Abstract
Overexpression studies have revealed a role for silent information regulator of transcription 1 (SIRT1) lysine deacetylase in cardioprotection against ischemia-reperfusion injury via long-term transcriptional effects. However, short-term SIRT1-mediated lysine deacetylation, within the context of acute cardioprotection, is poorly understood. In this study, the role of SIRT1 in the acute cardioprotective paradigm of first window ischemic preconditioning (IPC) was studied using SIRT1-deficient (SIRT1+/−) and SIRT1-overexpressing (SIRT1+++) mice. In wild-type hearts, cytosolic lysine deacetylation was observed during IPC, and overacetylation was observed upon pharmacological SIRT1 inhibition. Consistent with a role for SIRT1 in IPC, SIRT1+/− hearts could not be preconditioned and exhibited increased cytosolic lysine acetylation. Furthermore, SIRT1+++ hearts were endogenously protected against ischemia-reperfusion injury and exhibited decreased cytosolic acetylation. Both of these effects in SIRT1+++ mice were reversed by pharmacological SIRT1 inhibition on an acute timescale. Several downstream targets of SIRT1 were examined, with data suggesting possible roles for endothelial nitric oxide synthase phosphorylation, NF-κB, and stimulation of autophagy. In conclusion, these data suggest that SIRT1, acting on nontranscriptional targets, is required for cardioprotection by acute IPC and that SIRT1-dependent lysine deacetylation occurs during IPC and may play a role in cardioprotective signaling.
Keywords: ischemic preconditioning, myocardial infarction, nicotinamide adenine dinucleotide, lysine deacetylation, silent information regulator of transcription
although the etiology of cardiac ischemia-reperfusion (I/R) injury has been well characterized, extensive knowledge of the mechanisms of myocardial damage has not successfully translated into methods for cardioprotection (3). Among the experimental protective strategies that have been studied, ischemic preconditioning (IPC) is one of the most effective (46). However, its limited clinical applicability has led to a focus on the mechanisms of IPC, with the goal of designing IPC mimetics to trigger protection.
IPC initiates a number of cardioprotective events, and the mechanism of protection appears to vary depending on the intervening time period between the protective stimulus (IPC) and the index I/R injury. Acute IPC is usually termed “first window” IPC (minutes to hours) and is mediated by the posttranslational modification of proteins. In contrast, “second window” IPC (days) induces protection mostly via de novo protein synthesis (33).
The focus of this study was posttranslational modifications in acute IPC. While protein phosphorylation has been extensively studied in IPC, more recently, lysine acetylation has emerged as a posttranslational modification of potential importance (27). In recent years, many nonhistone proteins that exhibit both reversible lysine acetylation and play critical roles in I/R injury have been discovered, including p53 (14), NF-κB (48), endothelial nitric oxide (NO) synthase (eNOS) (28), and Ku70 (42).
The silent information regulator of transcription (SIRT) family of proteins comprises class III lysine deacetylases that regulate various intracellular events (2). The archetypal family member is SIRT1, and a recent study (17) has highlighted an emerging role for SIRT1-mediated transcriptional events in cardioprotection. These effects are mediated via deacetylation of transcription factors, upregulation of antioxidant enzymes, and other downstream gene targets (1, 17, 19, 43).
In contrast, acute protein deacetylation by SIRT1 is also known to stimulate several protective signaling pathways, including NO· production (28), insulin signaling (49), and autophagy (26). We (31) have recently shown that SIRT1 plays a role in acute cardioprotection. However, those studies, as well as another implicating SIRT1 in neuronal IPC (36), relied on pharmacological manipulation of SIRT1 activity. In this study, we further established a role for SIRT1 in acute IPC cardioprotection using SIRT1-deficient (SIRT1+/−) and SIRT1-overexpressing (SIRT1+++) mice.
