Abstract
Methionine sulfoxide reductase A (MsrA) catalytically scavenges reactive oxygen species and also repairs oxidized methionines in proteins. Increasing MsrA protects cells and organs from a variety of oxidative stresses while decreasing MsrA enhances damage, but the mechanisms of action have not been elucidated. A single gene encodes MsrA of which ∼25% is targeted to the mitochondria, a major site of reactive oxygen species production. The other ∼75% is targeted to the cytosol and is posttranslationally modified by myristoylation. To determine the relative importance of MsrA in each compartment in protecting against ischemia-reperfusion damage, we created a series of transgenic mice overexpressing MsrA targeted to the mitochondria or the cytosol. We used a Langendorff model of ischemia-reperfusion and assayed both the rate pressure product and infarct size following ischemia and reperfusion as measures of injury. While the mitochondrially targeted MsrA was expected to be protective, it was not. Notably, the cytosolic form was protective but only if myristoylated. The nonmyristoylated, cytosolic form offered no protection against injury. We conclude that cytosolic MsrA protects the heart from ischemia-reperfusion damage. The requirement for myristoylation suggests that MsrA must interact with a hydrophobic domain to provide protection.
Keywords: myristoylation, oxidative stress, reactive oxygen species
virtually all organisms from bacteria to mammals have several methionine sulfoxide reductases (Msr) that catalyze the reduction of methionine sulfoxide to methionine. Oxidation by hydrogen peroxide, hypochlorous acid, and other reactive species creates a chiral center at the sulfur so that the methionine sulfoxide (MetO) produced is a mixture of the S and R epimers (41). Thus two distinct classes of Msr are required with one acting to reduce the S-epimer (MsrA) and one acting to reduce the R-epimer (MsrB). MsrA is virtually ubiquitous in organisms exposed to oxygen, and a variety of studies establish its importance. Reducing or knocking out MsrA in cultured cells and several lower organisms caused increased susceptibility to oxidative stress (10, 22, 23, 34, 37). Mice lacking MsrA are also more susceptible to oxidative challenge, although their life span is not altered (35). MsrA is downregulated in human breast cancer cells, and this downregulation causes a more aggressive phenotype primarily due to increased oxidative stress (9).
Conversely, overexpression of MsrA increases resistance to oxidative stress (24, 33, 34, 46). Increased expression of MsrA in WI-38 fibroblasts was shown to reduce the level of oxidatively damaged proteins after a hydrogen peroxide challenge (5). Accumulation of oxidatively damaged proteins has been hypothesized to be an important mechanism of the aging process (17), and it is notable that overexpression of MsrA in Drosophila doubled the lifespan of the flies (34).
Given the considerable evidence that reactive oxygen species (ROS) play a causal role in cardiac ischemia-reperfusion (I/R) injury (25, 43), one might expect MsrA to protect against such injury. Prentice et al. (29) reported that overexpression of MsrA in cultured cardiac myocytes provided strong protection against injury in their hypoxia-reoxygenation model. Conversely, myocytes from the heart of knockout mice lacking MsrA cannot mount a normal defense against stresses (26). Mammalian MsrA is encoded by a single gene that targets MsrA to both the cytosol and the mitochondria, with ∼75% targeted to the cytosol and ∼25% to the mitochondria (16, 44). The cytosolic form is myristoylated on its amino-terminal glycine while the mitochondrial form is not myristoylated.
To test the hypothesis that increased MsrA will protect the heart from I/R injury, we created transgenic mice with MsrA overexpression in the heart. We targeted overexpression to either the mitochondria or to the cytosol. We created two different lines targeted to the cytosol, one in which the MsrA is myristoylated and another in which it is not. These three models allowed us to dissect the roles of subcellular compartmentalization and whether myristoylation affects protection against oxidative damage. Since mitochondria are the major source of ROS, we expected that mitochondrially targeted MsrA overexpression would be protective in the I/R model. However, we found that it was not protective, while the cytosolic form was protective provided that it was myristoylated.
METHODS
Generation of transgenic mice.
