Abstract
Background and Aims
The major objective was to identify plant traits functionally important for optimization of shoot growth and nitrogen (N) economy under drought. Although increased leaf N content (area basis) has been observed in dry environments and theory predicts increased leaf N to be an acclimation to drought, experimental evidence for the prediction is rare.
Methods
A pedigree of 200 full-sibling hybrid willows was pot-grown in a glasshouse in three replicate blocks and exposed to two water regimes for 3 weeks. Drought conditions were simulated as repeated periods of water shortage. The total leaf mass and area, leaf area efficiency (shoot growth per unit leaf area, EA), area-based leaf N content (NA), total leaf N pool (NL) and leaf N efficiency (shoot growth per unit leaf N, EN) were assessed.
Key Results
In the water-stress treatment, shoot biomass growth was N limited in the genotypes with low NL, but increasingly limited by other factors in the genotypes with greatest NL. The NA was increased by drought, and drought-induced shift in NA varied between genotypes (significant G × E). Judged from the EA–NA relationship, optimal NA was 16 % higher in the water-stress compared with the well-watered treatment. Biomass allocation to leaves and shoots varied between treatments, but the treatment response of the leaf : shoot ratio was similar across all genotypes.
Conclusions
It is concluded that N-uptake efficiency and leaf N efficiency are important traits to improve growth under drought. Increased leaf N content (area basis) is an acclimation to optimize N economy under drought. The leaf N content is an interesting trait for breeding of willow bioenergy crops in a climate change future. In contrast, leaf biomass allocation is a less interesting breeding target to improve yield under drought.
Keywords: Biomass allocation, biomass production, drought, leaf nitrogen, plant breeding, trait functionality
INTRODUCTION
Knowledge on the functionality of plant traits affecting growth and resource economy is important for the basic understanding of plants, and relevant for breeding research. The availability of water and mineral nutrients, among them nitrogen (N), are important determinants of plant growth, and crop breeding programmes frequently target water and N use efficiency along with drought resistance. Water and N use are interrelated, and it is often unclear to what extent growth reduction under drought is a consequence of reduced stomatal conductance and CO2 uptake, or reduced N uptake and accumulation in dry soils (Chaves et al., 2003). In a field survey performed in areas receiving different amounts of precipitation, low-rainfall species have been observed to operate with higher leaf N content (area basis) than species grown in high-rainfall environments (Wright et al., 2003). In a theoretical approach, total assimilation was suggested to be a scaled linear sum of total N and total transpiration (Farquhar et al., 2002). Hence, limited water supply is expected to go along with reduced leaf area to lower transpiration, and optimization of N economy under limited water supply may be accomplished by increased leaf N content per area (Farquhar et al., 2002). The prediction has rarely been evidenced experimentally, but could be tested by using the definition of optimal leaf N content suggested by Hirose (1984).
Functional adaptation to the availability of growth-limiting resources frequently implies changed biomass allocation. For example, ‘functional equilibrium’ or ‘resource balancing’ theory predicts that plants invest abundant resources in order to increase the gain of scarcer resources, and thus reduce the risk of being growth-limited by a single resource (Brouwer, 1962; Bloom et al., 1985; Ericsson, 1995). Assuming resource balancing to be an adaptive mechanism for growth under strong water limitation, plants should allocate biomass predominantly to roots (to counteract water/nutrient limitation), at the cost of reduced biomass allocation to leaves. Biomass allocation to different plant organs is affected by a combination of environmental factors, such as resource availability, ontogeny/plant size and genotype (Poorter and Nagel, 2000). Reduced leaf biomass allocation in response to limited water supply, as reported in many studies (review by Poorter and Nagel, 2000), could therefore reflect functional optimization independent of plant ontogeny/size and/or the outcome of drought-induced size difference. This distinction is important when functional adaptation of plants is discussed in the context of plant breeding. Reduced leaf biomass allocation in response to drought is interesting for breeding only if it is the result of optimized plant function and independent of plant size. In this case, an allometric analysis using log–log relations between biomass of different plant organs is an appropriate analysis (Farrar and Gunn, 1998; Poorter and Nagel, 2000). Theory and also experimental evidence indicate a linear log–log relationship between leaf, stem and root biomass across a broad range of taxa inhabiting diverse ecological habitats including dry sites (Enquist and Niklas, 2002). If the biomass allocation rule reported by Enquist and Niklas (2002) applies for intraspecific comparisons within, for example breeding populations, drought-induced differences in biomass allocation cannot be interpreted by means of functional optimization independent of plant size, and are thus less relevant for plant breeding. ‘Resource balancing’ might apply for the allocation of biomass and also mineral nutrients within plants. In that case, we expect that plants predominantly growth-limited by water (rather than N) would allocate more N per unit leaf area to increase assimilation at reduced total leaf area and stomatal conductance (Farquhar et al., 2002). High N accumulation capacity under drought and optimized leaf N per unit area in response to drought would then be desirable traits for plant breeding.