METHODS
The generation of heterozygous SIRT1+/− and transgenic SIRT1+++ mice was as previously described, with their background wild-type (WT) mice being the 129/SvJ and mixed C57BL/6J×129/SvJ strains, respectively (4, 29). SIRT1+/− mice were backcrossed to WT C57BL/6J mice for at least two generations. SIRT1+++ mice were bred with WT mixed C57BL/6J×129/SvJ mice. For breeding, WT mice of C57BL/6J or mixed C57BL/6J×129/SvJ background were purchased from Jackson Laboratory (Bar Harbor, ME) at 8–12 wk of age. WT littermates were used as controls in all experiments. All mice were bred and maintained in a pathogen-free vivarium under recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals with a 12:12-h light-dark cycle and food and water available ad libitum. All experimental protocols were approved by the American Association for Accreditation of Laboratory Animal Care-accredited University of Rochester Committee on Animal Resources.
Mouse hearts (n = 43) were subjected to Langendorff perfusion as previously described (30). I/R injury comprised 25 min of index ischemia followed by 60 min of reperfusion. IPC comprised 3 × 5-min cycles of ischemia interspersed with 5 min of reperfusion before index I/R. Perfused hearts isolated from SIRT1+/− mice and their corresponding littermate controls (WT mice) were divided into the following four groups: 1) WT I/R (n = 7), 2) WT IPC + I/R (n = 6), 3) SIRT1+/− I/R (n = 5), and 4) SIRT1+/− IPC + I/R (n = 5).
In a separate set of experiments, both SIRT1+++ and WT hearts were perfused with the SIRT1 inhibitor splitomicin (Sp; 10 μM), which was delivered via a port above the aortic perfusion cannula for 20 min before I/R injury. The following four groups were tested: 1) WT I/R (n = 5), 2) I/R + Sp (n = 5), 3) SIRT1+++ I/R (n = 5), and 4) SIRT1+++ I/R + Sp (n = 5). Left ventricular pressure was monitored throughout by a balloon-linked transducer, and coronary root pressure was monitored by an in-line transducer. After reperfusion, infarct size was measured by 2,3,5-triphenyltetrazolium chloride staining as previously described (30).
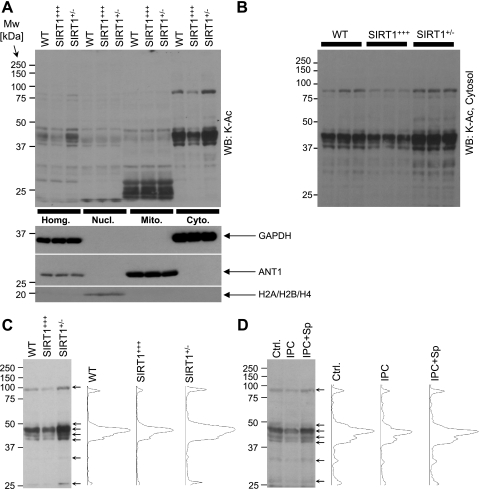
Lysine acetylation (K-Ac) was analyzed by immunoblot analysis of cell fractions prepared as previously described (8). Lysis buffer contained trichostatin A (5 μM) to inhibit class I/II histone deacetylases. Subcellular fractionation was verified by immunoblotting histones (H2A, H2B, and H4), adenine nucleotide translocator 1 (ANT1), and GAPDH (see Fig. 2A). Samples were separated by SDS-PAGE and transferred to nitrocellulose. Membranes were blocked with Tris-buffered saline containing 0.05% (wt/vol) Tween 20 and 5% nonfat dry milk. The primary antibodies were anti-K-Ac, anti-Ac-p65, anti-eNOS, anti-phospho-eNOS (Ser1177, Cell Signaling, Danvers, MA), anti-histone (H2A, H2B, and H4), anti-GAPDH (Chemicon, Billerica, MA), anti-LC3 (Sigma, St. Louis, MO), anti-SIRT1 (Abcam, Cambridge, MA), anti-ANT1 (MitoScience-Abcam, Cambridge, MA), anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-actin (Calbiochem/EMD, Gibbstown, NJ) at 1:1,000 to 1:10,000 dilutions in blocking buffer. All blots were developed with horseradish peroxidase-linked anti-mouse or anti-rabbit secondary antibodies and enhanced chemiluminescence (31). Densitometry of K-Ac blots and corresponding Ponceau S-stained membranes was performed in the molecular mass range of 25–100 kDa using Scion Image software. Statistical significance between groups was determined using multivariate ANOVA.