All animals were treated humanely in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, 1996). The study was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. Transgenic mice were generated using the C57BL/6 inbred strain following pronuclear microinjection method as described previously (47). MsrA residues 21–233 were included in the construct for the myristoylatable cytosolic targeted transgenic mice. The construct for the nonmyristoylatable cytosolic targeted mouse was the same except that residue 22 was changed from a glycine to an alanine. We made two mitochondrially targeted constructs because we were uncertain which might best target to the mitochondria. The first was the full-length wild-type sequence with the nucleotide at the −3 position changed from C to A to generate a strong Kozak sequence to drive initiation at Met1 (16). For the second construct, the endogenous MsrA mitochondrial targeting sequence was replaced by the strong mitochondrial targeting sequence from human ornithine transcarbamoylase (14). Possible transgenic mice were screened for the presence of transgene by PCR as described previously (47), and the founders were further bred with wild-type C57BL/6 mice to generate transgenic lines. For each line, the level of transgene expression was analyzed by Western blotting using heart, brain, liver, kidney, and the quadriceps muscle tissues.
Langendorff preparation.
Female mice between 12 and 16 wk of age were studied; cardiac hypertrophy was not observed in any mice. Hearts were Langendorff perfused as described previously (20, 40). After an equilibration period of 20 min, hearts were subjected to 20 min of no-flow ischemia followed by 120 min of reperfusion. Functional recovery and infarct size were assessed as described previously (40). All values are presented as means ± SE. Statistical significance was assessed by one-way ANOVA, followed by post hoc multiple comparison procedures using the Holm-Sidak method. A P value <0.05 was considered to be statistically significant.
Immunoquantitation, enzymatic activity, and subcellular fractionation of tissue MsrA.
Mice were euthanized with carbon dioxide, and hearts were removed and rinsed in ice-cold PBS. Tissue was finely minced with scissors and then homogenized with a hand-held micro grinder (Kontes Pellet Pestle) in 10 vol of RIPA lysis buffer (R0278; Sigma) supplemented with a 1:1,000 dilution of protease inhibitor cocktail (P8340; Sigma). Protein concentration was determined by the BCA assay (Pierce-Thermo Scientific). Equal amounts of total protein were loaded into lanes of 10–20% gradient SDS-PAGE gels in Tris-glycine buffer (Invitrogen). Following electrophoresis, proteins were transferred onto 0.45 μM nitrocellulose membrane (LC2001; Invitrogen). Membranes were incubated for 30 min in Odyssey blocking buffer (927–40000; Li-Cor) and then overnight at 4°C with a 1:25,000 dilution of polyclonal rabbit anti-MsrA (this laboratory) and a 1:20,000 dilution of monoclonal mouse anti-GAPDH (G8795; Sigma). Incubation with the secondary antibody was for 1 h at room temperature with either a 1:10,000 dilution of Dylight 680 goat-anti-rabbit IgG (072–06-15–06; KPL) or IRDye 800CW goat-anti-mouse IgG (926–32210; Li-Cor). Quantitation of fluorescence was performed with a Li-Cor Odyssey infrared scanner in the 700-nm for MsrA and the 800-nm channel for GAPDH.
Heart MsrA activity was measured using l-methionine [R,S] sulfoxide (Sigma) as the substrate. The reaction mixture for the activity assay contained 10 mM MetO, 10 mM dithiothreitol, and 50 mM sodium phosphate buffer at pH 7.4. Heart tissue lysate containing 200 μg protein was added to the reaction mixture, incubated at 37°C for 30 min, and the reaction was terminated by addition of 1/20th vol of 10% (vol/vol) trifluoroacetic acid. The reaction product was analyzed by amino acid analysis as described previously (21).