Many species of the genus Salix are fast growing, easy to propagate vegetatively and commercially used for biomass production (Kuzovkina et al., 2008). Breeding programmes for Salix have been established (Gullberg, 1993; Karp et al., 2011), linkage maps are available (Rönnberg-Wästljung et al., 2003; Hanley et al., 2006; Berlin et al., 2010) and candidate genetic markers have been identified for various traits, among them growth performance under drought (Rönnberg-Wästljung et al., 2005; Weih et al., 2006). Breeding populations are available and allow the comparison of a range of genotypes that may vary in the traits of interest, but are similar in confounding attributes associated with, for example, leaf longevity and functional type. A mapping population, here of Salix, grown under suboptimal conditions, offers an opportunity to test hypotheses regarding the functionality of traits thought to be adaptive.
The objective here was to identify plant traits functionally important for optimization of shoot growth and N economy under drought. Several hypotheses were tested: (1) plant growth is N-limited under drought, and N-uptake efficiency is a critical trait for growth both under well-watered and water-stress conditions; (2) biomass allocation to leaves and shoots across a range of closely related genotypes grown in well-watered and water-stressed conditions, follows a linear log–log relationship similar to the relationship reported by Enquist and Niklas (2002); and (3) optimal leaf N content (area basis; sensu Hirose, 1984) is higher in drought-treated compared with well-watered plants. These hypotheses were tested by using a pedigree of 200 full-sibling willow (Salix) genotypes exposed to two contrasting environments differing in irrigation.
MATERIALS AND METHODS
Plant material, growth conditions and harvest procedure
A subset of 200 genotypes from a mapping population with, in total, 463 F1 offspring originating from a cross between a male diploid hybrid Salix viminalis × S. schwerinii variety (‘Björn’) and a female diploid S. viminalis variety (‘L78183’) was used. The subset of 200 genotypes included the parents of the pedigree. The hybrid variety ‘Björn’, as well as its full-sib ‘Tora’, was bred for high productivity under favourable growth conditions, while the natural variety ‘L78183’ was collected in southern Sweden. Based on the results from previous studies comparing the parental genotypes or their full-sib relative [e.g. ‘Tora’ and ‘L78183’ compared by Weih (2001) and Weih and Nordh (2002)], we expected great variation in drought tolerance across the F1 offspring. The parents and 198 F1 offspring genotypes were vegetatively propagated using 5-cm cuttings.
The cuttings were planted in 2-L plastic pots filled with a mixture of two-thirds of the growth medium Weibulls ‘Kron Mull’ (organic matter 95 %; pH 5·5–6·5; 180 g m−3 N, 110 g m−3 P, 195 g m−3 K, 260 g m−3 Mg, 100 g m−3 S, 2000 g m−3 Ca) and one-third of perlite in mid-April 2009. All plants were grown in a ventilated glasshouse in Uppsala, central Sweden, at slightly (approx. 2–6 K) above-ambient temperature (mean temperature 21·8 °C during the treatment period) and ambient light conditions (>12 h photoperiod). During daytime photosynthetically active radiation (400–700 nm) at plant height varied between 280 and 350 µmol m−2 s−1 during the treatment. The pots were arranged in three blocks and individual genotypes (2 × 200 in each block) were positioned randomly within two randomly assigned treatments in each block. During the first 5 weeks all plants were watered daily to field capacity of the substrate (‘well-watered’). Competing shoots were regularly removed before they reached 1 cm length to keep shoot numbers similar and reduce effects on shoot number caused by heterogeneous bud development from cuttings. After 5 weeks of growth initial non-destructive assessments of stem height and leaf chlorophyll content (SPAD-502 leaf chlorophyll meter; Konica Minolta Sensing, Japan) were carried out and two experimental treatments (well-watered, WW; water-stress, WS) were started. Plants in the WW treatment received about 120 mL water per pot per day. In the WS treatment the plants were exposed to repeated periods of water shortage. Thus, the plants in the WS treatment were watered to field capacity of the soil (approx. 120 mL per pot) on days 8, 14, 15 and 19 from the start of the experimental treatments. Individual plants were supplied with a complete fertilizer solution (12·6 mg N per pot) on days 0 and 14 from the start of the experimental period. The number of leaves abscised during the treatment was recorded. The final harvest of leaves and shoots, in total 1200 individuals, began after 20 d of treatment, and blocks containing plants from both treatments were harvested successively during a period of 2 weeks.