Fig. 2.
Lysine acetylation in WT, SIRT1-overexpressing (SIRT1+++), and SIRT1+/− hearts. A, top: cardiac tissues were fractionated, proteins were separated by SDS-PAGE, and lysine acetylation (K-Ac) was detected by immunoblot analysis. Numbers on the left indicate molecular mass (in kDa). The bottom blots show Western blots for GAPDH (cytosol), adenine nucleotide translocator 1 (ANT1; mitochondria), and histones (nucleus), indicating the purity of the cell fractions. B: since A shows only one sample (lane) for each genotype, from four different tissue fractions, here three separate samples are shown for each genotype, from the cytosol alone. Densitometry was performed in the range of 25–100 kDa for A and B and normalized to protein across the same molecular mass range from Ponceau S-stained membranes (not shown). The ratio of K-Ac to protein is shown in Table 1. C: densitometry of cytosolic fractions from WT, SIRT1+++, and SIRT1+/− hearts was performed. Left, typical blot image; right, densitometry profiles of the three lanes. D: cytosolic fractions were obtained from hearts subjected to control perfusion (Ctrl.), IPC, and IPC + splitomicin (Sp; 10 μM). Lysine acetylation was detected by immunoblot analysis. Left, typical Western blot image; right, densitometry profile of the lanes.
RESULTS
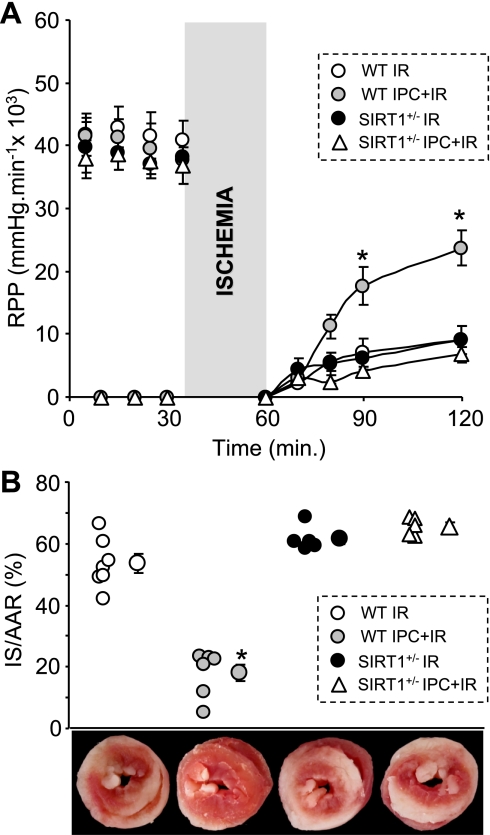
Our previous investigation (31), in which the SIRT1 inhibitor Sp blocked cardioprotection by acute IPC, suggested a role for SIRT1 in cardioprotection. In this study, we applied a genetic approach to cement this role. First, we investigated whether SIRT1+/− hearts were refractory to IPC using Langendorff perfusion to expose hearts to I/R injury and IPC. Figure 1 shows that IPC protected WT hearts against I/R injury, preserving cardiac function (rate-pressure product) during reperfusion (A) and decreasing infarct size (B). In contrast, IPC failed to preserve cardiac function and decrease infarct size in SIRT1+/− hearts. Importantly, the outcome of I/R injury alone (without IPC) was no different between SIRT1+/− and WT hearts, suggesting that resistance to IPC was not due to additional damage in SIRT1+/− hearts. These data establish that SIRT1 is required for acute IPC cardioprotection and that the cardioprotective role of SIRT1 extends beyond merely a transcriptional regulatory effect.
Fig. 1.
Silent information regulator of transcription 1 (SIRT1) is required for ischemic preconditioning (IPC). A: wild-type (WT) and SIRT-deficient (SIRT1+/−) hearts were perfused in Langendorff mode and subjected to ischemia-reperfusion (I/R) injury with or without IPC. The rate-pressure product (RPP; equal to left ventricular developed pressure × heart rate) indicated functional recovery during I/R injury and was monitored thought the whole experiment. Data are meanss ± SE; n ≥ 5. *P < 0.05 vs. I/R alone (by ANOVA). B: infarct size (IS) was quantified by 2,3,5-tetraphenyltetrazolium chloride (TTC) staining. AAR, area at risk. The top graph shows individual data points for each condition on the left, with means ± SE on the right. *P < 0.05 vs. I/R alone (by ANOVA). The bottom images show representative TTC-stained hearts from each experimental group.
