Abstract
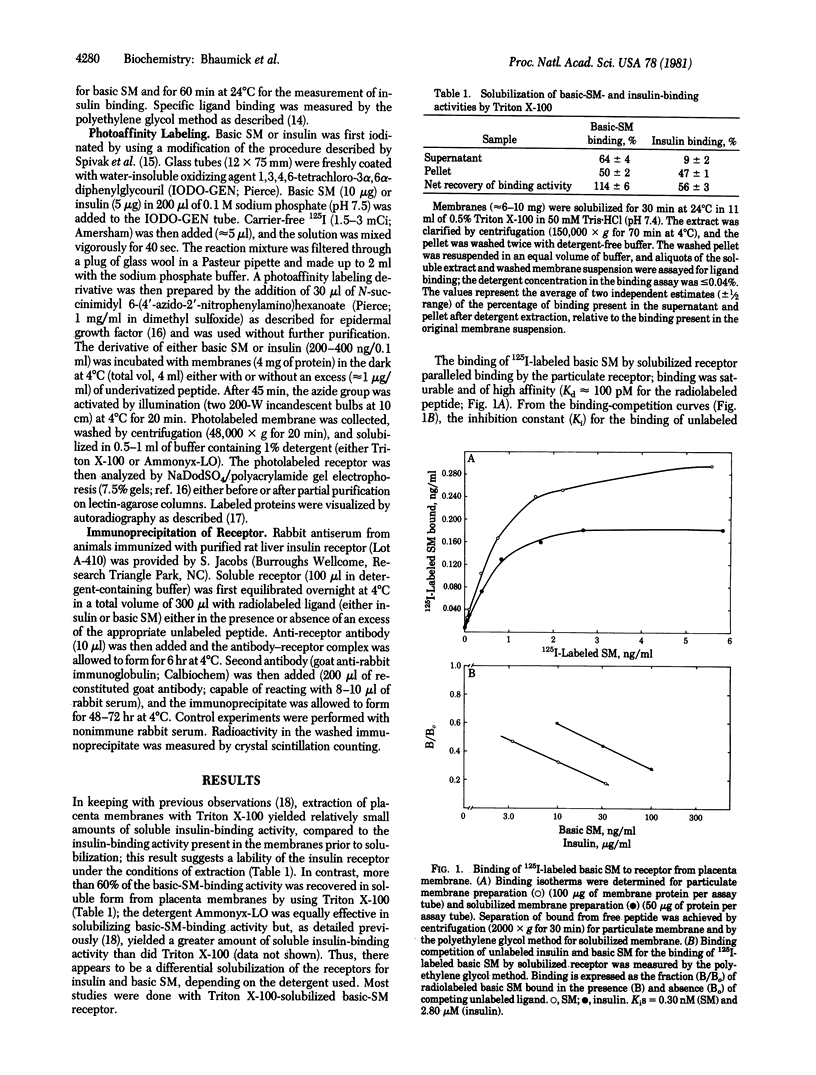
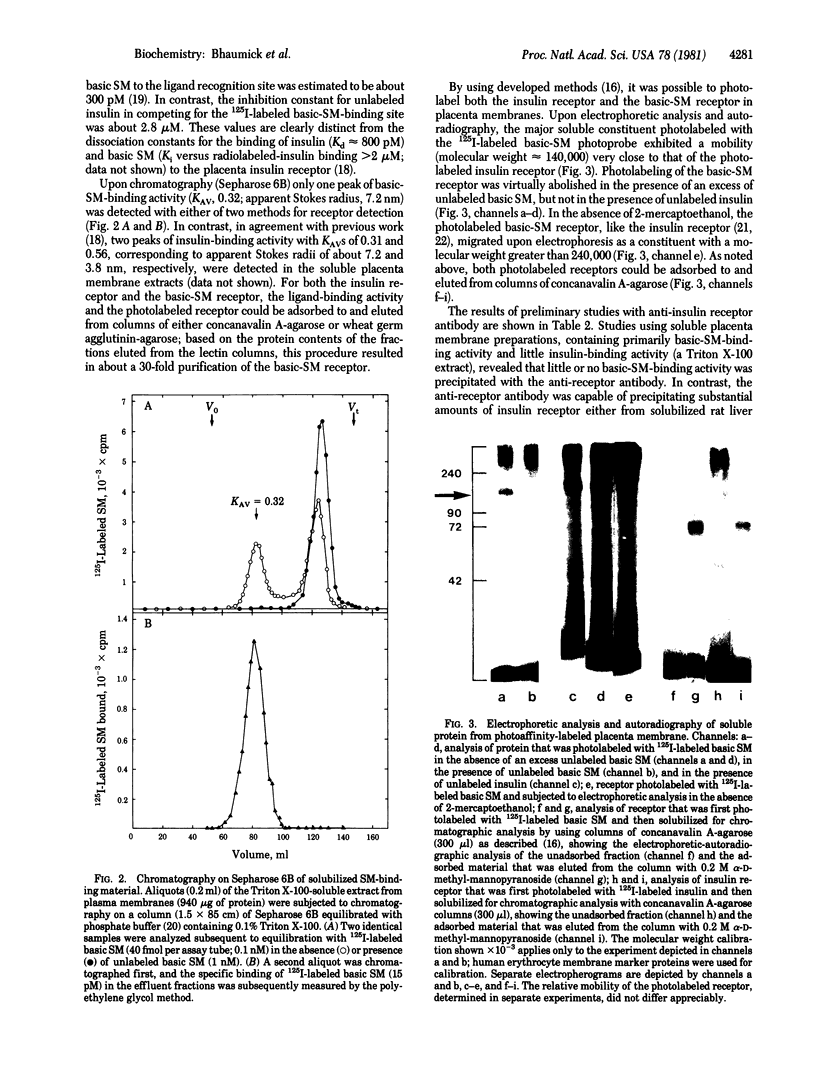
Using a recently isolated human basic somatomedin (basic SM) similar to insulin-like growth factor I (IGF-I), we studied both the photoaffinity-labeled and unlabeled basic-SM receptor solubilized from human placental cell membranes. Unlike the result with the insulin receptor, high yields of soluble basic-SM-binding activity are obtained with Triton X-100. The soluble basic-SM receptor retains high-affinity (Kd approximately 0.3 nM) peptide-specific binding of basic SM, similar to the binding present in particulate placenta membranes; the receptor exhibits a comparatively low affinity for insulin (Kd approximately 3 microM). On Sepharose 6B, like the crude soluble insulin receptor, the basic-SM receptor migrates as a species with an apparent Stokes radius of 7.2 nm; unlike the insulin receptor, the basic-SM receptor does not, under similar conditions, yield a smaller binding species (apparent Stokes radius 3.8 nm). Upon photoaffinity labeling with 125I-labeled basic SM, one principal specifically labeled constituent is detected. Upon gel electrophoresis in the presence of 2-mercaptoethanol, the photolabeled constituent, like the insulin receptor, migrates as a species with an apparent molecular weight of about 140,000; in the absence of reducing agent, a molecular weight greater than 240,000 is observed. Lectin-agarose affinity chromatography yields a 30-fold purification both of the basic-SM-binding activity and the photolabeled constituent. Anti-insulin receptor antibody does not appear to precipitate the basic-SM receptor. We conclude that the basic-SM receptor of human placenta is a glycoprotein, remarkably similar to (an isoreceptor) but distinct from the insulin receptor previously characterized in this tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Levitzki A. Tentative identification of beta-adrenoreceptor subunts. Nature. 1978 Mar 23;272(5651):370–371. doi: 10.1038/272370a0. [DOI] [PubMed] [Google Scholar]
- Bala R. M., Bhaumick B. Purification of a basic somatomedin, from human plasma Cohn fraction IV-1, with physicochemical and radioimmunoassay similarity to somatomedin-C and insulin-like growth factor. Can J Biochem. 1979 Nov;57(11):1289–1298. doi: 10.1139/o79-172. [DOI] [PubMed] [Google Scholar]