Mitochondria are often tethered to the cellular cytoskeleton, facilitating both intracellular motility and anchoring at sites with high ATP requirement (3). The classical subcellular fractionation protocols were designed to yield mitochondria free of cytoskeletal contaminants, but use of such protocols can give erroneous assessment of the amount of MsrA in mitochondria because they discard the large fraction of cellular mitochondria associated with the cytoskeleton (3, 30, 45). We therefore employed a protocol for subcellular fractionation designed to recover the total cellular content of mitochondria. A freshly harvested heart was washed with ice-cold buffer (10 mM HEPES, pH 7.5, 200 mM mannitol, 70 mM sucrose, and 1 mM diethylenetriamine pentaacetic acid) and minced to fine cubes with scissors. Minced samples were then incubated on ice for 20 min in 10 vol of the same buffer supplemented with 0.25 mg/ml trypsin (T9201; Sigma) followed by addition of 1:500 (vol/vol) protease inhibitors cocktail (P8340; Sigma) to inactivate the trypsin. The sample was homogenized gently with 15 strokes of a loose-fitting Dounce homogenizer. The homogenate was centrifuged at 800 g for 10 min, and the resulting “800-g pellet” containing cytoskeleton, adherent mitochondria, and cell debris were saved. The supernatant was centrifuged at 8,000 g for 15 min to generate the “8,000-g pellet” containing mitochondria and a supernatant containing the “8,000-g supernatant.” The pellets were washed three times with buffer, resuspended in RIPA lysis buffer (R0278, Sigma), and sonicated briefly on ice. The final volume of each fraction was measured to allow calculation of the content of MsrA in each subcellular compartment. The purity of each fraction was evaluated by Western blot using a mouse monoclonal anti-GAPDH (G8795; Sigma) as a cytosolic marker and rabbit monoclonal anti-human voltage-dependent anion channel (#4661; Cell Signaling) and rabbit polyclonal anti-human cytochrome c oxidase subunit II (#ab91317; Abcam) as mitochondrial probes.
For separation of microsomes from cytosol, the “8,000-g supernatant” above was centrifuged at 100,000 g for 1 h at 4°C. The resulting supernatant was saved as the “100,000-g supernatant” fraction and the pellet as the “100,000-g pellet”. After being washed, the pellet was solubilized with RIPA lysis buffer containing 1% TritonX-100 and a 1:500 dilution of protease inhibitors (P8340; Sigma).
RESULTS
MsrA transgenic mice constructs.
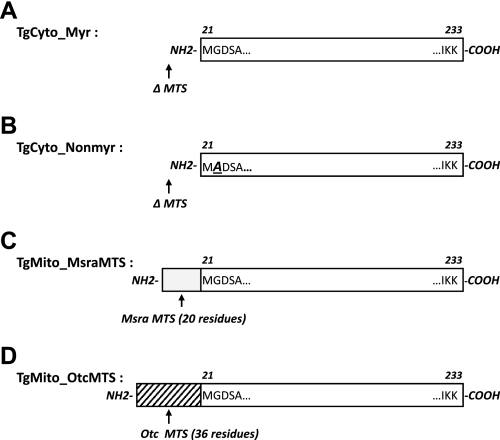
We made constructs to generate transgenic mice overexpressing MsrA in either the cytosol or mitochondria. For the cytosolic targeted MsrA, we created constructs to produce myristoylated or nonmyristoylated MsrA (Fig. 1). Cytoplasmic targeting was accomplished simply by deleting the mitochondrial targeting sequence consisting of residues 1–20. The initiating methionine at position 21 is removed in vivo, leaving Gly22 as the amino-terminal residue that is normally myristoylated (16). The nonmyristoylated construct was altered to encode alanine at residue 22 to prevent myristoylation. For the mitochondrially targeted MsrA, we made two different constructs. The first (TgMito_MsraMTS) was simply the full-length wild-type sequence with the nucleotide at the −3 position changed from C to A to generate a strong Kozak sequence to drive initiation at Met1 (16). Since we were unsure whether this would be sufficient to target the transgenic protein exclusive to the mitochondria, a second construct (TgMito_OtcMTS) was made in which the endogenous MsrA mitochondrial targeting sequence was replaced by the very strong mitochondrial targeting sequence from human ornithine transcarbamoylase (14). All transgenic mice were viable, had postnatal growth rates equal to that of nontransgenic controls, and had no observed phenotypic differences from the nontransgenic mice.
Fig. 1.
Scheme illustrates the differences among transgenic methionine sulfoxide reductase A (MsrA) constructs. A: TgCyto_Myr. Mitochondria targeting signal (MTS) sequence was deleted (ΔMTS), thus targeting the MsrA only to the cytosol. B: TgCyto_Nonmyr. MTS sequence was deleted (ΔMTS), and the endogenous glycine at position 22 was mutated to alanine to prevent myristoylation. C: TgMito_MsraMTS. Endogenous (1–233) mouse MsrA sequence was used for overexpressing full-length MsrA in transgenic mice. Endogenous MTS (residues 1–20, gray bar) was intact. D: TgMito_OtcMTS. Mouse MTS was replaced with the MTS (36 residues) from human ornithine transcarbamoylase (hatched bar).