Next, we sought to investigate lysine acetylation in WT, SIRT1+/−, and SIRT1+++ hearts. Homogenate, nuclear, mitochondrial, and cytosolic extracts were immunoblotted with anti-K-Ac antibodies. Figure 2A shows that changes in protein acetylation in homogenates were mostly due to changes in the cytosolic compartment, with minimal impact from the nucleus or mitochondria. This is consistent with previous observations showing that the majority of SIRT1 is cytosolic in the adult heart (31, 44). Figure 2B shows that the observed changes in cytosolic lysine acetylation between genotypes were reproducible across multiple independent samples. Quantitative densitometry is shown in Table 1 and revealed a robust decrease in cytosolic protein acetylation in SIRT1+++ hearts (compared with both WT and SIRT1+/− hearts), whereas the opposite effects were seen in SIRT1+/− hearts, i.e., acetylation was significantly higher compared with WT and SIRT1+++ hearts. These data are consistent with the known enzymatic role of SIRT1 as a lysine deacetylase.
Table 1.
| WT | SIRT1+/− | SIRT1+++ | |
|---|---|---|---|
| Figure 2, A and B | |||
| Lysine acetylation/protein | |||
| Homogenate | 0.26 ± 0.01 | 0.35 ± 0.005 | 0.20 ± 0.01* |
| Nucleus | 0.18 ± 0.04 | 0.21 ± 0.05 | 0.19 ± 0.05 |
| Mitochondria | 0.49 ± 0.09 | 0.55 ± 0.06 | 0.54 ± 0.10 |
| Cytosol | 0.48 ± 0.01* | 0.71 ± 0.03† | 0.31 ± 0.01*† |
| Figure 3 | |||
| SIRT1/actin | 1.02 ± 0.18 | 0.54 ± 0.05 | 2.08 ± 0.34* |
| Phospho-eNOS/eNOS | 0.67 ± 0.11 | 0.44 ± 0.05 | 1.58 ± 0.17* |
| Ac p65/p65 | 0.49 ± 0.02* | 0.64 ± 0.02 | 0.49 ± 0.01* |
| MnSOD/actin | 1.38 ± 0.05 | 1.36 ± 0.09 | 1.23± 0.07 |
| LC3 II/I | 2.78 ± 0.64 | 3.59 ± 0.50 | 4.44 ± 1.20 |
Data are means ± SE; n ≥ 3. WT, wild type; SIRT1+/−, silent information regulator of transcription 1 (SIRT1) deficient; SIRT1+++, SIRT overexpressing. For Fig. 2, A and B, lysine acetylation densitometry was performed in the range of 25–100 kDa and normalized to protein across the same molecular mass range from Ponceau S-stained membranes. For Fig. 3, SIRT1 and MnSOD were normalized to actin; phospho-endothelial nitric oxide synthase (eNOS), Ac p65, and LC3 II were normalized to eNOS, p65, and LC3 I, respectively.
P < 0.05 vs. SIRT1+/−;
P < 0.05 vs. WT (by ANOVA).
We (31) recently demonstrated an association between SIRT1 activity and cytosolic protein deacetylation during IPC. To investigate this association in more detail, densitometric analysis was performed to compare cytosolic acetylation patterns between two groups of samples: 1) WT, SIRT1+++, and SIRT1+/− hearts without IPC (Fig. 2C) and 2) WT hearts upon control perfusion, IPC, and IPC + Sp (Fig. 2D). Notably, deacetylated bands from SIRT1+++ hearts (arrows) matched with those found in WT hearts after IPC. Importantly, these same bands were also overacetylated in both SIRT1+/− hearts (no IPC) and in WT hearts with IPC + Sp. These data suggest that cytosolic protein deacetylation is concomitant with increased SIRT1 activity. Moreover, having established that IPC was inhibited in both SIRT1+/− hearts (Fig. 1) and WT hearts + Sp (31), these data indicate that SIRT1 activity correlates with the ability of the heart to be preconditioned.