Harvested plant parts were separated into shoots and leaves. For all individuals, leaf area and leaf chlorophyll content (measured as SPAD units) were determined on three samples of fresh leaves (top, middle and bottom of canopy) with a LI-COR LI-3100 leaf area meter (Lambda Instruments, Nebraska, USA). The sample leaves were dried to constant weight, and specific leaf area (SLA) was calculated as mean for the three sample leaves of individual plants. All leaves and shoots were dried at 70 °C for 48 h and weighed. Total leaf area of individual plants was estimated by multiplying the SLA of sample leaves with the total leaf biomass of individual plants. In general, the SPAD value can be used as an estimate of leaf N content [Weih and Rönnberg-Wästljung (2007) for Salix]. The N content of a subset of sample leaves was determined with a mass spectrometer (ANCA-MS, Europe Scientific Ltd, Crewe, UK) to establish the relationship between area-based leaf N content (NA) and SPAD in this study. A significant relationship between SPAD values and NA was confirmed on a subset of 108 sample leaves selected randomly among all genotypes and representing the whole range of observed SPAD values (logarithmic regression, P < 0·001). The relationship was used to estimate the NA of sample leaves for all individuals. The total leaf N pool (NL) of individual plants was calculated by multiplying the NA with the total leaf area (AL).
Analysis of growth and N economy
Growth and N economy analysis involved the assessment of shoot and leaf biomass (BS and BL, respectively), leaf area efficiency (shoot biomass per unit of mean leaf area during the experiment, EA), N-uptake efficiency (here equivalent to N accumulation in leaves at final harvest, NL) and leaf N efficiency (shoot biomass per unit mean leaf N during the experiment, EN) according to the general concept by Weih et al. (2011). The relationship between EA and NA was analysed to evaluate optimum NA according to the concept of Hirose (1984). Plant growth and N economy of the 1200 plants (200 genotypes × 3 replicates × 2 treatments) were assessed by means of the accumulation of biomass (shoots and leaves), leaf area and leaf N through the experiment.
Statistical analysis
Regression analysis was performed to assess the relationships between various traits separately for the two treatments. Analysis of covariance (ANCOVA) was used to assess the effects of treatment, genotype and block (covariate), and the treatment × genotype interaction, on the various traits recorded. The shoot height at the beginning of the experimental treatment period was used as an additional covariate in ANCOVA on BS and NL in order to statistically consider the effects of initial plant size on the two traits. Shoot biomass and leaf N pool were loge transformed prior to ANCOVA to ensure homogeneity of variances. The number of leaves abscised during the experiment was used as a covariate in ANCOVA on leaf : shoot biomass ratio. All statistics was computed using the SPSS statistical software package (Release 17·0; SPSS Inc., Chicago, IL, USA).
RESULTS
Parental genotypes
The natural female variety ‘L78183’ had higher leaf N content (NA), similar leaf N pool (NL), but lower shoot biomass (BS), leaf area (AL) and N efficiency (EN) than the hybrid male variety ‘Björn’ (Table 1). Treatment (WS and WW) responses ranged from strongly negative (BS, AL, EN) through neutral (SLA) to positive (NA) and varied between the two genotypes (ANOVA P ≤ 0·05 not shown). For example, ‘Björn’ showed stronger reduction of AL, NL and EN in response to water stress compared with ‘L78183’.
Table 1.
Abbreviations of the phenotypic traits measured and the mean values (± s.d.) for the two parents of the F1 pedigree exposed to two different water regimes (well-watered and water-stressed) in a glasshouse for 3 weeks
| Abbreviation | Trait (unit) | Well-watered (mean) |
Water-stressed (mean) |
||
|---|---|---|---|---|---|
| Female* | Male† | Female* | Male† | ||
| BS | Shoot biomass at final harvest (g) | 2·34 ± 0·39 | 2·93 ± 0·87 | 1·16 ± 0·35 | 1·49 ± 0·14 |
| NL | Total leaf N pool at final harvest (mmol) | 3·67 ± 0·66 | 3·80 ± 0·87 | 2·61 ± 0·43 | 2·41 ± 0·01 |
| AL | Total leaf area at final harvest (m2) | 0·088 ± 0·011 | 0·108 ± 0·026 | 0·056 ± 0·015 | 0·062 ± 0·002 |
| SLA | Specific leaf area (m2 kg−1) | 35·8 ± 2·3 | 37·8 ± 1·8 | 33·1 ± 1·5 | 36·5 ± 0·3 |
| NA | Area-based leaf N content (mmol m−2) | 42·4 ± 4·8 | 35·7 ± 1·3 | 47·6 ± 3·9 | 39·3 ± 1·2 |
| EN | Leaf N efficiency (g mmol−1) | 1·29 ± 0·15 | 1·51 ± 0·13 | 0·88 ± 0·16 | 1·27 ± 0·14 |
* Salix viminalis (‘L78183’), † S. viminalis × S. schwerinii (‘Björn’).