- Bala R. M., Bhaumick B. Radioimmunoassay of a basic somatomedin: comparison of various assay techniques and somatomedin levels in various sera. J Clin Endocrinol Metab. 1979 Nov;49(5):770–777. doi: 10.1210/jcem-49-5-770. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Bedarkar S., Rinderknecht E., Humbel R. E. Insulin-like growth factor: a model for tertiary structure accounting for immunoreactivity and receptor binding. Proc Natl Acad Sci U S A. 1978 Jan;75(1):180–184. doi: 10.1073/pnas.75.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Fryklund L., Uthne K., Sievertsson H. Indentification of two somatomedin A active polypeptides and in vivo effects of a somatomedin A concentrate. Biochem Biophys Res Commun. 1974 Dec 11;61(3):957–962. doi: 10.1016/0006-291x(74)90248-4. [DOI] [PubMed] [Google Scholar]
- Hintz R. L., Liu F., Marshall L. B., Chang D. Interaction of somatomedin-C with an antibody directed against the synthetic C-peptide region of insulin-like growth factor-I. J Clin Endocrinol Metab. 1980 Feb;50(2):405–407. doi: 10.1210/jcem-50-2-405. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Hollenberg M. D. Characterization of the receptor for epidermal growth factor-urogastrone in human placenta membranes. J Biol Chem. 1980 Nov 25;255(22):10731–10736. [PubMed] [Google Scholar]
- Hock R. A., Nexø E., Hollenberg M. D. Isolation of the human placenta receptor for epidermal growth factor-urogastrone. Nature. 1979 Feb 1;277(5695):403–405. doi: 10.1038/277403a0. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Nexø E., Hollenberg M. D. Solubilization and isolation of the human placenta receptor for epidermal growth factor-urogastrone. J Biol Chem. 1980 Nov 25;255(22):10737–10743. [PubMed] [Google Scholar]
- Hollenberg M. D., Fryklund L. Insulin and somatomedins A and B: comparisons of biological activities in cultured human skin-derived fibroblasts. Life Sci. 1977 Oct 1;21(7):943–950. doi: 10.1016/0024-3205(77)90260-0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
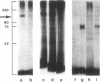