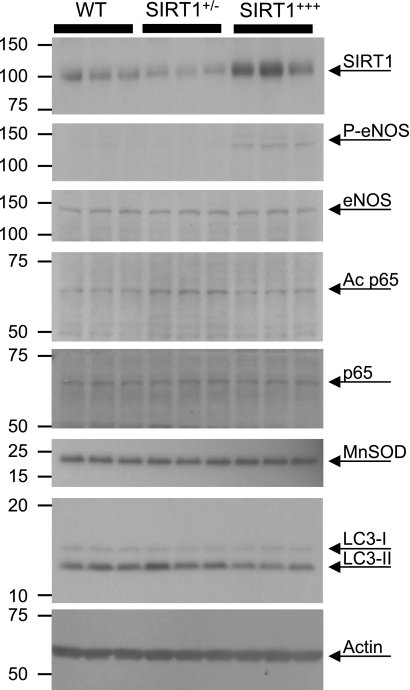
Next, we tested several commonly studied downstream targets of SIRT1 that might potentially be involved in acute protection. One such signaling mechanism is the SIRT1-mediated activation of eNOS via Akt signaling phosphorylation at eNOS (Ser1177) (9, 41). In this regard, we found that phospho-eNOS was significantly higher in SIRT1+++ hearts compared with both WT and SIRT1+/− hearts (Fig. 3 and Table 1). Alternatively, SIRT1 is known to deacetylate and inhibit NF-κB p65 (48), which may impact on inflammation or apoptotic signaling (47). Figure 3 shows the significant increase in the level of p65 acetylation in SIRT1+/− hearts relative to both WT and SIRT1+++ hearts.
Fig. 3.
SIRT1 downstream targets in WT, SIRT1+/−, and SIRT1+++ hearts. Whole heart homogenates were prepared, proteins were separated by SDS-PAGE, and SIRT1, phosphorylated (P) endothelial nitric oxide synthase (eNOS; Ser1177), total eNOS, Ac p65, p65, MnSOD, LC3, and actin were all detected by immunoblot analysis. Three separate samples are shown for each genotype. Densitometry is shown in Table 1.
Another known target of SIRT1 is the FOXO1-dependent transcription of MnSOD (17). However, we did not observe any significant differences in MnSOD levels between WT, SIRT1+/−, or SIRT1+++ hearts (Fig. 3). This observation contrasts with another study (17), and this discrepancy may be due to the comparatively small differences in SIRT1 levels between WT and SIRT1+++ mice in this study (∼2-fold; Table 1) versus the much larger degree of SIRT1 overexpression in Ref. 17.
Another critical event in I/R injury is autophagy (38), but, more recently, it has also been shown that autophagy plays a role in IPC cardioprotection (18). Surprisingly, autophagy was slightly stimulated in both SIRT1+/− and SIRT1+++ hearts, as suggested by the increase in the LC3 II-to-I ratio (Fig. 3 and Table 1). These data are in agreement with other studies (15, 16, 20) showing modulation of the autophagic machinery by SIRT1.