Growth and N economy under well-watered and water-stressed conditions
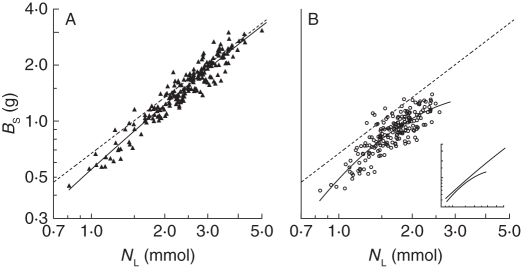
Shoot biomass (BS) was an almost linear function (slope close to 1·0) of the leaf N pool (NL) in the WW treatment, indicating that N accumulation was the major determinant of shoot growth in these plants (Fig. 1A). In the WS treatment, BS increased with NL in a similar relationship compared with the WW treatment up to an NL of around 2 mmol (Fig. 1B). At greater values of NL, diminishing slope indicated that the shoot biomass growth of the WS plants was increasingly limited by factors other than N accumulation. The pattern in shoot biomass was similar to the pattern in total above-ground biomass (not shown). The BS, SLA, NL and NA were all significantly affected by treatment and genotype (Tables 2 and 3). Also block affected these traits (Table 3): The BS, SLA and NL increased from the first to the least-harvested block, whereas NA decreased. The reduction in BS by water stress was the result of a greater reduction in NL than EN (Table 2). The effect of water availability on BS and NA varied between genotypes (Table 3, interaction effects).
Fig. 1.
Relationships between final shoot biomass (BS) and leaf N pool (NL) of 200 willow genotypes (means of three replicates) grown experimentally in a glasshouse under well-watered (A) and water-stressed (B) conditions. For ease of comparison, the regression lines for the two water regimes are shown in the same figure in the inset. Broken lines indicate slope of 1, i.e. a hypothetically constant leaf N efficiency (g mmol−1) across all genotypes and treatments. Quadratic regressions: (A) loge y = 1·192 loge x – 0·056 (loge x)2 – 0·597, r2 = 0·92, P < 0·001, n = 200; (B) loge y = 1·410 loge x – 0·480 (loge x)2 – 0·715, r2 = 0·71, P < 0·001, n = 200. Note the loge scales.
Table 2.
Means (± s.d.) of major growth and N economy traits for 200 willow genotypes (three replicates) grown in two water regimes (well-watered and water-stressed)
| Trait | Unit | Well-watered | Water-stressed |
|---|---|---|---|
| BS | g | 1·67 ± 0·77 | 0·92 ± 0·29 |
| NL | mmol | 2·62 ± 1·07 | 1·75 ± 0·52 |
| AL | m2 | 0·072 ± 0·025 | 0·042 ± 0·011 |
| SLA | m2 kg−1 | 41·0 ± 5·9 | 36·3 ± 5·5 |
| NA | mmol m−2 | 35·8 ± 6·9 | 41·4 ± 8·5 |
| EN | g mmol−1 | 1·25 ± 0·19 | 1·05 ± 0·18 |
The plants were grown in a glasshouse and exposed to the treatments for 3 weeks.
Table 3.
Analysis of covariance for the fixed effects of genotype and irrigation, and the interactions between them, on shoot biomass (BS), leaf N pool (NL), leaf N content (NA) and specific leaf area (SLA) assessed in 200 willow genotypes pot-grown in the glasshouse
| Source of variation | d.f. |
BS (g) |
NL (mmol) |
NA (mmol m−2) |
SLA (m2 kg−1) |
||||
|---|---|---|---|---|---|---|---|---|---|
| MS | P | MS | P | MS | P | MS | P | ||
| Block | 1 | 0·49 | 0·019 | 0·37 | 0·019 | 3888 | <0·001 | 1375 | <0·001 |
| Initial shoot height | 1 | 70·34 | <0·001 | 48·36 | <0·001 | – | – | – | – |
| Genotype (G) | 199 | 0·11 | 0·034 | 0·10 | <0·001 | 101 | <0·001 | 45 | <0·001 |
| Irrigation (I) | 1 | 88·76 | <0·001 | 31·40 | <0·001 | 9150 | <0·001 | 7093 | <0·001 |
| G × I | 199 | 0·12 | 0·049 | 0·07 | 0·185 | 57 | 0·003 | 24 | 0·150 |
| Error | 780* | 0·09 | 0·07 | 42 | 22 | ||||
Block and initial shoot height (cm) at the start of the treatments were used as covariates. BS and NL were loge transformed prior to analysis.
Significant differences (P < 0·05) are highlighted in bold.
d.f., Degrees of freedom; MS, mean squares (variance).
* d.f. = 718 for the analyses of NA and SLA, in which no covariate was used and individuals with BS < 0·5 g were excluded.
The BL increased proportional to BS (Fig. 2A). When assessed across both treatments, the slope of the linear log–log relationship between BL and BS was 0·77 and similar to the predicted slope of 0·75 by Enquist and Niklas (2002). Separate regressions within the two treatments, and considering only data in the plant-size range common to the two treatments, resulted in a significantly reduced slope in the WS compared with the WW treatment (t-test of slopes; Fig. 2B–C). In addition, water stress and genotype significantly affected loge-transformed leaf : shoot biomass ratio, but the treatment response was similar across genotypes (ANCOVA with covariate number of abscised leaves, P < 0·001 irrigation, P < 0·001 genotype, P = 0·973 genotype × irrigation).