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. N., Underwood L. E., Voina S. J., Foushee D. B., Van Wyk J. J. Characterization of the insulin and somatomedin-C receptors in human placental cell membranes. J Clin Endocrinol Metab. 1974 Aug;39(2):283–292. doi: 10.1210/jcem-39-2-283. [DOI] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptors in transformed fibroblasts and in adipocytes: a comparative study. Can J Biochem. 1979 Jun;57(6):497–506. doi: 10.1139/o79-063. [DOI] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Shackelford W. H., Hollenberg M. D. Characteristics of the solubilized insulin receptor of human placenta. Life Sci. 1978 Nov 13;23(20):2063–2071. doi: 10.1016/0024-3205(78)90240-0. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., Podskalny J. M. Purification and characterization of multiplication-stimulating activity. Insulin-like growth factors purified from rat-liver-cell-conditioned medium. Eur J Biochem. 1980 Jan;103(2):387–400. doi: 10.1111/j.1432-1033.1980.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P., Podskalny J. M., Moses A. C., Fryklund L. Identification of a receptor for somatomedin-like polypeptides in human fibroblasts. J Clin Endocrinol Metab. 1977 May;44(5):820–831. doi: 10.1210/jcem-44-5-820. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Polypeptides with nonsuppressible insulin-like and cell-growth promoting activities in human serum: isolation, chemical characterization, and some biological properties of forms I and II. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2365–2369. doi: 10.1073/pnas.73.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak J. L., Small D., Hollenberg M. D. Erythropoietin: isolation by affinity chromatography with lectin-agarose derivatives. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4633–4635. doi: 10.1073/pnas.74.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M. E., Van Wyk J. J., Klapper D. G., Fellows R. E., Grissom F. E., Schlueter R. J. Purification of somatomedin-C from human plasma: chemical and biological properties, partial sequence analysis, and relationship to other somatomedins. Biochemistry. 1980 Feb 19;19(4):790–797. doi: 10.1021/bi00545a027. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]