–1791, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest 116: 1005–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palazzesi S, Musumeci M, Catalano L, Patrizio M, Stati T, Michienzi S, Di Certo MG, Mattei E, Vitelli L, Marano G. Pressure overload causes cardiac hypertrophy in beta1-adrenergic and beta2-adrenergic receptor double knockout mice. J Hypertens 24: 563–571, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Perlini S, Ferrero I, Palladini G, Tozzi R, Gatti C, Vezzoli M, Cesana F, Janetti MB, Clari F, Busca G, Mancia G, Ferrari AU. Survival benefits of different antiadrenergic interventions in pressure overload left ventricular hypertrophy/failure. Hypertension 48: 93–97, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest 116: 1547–1560, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 14: 34–44, 2000 [PMC free article] [PubMed] [Google Scholar]
- 35. Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem 274: 16701–16708, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA 93: 7375–7380, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63: 423–432, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, Yoo KD, Demetroulis E, Gimbel M, Cabuay B, Karimi M, Hill JA. Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure. Physiol Genomics 23: 18–27, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446: 444–448, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Schneider M, Kostin S, Strom CC, Aplin M, Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E, Lerche Hansen J, Sheikh SP. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res 75: 40–50, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Shusterman V, Usiene I, Harrigal C, Lee JS, Kubota T, Feldman AM, London B. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol 282: H2076–H2083, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281: 1690–1693, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Tabibiazar R, Wagner RA, Liao A, Quertermous T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circ Res 93: 1193–1201, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wagner RA, Tabibiazar R, Liao A, Quertermous T. Genome-wide expression dynamics during mouse embryonic development reveal similarities to Drosophila development. Dev Biol 288: 595–611, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Wagner RA, Tabibiazar R, Powers J, Bernstein D, Quertermous T. Genome-wide expression profiling of a cardiac pressure overload model identifies major metabolic and signaling pathway responses. J Mol Cell Cardiol 37: 1159–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322: 1178–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Wilson CL, Pepper SD, Hey Y, Miller CJ. Amplification protocols introduce systematic but reproducible errors into gene expression studies. Biotechniques 36: 498–506, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest 112: 1395–1406, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang D, Ma S, Tan Y, Li D, Tang B, Chen J, Su X, Li G, Zhang X, Yang Y. Adrenergic receptor blockade-induced regression of pressure-overload cardiac hypertrophy is associated with inhibition of the calcineurin/NFAT3/GATA4 pathway. Mol Med Report 3: 497–501, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Yoshioka J, Schulze PC, Cupesi M, Sylvan JD, MacGillivray C, Gannon J, Huang H, Lee RT. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation 109: 2581–2586, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Zhao M, Chow A, Powers J, Fajardo G, Bernstein D. Microarray analysis of gene expression after transverse aortic constriction in mice. Physiol Genomics 19: 93–105, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of beta2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ Res 97: 507–509, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest 111: 617–625, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA 98: 1607–1612, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]