Fig. 2.
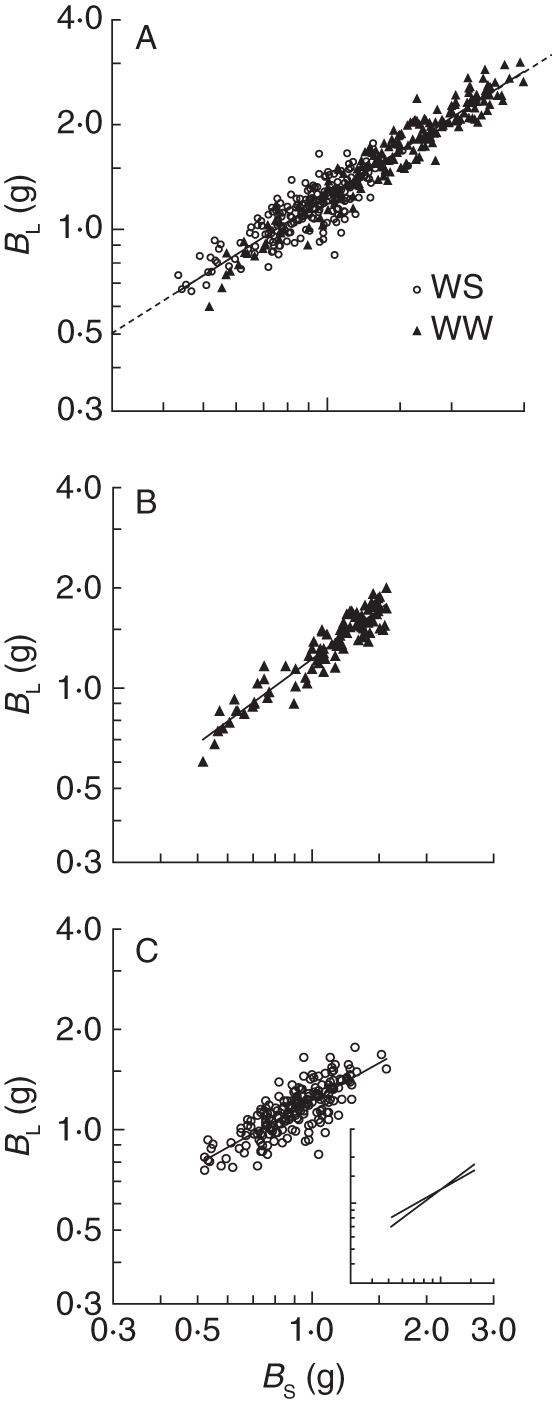
Relationships between final leaf biomass (BL) and shoot biomass (BS) in 200 willow genotypes (means of three replicates) grown experimentally in a glasshouse in two water regimes (WS, water stress; WW, well-watered). (A) The BL vs. BS relationship across all treatments, genotypes and plant sizes. Linear regression: loge y = 0·76 loge x + 0·217, r2 = 0·91, P < 0·001, n = 400. The broken line in (A) indicates a slope of 0·75, which was predicted by Enquist and Niklas (2002). In (B) and (C) the BL vs. BS relationship is plotted separately for the two treatments and including only plants of common plant size in the two treatments. Linear regressions: (B) loge y = 0·84 loge x + 0·201, r2 = 0·89, P < 0·001, n = 189; (C) loge y = 0·64 loge x + 0·202, r2 = 0·61, P < 0·001, n = 193. For ease of comparison, the regression lines for the two water regimes (B, C) are shown in the same figure in the inset in (C). t-test of slopes (B) vs. (C): t = –4·39, P < 0·001. Note the loge scales.
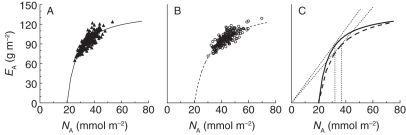
The leaf area efficiency (EA) increased with NA, but the curve levelled off at higher NA in the WS treatment compared with the WW treatment (Fig. 3). In Fig. 3, the tangent from the origin to the curve indicates the transition from being N-limited to C-limited and, in that sense, optimal N concentration according to Hirose (1984). Applying this definition, optimal NA was 16 % higher in the WS (37 mmol m−2) compared with the WW treatment (32 mmol m−2) (Fig. 3). Increasing NA was generally associated with decreasing leaf N efficiency (EN) (Pearson correlation r = – 0·39, P < 0·001, n = 386). Despite lower NL in WS plants, mean NA was greater than in the WW plants (Tables 2 and 3).
Fig. 3.