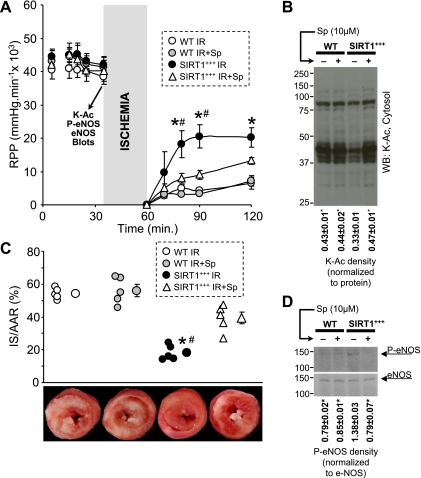
Next, we investigated cardioprotection in SIRT1+++ mice. In agreement with Hsu et al. (17), we found that SIRT1+++ hearts were endogenously protected against I/R injury (Fig. 4, A and C). To determine whether endogenous cardioprotection in SIRT1+++ mice was due to long-term effects, such as the upregulation of prosurvival proteins (17, 31) or stimulation of FOXO3 signaling (19), or was mediated by acute lysine deacetylation, we administered Sp immediately before I/R in SIRT1+++ hearts. Notably, in SIRT1+++ mice, acute pharmacological inhibition of SIRT1 enzymatic activity brought lysine acetylation back up to control levels (Fig. 4B). Consistent with its effects on lysine acetylation, Sp also inhibited cardioprotection in SIRT1+++ hearts (Fig. 4, A and C). These results suggest that a significant portion of the endogenous cardioprotection in SIRT1+++ hearts is due to immediate deacetylase activity of SIRT1 within the 20-min time frame of Sp delivery and not due to transcriptional effects of SIRT1. In other words, the cardioprotective benefit of a lifetime of SIRT1 overexpression can be abolished with 20 min of inhibition of SIRT1 enzymatic activity.
Fig. 4.
SIRT1+++ hearts are endogenously protected. A: WT and SIRT1+++ hearts were subjected to I/R injury with or without an infusion of Sp (10 μM). RPP was monitored throughout the experiment. Data are means ± SE; n = 5. *P < 0.05 vs. SIRT1+++ I/R; #P < 0.05 vs. SIRT1+++ I/R + Sp (by ANOVA). B: both WT and SIRT1+++ perfused hearts were subjected to control perfusion with or without Sp (10 μM). Before index ischemia, cytosolic fractions were prepared and immunoblotted for K-Ac (as indicated by the arrow in A). Densitometry was performed exactly as described in Fig. 2, with normalization to Ponceau S-stained membranes (not shown). Data are means ± SE; n = 3. *P < 0.05 vs. SIRT1+++ (by ANOVA). C: IS was quantified by TTC staining. In the top graph, individual data points for each condition are shown on the left, with means ± SE on the right (n = 5). *P < 0.05 vs. SIRT1+++ I/R; #P < 0.05 vs. SIRT1+++ I/R + Sp (by ANOVA). The bottom images show representative TTC-stained hearts from each experimental group. D: eNOS phosphorylation was tested in whole heart homogenates. Experimental groups were as in B. Densitometry data (P-eNOS/total eNOS) are shown below the blots. Data are means ± SE; n = 3. *P < 0.05 vs. SIRT1+++ (by ANOVA).
Given our intriguing findings regarding eNOS phosphorylation in SIRT1+++ hearts (Fig. 3) and the known cardioprotective role of NO· (23), we next examined whether acute Sp delivery inhibited eNOS phosphorylation. Figure 4D shows that in SIRT1+++ hearts, Sp lowered eNOS (Ser1177) phosphorylation down to WT levels. Together, these data support a role for SIRT1 in endogenous acute cardioprotection by a nontranscriptional mechanism.
DISCUSSION
This study determined an important role for SIRT1 in acute cardioprotection against I/R injury. We showed that SIRT1+/− hearts are refractory to first window IPC; moreover, we demonstrated that the SIRT1 inhibitor Sp acutely inhibited the endogenous cardioprotection observed in SIRT1+++ hearts. Thus, our study establishes a clear relationship between SIRT1 activity and cardioprotection on a short timescale.
The SIRT1 protection seen herein is distinct from the cardioprotection previously assigned to SIRT1 acting at a transcriptional regulatory level (17). The latter is likely important in second window or delayed preconditioning and may involve FOXO3 (de)acetylation (5). FOXO3 is an important regulator of antioxidant genes and is directly involved in the cell stress response (19, 34). Further studies are required to address the involvement of a SIRT1-FOXO3 axis in cardioprotection.