Transgenic MsrA protein, activity, and subcellular localization.
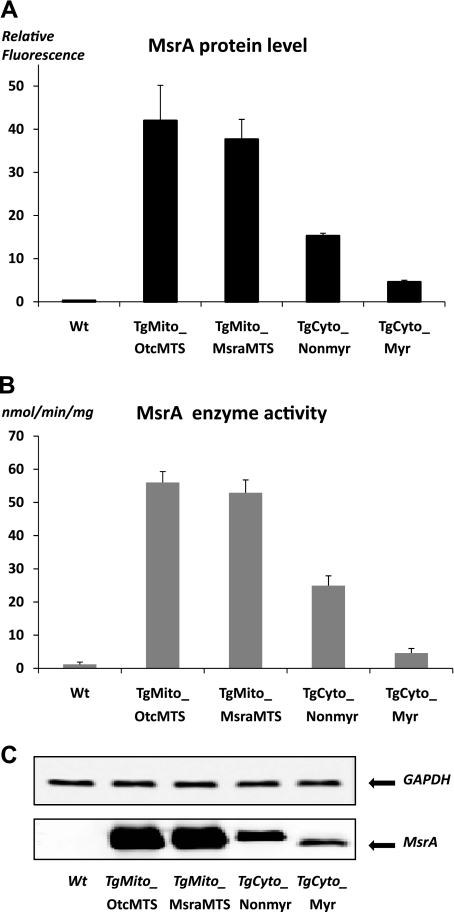
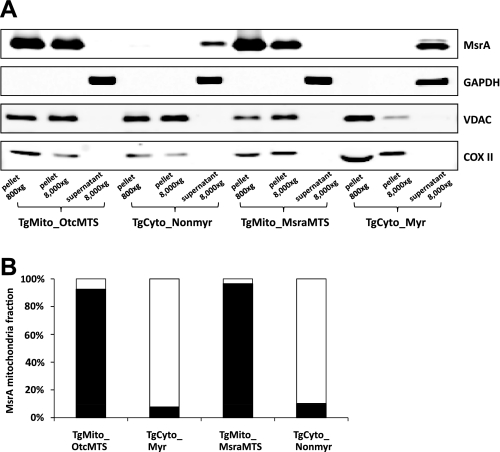
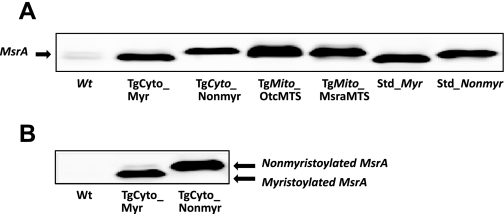
We found by quantitative immunoblotting that each transgenic line overexpressed MsrA in the tissues examined, namely liver, kidney, brain, quadriceps muscle, and heart. The endogenous protein level and MsrA activity were relatively low in the heart, and both were increased markedly in the transgenic mice (Fig. 2). Overexpression in heart tissue was approximately three times greater for the mitochondrially targeted constructs than for the cytosolic targeted constructs, perhaps because of the relative strengths of the Kozak initiation signals. Enzymatic activity correlated very well with protein levels (Fig. 2). Subcellular fractionation established that the desired localization of MsrA was achieved (Fig. 3). Myristoylation status can conveniently be evaluated by mobility differences on an SDS gel (16), and Fig. 4 shows that the MsrA in the TgCyto_Myr line is myristoylated while the TgCyto_Nonmyr line is not. As expected, the MsrA in the transgenic mitochondrially targeted mouse hearts was not myristoylated. Since the two mitochondrially targeted constructs had similar levels of overexpression and response to I/R, results were combined and the combined group was designated as TgMito.
Fig. 2.
MsrA protein level and enzymatic activity in mouse heart. A: quantitation of MsrA protein levels in heart. B: MsrA enzymatic activity in heart. C: representative Western blot of MsrA levels in heart. An equal amount of protein was loaded in each lane. GAPDH served as a loading control. Error bars show the SE from 3 separate experiments.