The leaf area efficiency (EA) against the area-based leaf-nitrogen (NA) across 200 willow genotypes (means of three replicates) grown experimentally in a glasshouse under well-watered (A) and water-stress (B) conditions. The relationships were fitted by hyperbolic functions of the form: (A) EA = a · (NA – 20)/[1 + b · (NA – 20)]; a = 19·20, b = 0·136, r2 = 0·70, P < 0·001, n = 200; (B) a = 13·06, b = 0·088, r2 = 0·66, P < 0·001, n = 199. In (C), the curves for the two water regimes are shown in the same figure, and tangents from the origin to the curves indicating optimal NA according to Hirose (1984), are drawn with dotted lines.
DISCUSSION
Using 200 different Salix genotypes, this study shows how the functionality of key adaptive plant leaf traits varies according to water-stress. The large number of plants included, made it unfeasible to harvest below-ground plant parts. The lack of root data certainly is a limitation, but it should not have affected the general conclusions on allocation pattern made only with respect to the leaf-to-shoot allocation. Leaf N content (area basis) was generally low compared with willows grown outdoors (cf. Weih, 2001), which reflects acclimation to relatively low irradiance in the glasshouse (cf. Niinemets, 1997). A general pattern of increased leaf N content (area basis) in response to water shortage was also found in full-sib parental relatives of the same willow pedigree as was used here when grown outdoors at ambient temperature (Weih, 2001). We therefore believe that the conclusions made here with respect to the leaf N content and N economy under drought are relevant for field conditions.
Optimization of N economy under drought
The fact that nitrogen was a major determinant of shoot growth for the majority of genotypes grown under water stress supports our first hypothesis. The N-uptake efficiency is therefore a critical trait for these plants, at least when grown with moderate N availability as in this study. Contrary to our initial hypothesis, the shoot growth of some genotypes, i.e. the ones with greatest N accumulation in the WS treatment, was increasingly limited by factor(s) other than N, likely CO2 through reduced stomatal conductance (e.g. Chaves et al., 2003; Wikberg and Ögren, 2007). A slope of around 1·0 in the BS vs. NL relationship indicates similar leaf N efficiency (EN), and the diminishing slope for the genotypes with the greatest N accumulation in the WS treatment indicates decreased EN (Fig. 1). The EN values reported here are generally lower than the EN of field-grown willow stands reported by Weih et al. (2011), probably due to the juvenile stage and the short experimental period in this study compared with the adult plants and whole growing season used for the assessment of EN in the field-grown willow.
The leaf area efficiency (EA) is an approximation of the net assimilation rate by Hirose (1984) and indicates a net photosynthesis minus respiration loss. Light-saturated photosynthesis is N-limited at low leaf N content and becomes C-limited at higher leaf-nitrogen (Hirose, 1984). When stomatal conductance is reduced, such as under drought conditions, light-saturated photosynthesis should become C-limited at a higher leaf N content than when stomatal conductance is higher. Furthermore under drought, optimal leaf N content should increase in association with the decreased slope of the photosynthesis–leaf-N relationship (Schieving, 1998). We found a decreased slope of the EA–NA relationship when 200 taxonomically closely related willow genotypes with variable NA were exposed to a water-stress treatment. Our results support the view that the high leaf N frequently observed in drought-exposed plants is an acclimation to water stress (Hirose, 1984; Schieving, 1998; Farquhar et al., 2002). The decrease in leaf N after termination of treatments and from early- to late-harvested plants indicates that the acclimation is probably an opportunistic short-term event in these plants. The acclimation enables plants to make better use of the available N resources when leaf area and stomatal conductance are greatly reduced (Farquhar et al., 2002; Wright et al., 2003). The results confirm our third hypothesis and are in line with the model predictions by Farquhar et al. (2002). Increased leaf N content (area basis) is therefore concluded to be a functional adaptation to, and not a passive consequence of, water shortage and therefore potentially relevant as a breeding target for crops to be grown in a climate change future with increased risk of drought.
Drought-induced shift in biomass allocation to shoots and leaves
Drought-induced differences in biomass allocation to shoots and leaves of the 200 genotypes assessed here closely followed the corresponding general allometric rule predicted by Enquist and Niklas (2002), which is in support of our second hypothesis. In a separate analysis for the two irrigation treatments (Fig. 2B, C) we followed Poorter and Nagel (2000) and considered only data for plants of common plant size range in the two treatments. The separate analysis resulted in different biomass allocation between leaves and shoots in the two irrigation treatments, which means that the allocational shift in response to drought is a functional adaptation to drought independent of plant size (Poorter and Nagel, 2002). The results are in line with the ‘functional equilibrium’ (or ‘resource balancing’) theory (Brouwer, 1962; Bloom et al., 1985; Ericsson, 1995), but partly conflict with some studies on forest trees and at stand level (Coyle et al., 2008).