An interesting finding from this study is the striking similarity between the patterns of cytosolic protein acetylation observed in IPC and with genetic manipulation of SIRT1 activity. It is commonly recognized that the field of sirtuin biology is limited by the poor availability of reliable assays for SIRT1 activity (32). These data suggest that modulation of SIRT1 activity may be estimable based on cytosolic protein acetylation patterns, at least in the heart. Our unpublished observations suggest these characteristic differences in cytosolic protein acetylation are also seen in both aging and diabetic hearts and upon treatment with the nonspecific SIRT1 activator resveratrol. Notably, SIRT1 is downregulated in both aged and diabetic hearts (11, 40). Thus, the further proteomic characterization of SIRT1 targets may enable a “signature” of cytosolic lysine acetylation to be developed as a surrogate marker to assay cardiac SIRT1 activity, in the same way that signaling kinase substrates are often used to assay upstream kinase activities. The recent development of technologies to immunoenrich acetylated peptides may be of use in the further characterization of the cardiac acetylated proteome (39). Regardless of the acetylation status in IPC, assignment of any SIRT1-mediated change in acetylation to a role in cardioprotection may be difficult, because SIRT1 may orchestrate multiple parallel signaling pathways.
To investigate downstream SIRT1 signaling, several well-known targets of SIRT1 were examined (Fig. 3). We demonstrated that eNOS might contribute to endogenous cardioprotection in SIRT1+++ hearts, because Sp removed both the protective effect against I/R injury and eNOS (Ser1177) phosphorylation (Fig. 4D). One mechanism by which eNOS may impact resistance to ischemia is via modulation of vascular tone. In this regard, we found no significant differences in coronary vascular resistance (CVR) between WT, SIRT1+/−, and SIRT1+++ hearts at baseline, although CVR was slightly elevated in the knockouts (Table 2). A significant rise in CVR was observed in WT hearts in response to I/R injury, and, notably, this elevation was blunted in SIRT1+++ hearts, suggesting preservation of vascular function. In SIRT1+/− hearts, CVR was already elevated at baseline (although not significantly) and thus did not rise further with I/R injury. Together, these CVR data match well with our observations on eNOS phosphorylation. It remains to be discovered whether SIRT1 can directly activate eNOS via deacetylation in the heart, as has been reported for other tissues (28).
Table 2.
Coronary resistance in WT, SIRT1+/−, and SIRT1+++ hearts
| WT | SIRT1+/− | SIRT1+++ | |
|---|---|---|---|
| Before ischemia | 32 ± 3 | 40 ± 4 | 33 ± 3 |
| 60 min of reperfusion | 38 ± 4* | 41 ± 7 | 35 ± 4 |
Values are means ± SE; n = 3. Coronary resistance (in mmHg · ml−1 · min−1) was calculated as the ratio of coronary root pressure (in mmHg) to coronary flow (in ml/min).
P < 0.05 vs. WT before ischemia (by t-test).
Other downstream SIRT1 signaling events that were tested herein and may affect IPC included autophagy and NF-κB activation. The ratio of LC3 II to I is used as an indicator of autophagosome formation (24), and we observed that the LC3 II-to-I ratio was slightly higher in both SIRT1+/− and SIRT1+++ hearts. It has been shown that SIRT1 regulates the expression of Rab7, which is required for autophagosome flux to lysosomes (15). Therefore, it is possible that the flux and intracellular waste disposal by lysosomes is impaired in SIRT1+/− hearts. Assuming a significant role of autophagy in cardioprotection (18, 38), it is also possible that the intracellular accumulation of autophagic material may partly be responsible for IPC resistance in SIRT1+/− hearts. Furthermore, inhibition of autophagic flux and stimulation of NF-κB (acetylation of p65) may predispose cardiomyocytes in SIRT1+/− hearts toward more apoptosis.
Two other SIRT1 targets of particular interest are isocitrate dehydrogenase and GAPDH (31). These same targets were also overacetylated in SIRT1+/− hearts compared with SIRT1+++ hearts (data not shown), and recent studies have suggested that deacetylation of these enzymes may be cardioprotective. Isocitrate dehydrogenase deacetylation stimulates its activity, which increases resistance to oxidative stress by keeping antioxidant systems in a reduced state (37), whereas deacetylation of GAPDH may prevent its translocation to the nucleus (45), where it can trigger apoptosis (7). Obviously, as more SIRT1 targets become identified and their roles in cardioprotection are tested by genetic or pharmacologic inhibition, the complexity of SIRT1 cardioprotective signaling will develop.