Fig. 3.
Subcellular distribution of MsrA in transgenic heart. A: Western blot for MsrA in cytosol and mitochondria. GAPDH was detected in the 800-nm channel while MsrA, voltage-dependent anion channel (VDAC), and cytochrome oxidase subunit II (COX II) were detected in the 700-nm channel. Volume loaded was adjusted to give approximately equal amounts of MsrA in each lane. B: percentage of total MsrA in mitochondria (solid bar) and the 8,000-g supernatant (open bar). This was calculated by taking into account the differences in volume of the fractions as well as the fraction loaded in A. Results shown are the average from 3 separate experiments.
Fig. 4.
Myristoylated MsrA mobility shift in SDS-PAGE. A: MsrA migration differences among transgenic and nontransgenic hearts in SDS-PAGE. Purified recombinant myristoylated (Std_Myr) and nonmyristoylated (Std_Nonmyr) MsrA were used as mobility standards. Only MsrA from TgCyto_Myr mice had the same mobility as the Std_Myr. B: comparison of MsrA mobility between TgCyto_Myr and TgCyto_Nonmyr in SDS-PAGE. Note that the myristoylated MsrA migrated faster.
Cytosolic, myristoylated MsrA protects the heart from I/R injury.
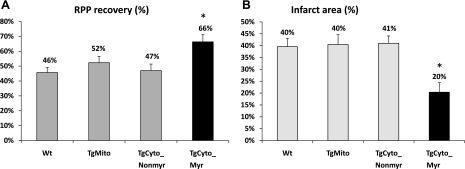
We examined whether increased cellular MsrA was protective against I/R injury in the Langendorff preparation. Hearts were perfused for 20 min and then subjected to 20 min of ischemia. After 2 h of reperfusion, postischemic functional recovery was assessed by measuring the rate pressure product (the product of left ventricular developed pressure × heart rate) followed by quantitation of infarct size. Hemodynamic parameters for each group are shown in Table 1. Since mitochondria are the major source of ROS, we expected that the mitochondrially targeted MsrA would provide protection against I/R injury, but it did not (Fig. 5). Notably, overexpression of cytosolic MsrA did protect from injury, provided that the protein was myristoylated. The postischemic rate pressure product recovery was 66.3 ± 4.8% compared with 45.7 ± 3.5% for the nontransgenic control (P < 0.001). Infarct size decreased from 39.6 ± 3.5 to 20.4 ± 4.0%. Myristoylation was essential as the nonmyristoylated, cytosolic MsrA experienced the same level of injury as the nontransgenic control mice.
Table 1.
Hemodynamic parameters
| Preischemic Hemodynamic Value |
Postischemic Hemodynamic Value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Samples | n | BW, g | HW, mg | HW/BW, mg/g | HR, beats/min | LVDP, cmH2O | +dp/dt, cmH2O/ms | −dp/dt cmH2O/ms | HR, beats/min | LVDP, cmH2O | +dp/dt, cmH2O/ms | −dp/dt, cmH2O/ms |
| Wt | 12 | 21.2 ± 0.2 | 107.1 ± 2.8 | 5.1 ± 0.2 | 357 ± 9 | 138 ± 4 | 8.2 ± 0.3 | −5.9 ± 0.3 | 309 ± 6 | 73 ± 3 | 6.1 ± 0.3 | −4.4 ± 0.2 |
| TgCyto_Myr | 6 | 21.5 ± 0.6 | 109.9 ± 3.2 | 5.1 ± 0.3 | 346 ± 16 | 123 ± 8 | 6.6 ± 0.2* | −4.4 ± 0.1* | 317 ± 16 | 89 ± 6 | 5.9 ± 0.3 | −3.6 ± 0.1 |
| TgCyto_Nonmyr | 5 | 21.6 ± 0.3 | 107.9 ± 1.7 | 5.0 ± 0.1 | 357 ± 5 | 116 ± 7 | 7.4 ± 0.5 | −5.2 ± 0.4 | 321 ± 8 | 60 ± 4 | 5.4 ± 0.3 | −4.2 ± 0.3 |
| TgMito | 8 | 20.9 ± 0.3 | 108.5 ± 1.3 | 5.2 ± 0.1 | 362 ± 10 | 137 ± 5 | 7.8 ± 0.5 | −5.7 ± 0.5 | 317 ± 8 | 82 ± 7 | 6.3 ± 0.7 | −4.8 ± 0.5 |
Values are means ± SE; n = number of hearts. BW, body weight; HW, heart weight; HR, heart rate (beats/min); LVDP, left ventricular developed pressure; ±dp/dt, rates of pressure rise and fall, respectively.