In summary, the major effects of decreased water supply on plant function include decreased shoot growth due to decreased leaf biomass and leaf area allocation, and increased leaf N content (area basis). In a study on birch, similar effects on growth, leaf allocation and leaf N content were observed in response to decreased air temperature (Weih and Karlsson 2001). It is also well known that leaf N content is higher in sunny than shady environments (Field et al., 1983; Niinemets, 1997). In natural environments, decreased water supply usually occurs in combination with increased air temperature and irradiance. In those circumstances, the overall effects of decreased water supply, elevated air temperature and increased irradiance on plant function might greatly interact. Such interaction might explain the shifting results in drought responses observed in natural environments (Chaves et al., 2003).
Implications for breeding
Crop breeding research often focuses on improved yield under drought and drought resistance (Monclus et al., 2005; Davies et al., 2010). The parental genotypes in this study varied considerably in many traits relevant for N economy under drought, indicating a potential for plant breeding when crossed. Historically, a changed biomass-allocation pattern has been a most successful breeding strategy (Khush, 2001). In general, decreased leaf biomass allocation in response to water shortage is an adjustment to limit transpiration and water loss. However, within the two treatments of our study, all genotypes allocated biomass to leaves and shoots according to similar rules determined by plant size, and genotype × irrigation interaction on the leaf : shoot ratio was far from significant. Based on the results, leaf biomass allocation per se can hardly be considered as an interesting breeding target to improve yield under drought. We have currently not analysed genetic markers (QTL) in this study, but it is interesting in this context that Weih et al. (2006) did not identify any significant QTL for leaf biomass allocation in Salix grown in an irrigation contrast.
The N-uptake efficiency and leaf N efficiency are both relevant breeding targets to improve growth under drought, and optimized leaf N content (area basis) can improve leaf N efficiency. We show here that increased leaf N content under drought is a functional optimization of N economy and possibly relevant for breeding. However, high leaf N under drought is apparently not enough to maximize growth performance under drought. The female parent ‘L78183’ had very high NA in the WS treatment, but was clearly outperformed in terms of growth by the male parent ‘Björn’ that had lower NA (Table 1). A high leaf N under drought needs to be combined with other plant characteristics to make use of the high photosynthetic capacity it is associated with, which needs to be considered in any plant breeding approach targeting growth improvement under drought. For example, N distribution in canopies can be optimized to increase canopy N use efficiency (Hikosaka, 2003; Weih and Rönnberg-Wästljung, 2007), and canopy N distribution varies between plants exposed to different water regimes (Bonosi et al., 2010). We found significant G × E interaction for NA in this study, and several significant QTL for NA were identified in another study on Salix grown in an irrigation contrast (Rönnberg-Wästljung et al., 2005; Weih et al., 2006). The results are promising, and further confirm that the leaf N content probably is an interesting trait for breeding of willow bioenergy crops in a future of climate change. In contrast, our results indicate that leaf biomass allocation is a less interesting breeding target to improve yield under drought.
ACKNOWLEDGEMENTS
We are grateful to N.-E. Nordh, who helped tend the plants and harvest the material. All authors would like to acknowledge funding from the Swedish Energy Agency.
LITERATURE CITED
- Berlin S, Lagercranz U, Von Arnold S, Öst T, Rönnberg-Wästljung AC. High-density linkage mapping and evolution of paralogs and orthologs in Salix and Populus. BMC Genomics. 2010;11:129. doi: 10.1186/1471-2164-11-129. doi:10.1186/1471-2164-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS, III, Mooney HA. Resource limitation in plants: an economic analogy. Annual Review of Ecology and Systematics. 1985;16:363–392. [Google Scholar]
- Bonosi L, Ghelardini L, Weih M. Growth responses of 15 Salix genotypes to temporary water stress are different from the responses to permanent water shortage. Trees. 2010;24:843–854. [Google Scholar]
- Brouwer R. Distribution of dry matter in the plant. Netherlands Journal of Agricultural Sciences. 1962;10:399–408. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Coyle DR, Coleman MD, Aubrey DP. Above- and below-ground biomass accumulation, production, and distribution of sweetgum and loblolly pine grown with irrigation and fertilization. Canadian Journal of Forest Research. 2008;38:1335–1348. [Google Scholar]
- Davies WJ, Zhang J, Yang J, Dodd IC. Novel crop science to improve yield and resource use efficiency in water-limited agriculture. Journal of Agricultural Science. 2010;149(Suppl. 1):123–131. [Google Scholar]