Another interesting observation arising from the current data set is the gene dose response of SIRT1-mediated cardioprotection. A single copy (SIRT1+/−) does not protect, even upon stimulation by IPC, whereas two doses (WT SIRT1+++) confer protection with IPC. This suggests a sharp threshold wherein a minimal amount of SIRT1 is required for IPC. Notably, multiple SIRT1 doses (SIRT1+++) confer protection even without IPC, suggesting that under baseline non-IPC-stimulated conditions, there may be an endogenous level of SIRT1 stimulus present. Further work is required to determine the nature of the upstream SIRT1 stimulus that is operational in cardiac cells under baseline, I/R, and IPC conditions.
Establishing a link between SIRT1 and IPC may provide some insight into the clinical problem of susceptibility to I/R injury in aging and diabetes. In addition to being major risk factors for myocardial infarction (10, 12, 22), aged and diabetic hearts are also refractory to protection by IPC (21, 25, 35). This loss of IPC is accompanied with a marked inhibition of SIRT1 activity (11, 40), which may contribute to the development of age-related disturbances such as a severe impairment of energy metabolism (6, 13), inhibition of autophagy (26), and inefficiency of the antioxidant machinery (17, 43). These pathological alterations may diminish the ability of heart to maintain endogenous cardioprotection. Thus, it is possible that preservation of SIRT1 activity might be a promising tool to restore IPC and provide cardioprotection in patients with cardiovascular disease and diabetes, as well as the aged.
In summary, SIRT1 plays an important role in endogenous cardioprotection and during acute IPC. Furthermore, SIRT1-mediated lysine deacetylation is a key posttranslational modification that occurs concurrent with acute cardioprotection against I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-071158 (to P. S. Brookes) and HL-092842 and HL-097751 (to I. Rahman).
ACKNOWLEDGMENTS
The authors thank Stephanie Uhrinek for technical assistance.
REFERENCES
- 1. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bao J, Sack MN. Protein deacetylation by sirtuins: delineating a post-translational regulatory program responsive to nutrient and redox stressors. Cell Mol Life Sci 67: 3073–3087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bordone L, Cohen D, Robinson A, Motta MC, van VE, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6: 759–767, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys 501: 79–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ 16: 1573–1581, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc 1: 1872–1878, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59: 418–458, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillum MP, Erion DM, Shulman GI. Sirtuin-1 regulation of mammalian metabolism. Trends Mol Med 17: 8–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90: 595–606, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Hariharan N, Maejima Y, Nakae J, Paik J, DePinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res 107: 1470–1482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 105: 481–491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122: 2170–2182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by parkin and p62/SQSTM1. PLos One 6: e20975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hwang J, Rajendrasozhan S, Yao H, Chung S, Sundar I, Huyck H, Pryhuber G, Kinnula V, Rahman I. FoxO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol 187: 987–998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys 500: 203–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol 103: 2120–2128, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 34: 1164–1170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von FK, Mizushima N, Saftig P, Uchiyama Y. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol 167: 1713–1728, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 47: 1716–1721, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105: 3374–3379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res 105: 830–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 23: 38–54, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46: 960–968, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res 89: 643–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci 69: 1–15, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115: 2382–2392, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Przyklenk K. Efficacy of cardioprotective “conditioning” strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging 28: 331–343, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab 26: 1141–1147, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol 382: 790–801, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol 32: 275–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaw PG, Chaerkady R, Zhang Z, Davidson NE, Pandey A. Monoclonal antibody cocktail as an enrichment tool for acetylome analysis. Anal Chem 83: 3623–3626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 298: H833–H843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4: ra46, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28: 6384–6401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem 285: 8375–8382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Ventura M, Mateo F, Serratosa J, Salaet I, Carujo S, Bachs O, Pujol MJ. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase is regulated by acetylation. Int J Biochem Cell Biol 42: 1672–1680, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther 24: 225–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeh CH, Chen TP, Wu YC, Lin YM, Jing LP. Inhibition of NF-κB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res 125: 109–116, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 282: 34356–34364, 2007 [DOI] [PubMed] [Google Scholar]