P < 0.05, vs. wild type (Wt).
Fig. 5.
Postischemic left ventricular rate pressure product (RPP) functional recovery and infarct size. A: postischemic RPP functional recovery, measured at the end of 120 min of reperfusion, was significantly higher in TgCyto_Myr mice compared with other transgenic and nontransgenic mice. B: myocardial infarct size, measured after 120-min reperfusion, was significantly decreased in TgCyto_Myr hearts. *P < 0.01 compared with the other groups. Number of animals in each group was Wt = 12; TgMito = 8; TgCyto_Nonmyr = 5; and TgCyto_Myr = 6.
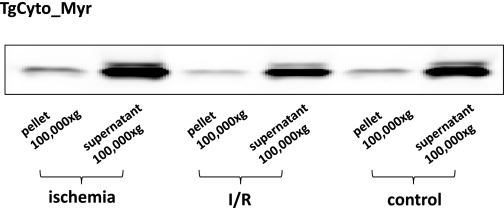
Myristoylated proteins are usually membrane bound when functional. While many myristoylated proteins are constitutively bound to the plasma membrane, there are now examples of reversible translocation of myristoylated proteins between compartments mediated by “myristoyl switches” (32). We considered the possibility that cytosolic, myristoylated MsrA was translocated as a consequence of ischemia or reperfusion (1, 11, 15, 42). However, subcellular fractionation demonstrated that >95% of both myristoylated and nonmyristoylated cytosolic MsrA remained in the 100,000-g supernatant rather than translocating to the plasma membrane, microsomes, or mitochondria (Fig. 6).
Fig. 6.
Subcellular distribution of MsrA in the TgCyto_Myr heart. This Western blot demonstrates no change in subcellular distribution during ischemia or reperfusion (I/R).
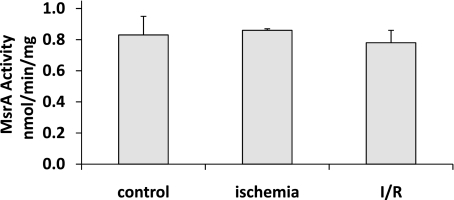
ROS formed during I/R, can be measured in the perfusate effluent (28, 48), and can damage macromolecules, usually with loss of function (18). MsrA activity was not affected by I/R (Fig. 7), indicating that this enzyme is relatively resistant to oxidative inactivation, perhaps in part because it is not metal dependent and thus lacks binding sites for metal-catalyzed oxidation (18).
Fig. 7.
I/R does not inactivate MsrA. Enzymatic activity was determined on 3 hearts in each group. Results shown are means ± SE.
DISCUSSION
We aimed to overexpress enzymatically active MsrA in either the mitochondria or the cytosol. Immunoblotting, subcellular fractionation, and activity assays demonstrated that we were successful. For the cytosolic targeted transgenic mice, we wanted lines that produced either unmodified or myristoylated enzyme, and this was also successful. These mice should assist us and other investigators in elucidating the roles of MsrA in different tissues. We began by studying the Langendorff model of I/R. ROS are produced after I/R, causing oxidation of molecules in the heart, including proteins. As noted in the Introduction, increasing MsrA levels protects against oxidative stresses and reduces protein oxidation. Since mitochondria are a major source of ROS, we expected that the mitochondrially targeted MsrA transgenic heart would be most protected from damage following I/R. Neither protection nor increased damage was observed in the mice with increased mitochondrial MsrA. However, substantial protection was afforded by increasing the cytosolic MsrA, measured both functionally and histochemically (Fig. 5). Protection occurred only when the cytosolic MsrA underwent its normal posttranslational modification of myristoylation. Nonmyristoylated, cytosolic MsrA did not protect.