- Enquist BJ, Niklas KJ. Global allocation rules for patterns of biomass partitioning in seed plants. Science. 2002;295:1517–1520. doi: 10.1126/science.1066360. [DOI] [PubMed] [Google Scholar]
- Ericsson T. Growth and shoot:root ratio of seedlings in relation to nutrient availability. Plant and Soil. 1995;168–169:205–214. [Google Scholar]
- Farquhar GD, Buckley TN, Miller JM. Optimal stomatal control in relation to leaf area and nitrogen content. Silva Fennica. 2002;36:625–637. [Google Scholar]
- Farrar JF, Gunn S. Allocation: allometry, acclimation – and alchemy? In. In: Lambers H, Poorter H, Van Muuren MMI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden, The Netherlands: Backhuys Publishers; 1998. pp. 183–198. [Google Scholar]
- Field C, Merino J, Mooney HA. Compromises between water use efficiency and nitrogen use efficiency in 5 species of Californian evergreens. Oecologia. 1983;60:384–389. doi: 10.1007/BF00376856. [DOI] [PubMed] [Google Scholar]
- Gullberg U. Towards making willows pilot species for coppicing production. Forestry Chronicle. 1993;69:721–726. [Google Scholar]
- Hanley SJ, Mallott MD, Karp A. Alignment of Salix linkage map to the Populus genomic sequence reveals macrosynteny between willow and poplar genomes. Tree Genetics and Genomes. 2006;3:35–48. [Google Scholar]
- Hikosaka K. A model of dynamics of leaves and nitrogen in a plant canopy: an integration of canopy photosynthesis, leaf life span and nitrogen use efficiency. American Naturalist. 2003;162:149–164. doi: 10.1086/376576. [DOI] [PubMed] [Google Scholar]
- Hirose T. Nitrogen use efficiency in growth of Polygonum cuspidatum Sieb. et Zucc. Annals of Botany. 1984;54:695–704. [Google Scholar]
- Karp A, Hanley SJ, Trybush SO, Macalpine W, Pei M, Shield I. Genetic improvement of willow for bioenergy and biofuels. Journal of Integrative Plant Biology. 2011;53:151–165. doi: 10.1111/j.1744-7909.2010.01015.x. [DOI] [PubMed] [Google Scholar]
- Khush GS. Green revolution: the way forward. Nature Reviews – Genetics. 2001;2:815–822. doi: 10.1038/35093585. [DOI] [PubMed] [Google Scholar]
- Kuzovkina Y, Weih M, Abalos Romero M, et al. Salix: botany and global horticulture. Horticultural Reviews. 2008;34:447–489. [Google Scholar]
- Monclus R, Dreyer E, Delmotte FM, et al. Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoides x P. nigra clones. New Phytologist. 2005;167:53–62. doi: 10.1111/j.1469-8137.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- Niinemets U. Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Functional Ecology. 1997;11:518–531. [Google Scholar]
- Poorter H, Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology. 2000;27:595–607. [Google Scholar]
- Rönnberg-Wästljung AC, Tsarouhas V, Semerikov V, Lagercranz U. A genetic linkage map of a tetraploid Salix viminalis × S. dasyclados hybrid based on AFLP markers. Forest Genetics. 2003;10:185–194. [Google Scholar]
- Rönnberg-Wästljung AC, Glynn C, Weih M. QTL analyses of drought tolerance and growth for a Salix dasyclados × Salix viminalis hybrid in contrasting water regimes. Theoretical and Applied Genetics. 2005;110:537–549. doi: 10.1007/s00122-004-1866-7. [DOI] [PubMed] [Google Scholar]
- Schieving F. Plato's plant: on the mathematical structure of simple plants and canopies. Leiden: Backhuys Publishers; 1998. [Google Scholar]
- Weih M. Evidence for increased sensitivity to nutrient and water stress in a fast-growing hybrid willow compared with a natural willow clone. Tree Physiology. 2001;21:1141–1148. doi: 10.1093/treephys/21.15.1141. [DOI] [PubMed] [Google Scholar]
- Weih M, Karlsson PS. Growth response of mountain birch to air and soil temperature: is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytologist. 2001;150:147–155. [Google Scholar]
- Weih M, Nordh NE. Characterising willows for biomass and phyto-remediation: growth, nitrogen and water use of 14 willow clones under different irrigation and fertilisation regimes. Biomass and Bioenergy. 2002;23:397–413. [Google Scholar]
- Weih M, Rönnberg-Wästljung AC. Shoot biomass is related to vertical leaf nitrogen gradient across Salix canopies. Tree Physiology. 2007;27:1551–1559. doi: 10.1093/treephys/27.11.1551. [DOI] [PubMed] [Google Scholar]
- Weih M, Rönnberg-Wästljung AC, Glynn C. Genetic basis of phenotypic correlations among growth traits in hybrid willow (Salix dasyclados × S. viminalis) grown under two water regimes. New Phytologist. 2006;170:467–477. doi: 10.1111/j.1469-8137.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Weih M, Asplund L, Bergkvist G. Assessment of nutrient use in annual and perennial crops: A functional concept for analyzing nitrogen use efficiency. Plant and Soil. 2011;339:513–520. [Google Scholar]
- Wikberg J, Ögren E. Variation in drought resistance, drought acclimation and water conservation in four willow cultivars used for biomass production. Tree Physiology. 2007;27:1339–1346. doi: 10.1093/treephys/27.9.1339. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Least-cost input mixtures of water and nitrogen for photosynthesis. American Naturalist. 2003;161:98–111. doi: 10.1086/344920. [DOI] [PubMed] [Google Scholar]