Myristoylation was originally discovered as a modification that anchors proteins to the plasma membrane, but myristoylated MsrA is a cytosolic enzyme (16). An earlier study (8) of transfected cells found no translocation to membranes in response to a variety of oxidative stresses, and in this study we detected no translocation induced by ischemia alone or I/R. While myristoylation is classically considered a mechanism for targeting proteins to the membrane, either constitutively or reversibly (31), recent investigations (13, 36) demonstrate that myristoylation plays a role in protein-protein interactions, translocation, and cell signaling. With this broader view of the functions of myristoylation, several explanations can be considered to account for the protective ability of myristoylated, cytosolic MsrA. Although mitochondria likely produce most of the ROS in cells, the ROS need not remain in the mitochondria. Mitochondria do release hydrogen peroxide to the cytosol (4), particularly when the integrity of the electron transport chain has been compromised (27, 38), as can occur as a consequence of I/R damage. Alternatively, ROS can be produced in the cytosol (2), for example, by “auto-oxidation” of the elevated levels of glutathione and NAD(P)H caused by ischemia. Cytosolic ROS may then damage proteins essential for cellular function. Increased MsrA can protect those critical proteins, either by scavenging the reactive species (39) or by directly reducing oxidized methionines in the damaged proteins. Myristoylation might promote interaction of MsrA with other proteins required for the protective reactions.
The transgenes were constructed with a strong promoter to assure good overexpression to improve chances of detecting any positive or negative effect of MsrA overexpression. Because the transgenic mice exhibit the expected high levels of overexpression of MsrA in the heart, the physiological significance of protection by the myristoylated, cytosolic enzyme is uncertain. It remains to be seen whether more modest increases in MsrA or the administration of MsrA-mimetic drugs are protective against I/R injury.
Although MsrA has been shown to protect cells and macromolecules against oxidative stress, we should note that increased MsrA may not be protecting through a classical antioxidant mechanism, that is, by scavenging reactive species or repairing oxidized proteins. MsrA might protect through regulation of the activity of other proteins. MsrA has recently been shown to function as a stereo-specific oxidase of methionine residues in proteins (19). Thus reversible oxidation and reduction of methionines in proteins may be a signaling mechanism analogous to reversible phosphorylation and dephosphorylation. If this occurs, overexpression of MsrA may protect against I/R injury by acting on the putative signaling pathway.
Experimental investigation of a possible regulatory role for MsrA has proven difficult because of the lack of methodology capable of detecting and quantitating methionine sulfoxide in proteins. Nevertheless, several proteins have been reported to be regulated by reversible oxidation and reduction of a methionine residue that can be mediated by MsrA. These include a voltage-dependent potassium channel (7), a magnesium channel (6), and the calcium/calmodulin-dependent protein kinase (12). The regulated methionine residue of such proteins could become oxidized during I/R, contributing to cardiac damage. Overexpression of MsrA could thus be protective, and myristoylation may assist in directing the repair enzyme to its target.
GRANTS
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Barbato R, Menabo R, Dainese P, Carafoli E, Schiaffino S, Di LF. Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res 78: 821–828, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555: 589–606, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol 17: 502–510, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J 128: 617–630, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cabreiro F, Picot CR, Perichon M, Mary J, Friguet B, Petropoulos I. Identification of proteins undergoing expression level modifications in WI-38 SV40 fibroblasts overexpressing methionine sulfoxide reductase A. Biochimie 89: 1388–1395, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cao G, Lee KP, van der Wijst J, de GM, van der Kemp A, Bindels RJ, Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem 285: 26081–26087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci USA 94: 9932–9937, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole NB, Daniels MP, Levine RL, Kim G. Oxidative stress causes reversible changes in mitochondrial permeability and structure. Exp Gerontol 45: 596–602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Luca A, Sanna F, Sallese M, Ruggiero C, Grossi M, Sacchetta P, Rossi C, De Laurenzi V, Di Ilio C, Favaloro B. Methionine sulfoxide reductase A down-regulation in human breast cancer cells results in a more aggressive phenotype. Proc Natl Acad Sci USA 107: 18628–18633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douglas T, Daniel DS, Parida BK, Jagannath C, Dhandayuthapani S. Methionine sulfoxide reductase A (MsrA) deficiency affects the survival of Mycobacterium smegmatis within macrophages. J Bacteriol 186: 3590–3598, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eaton P, Awad WI, Miller JI, Hearse DJ, Shattock MJ. Ischemic preconditioning: a potential role for constitutive low molecular weight stress protein translocation and phosphorylation? J Mol Cell Cardiol 32: 961–971, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi N, Titani K. N-myristoylated proteins, key components in intracellular signal transduction systems enabling rapid and flexible cell responses. Proc Jpn Acad Ser B Phys Biol Sci 86: 494–508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horwich AL, Kalousek F, Mellman I, Rosenberg LE. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J 4: 1129–1135, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 52: 2896–2904, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Kim G, Cole NB, Lim JC, Zhao H, Levine RL. Dual sites of protein initiation control the localization and myristoylation of methionine sulfoxide reductase A. J Biol Chem 285: 18085–18094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp Gerontol 36: 1495–1502, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Levine RL, Stadtman ER. Carbonylated proteins and their implication in physiology and pathology. In: Redox Proteomics: from Protein Modifications to Cellular Dysfunction and Diseases, edited by Dalle-Donne I, Scaloni A, Butterfield DA. New York: Wiley Interscience, 2006 [Google Scholar]
- 19. Lim JC, You Z, Kim G, Levine RL. Methionine sulfoxide reductase A is a stereospecific methionine oxidase. Proc Natl Acad Sci USA 108: 10472–10477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120: 245–254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J 23: 464–472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA 98: 12920–12925, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci USA 94: 9585–9589, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci USA 95: 14071–14075, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nan C, Li Y, Jean-Charles PY, Chen G, Kreymerman A, Prentice H, Weissbach H, Huang X. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun 402: 608–613, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol 69: 719–723, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Poderoso JJ, Peralta JG, Lisdero CL, Carreras MC, Radisic M, Schopfer F, Cadenas E, Boveris A. Nitric oxide regulates oxygen uptake and hydrogen peroxide release by the isolated beating rat heart. Am J Physiol Cell Physiol 274: C112–C119, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun 366: 775–778, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Rappaport L, Oliviero P, Samuel JL. Cytoskeleton and mitochondrial morphology and function. Mol Cell Biochem 184: 101–105, 1998 [PubMed] [Google Scholar]
- 31. Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451: 1–16, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol 2: 584–590, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Romero HM, Berlett BS, Jensen PJ, Pell EJ, Tien M. Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol 136: 3784–3794, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T, Chen ML, Joiner MA, Heinemann SH. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA 99: 2748–2753, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmon AB, Perez VI, Bokov A, Jernigan A, Kim G, Zhao H, Levine RL, Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J 23: 3601–3608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorek N, Bloch D, Yalovsky S. Protein lipid modifications in signaling and subcellular targeting. Curr Opin Plant Biol 12: 714–720, 2009 [DOI] [PubMed] [Google Scholar]
- 37. St John G, Brot N, Ruan J, Erdjument-Bromage H, Tempst P, Weissbach H, Nathan C. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc Natl Acad Sci USA 98: 9901–9906, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different dites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Stadtman ER, Moskovitz J, Berlett BS, Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem 234–235: 3–9, 2002 [PubMed] [Google Scholar]
- 40. Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101: 1155–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Toennies G, Kolb JJ. Methionine studies. J Biol Chem 128: 399–405, 1939 [Google Scholar]
- 42. van de Klundert FA, Gijsen ML, van den IJssel PR, Snoeckx LH, de Jong WW. alpha B-crystallin and hsp25 in neonatal cardiac cells–differences in cellular localization under stress conditions. Eur J Cell Biol 75: 38–45, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem 14: 1539–1549, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Vougier S, Mary J, Friguet B. Subcellular localization of methionine sulphoxide reductase A (MsrA): evidence for mitochondrial and cytosolic isoforms in rat liver cells. Biochem J 373: 531–537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yaffe MP. The machinery of mitochondrial inheritance and behavior. Science 283: 1493–1497, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci USA 101: 1159–1164, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao H, Kim G, Liu C, Levine RL. Transgenic mice overexpressing methionine sulfoxide reductase A: characterization of embryonic fibroblasts. Free Radic Biol Med 49: 641–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–1357, 1988 [PubMed] [Google Scholar]