Abstract
Mutants of Rhizobium trifolii strain 7012 defective in C4-dicarboxylate transport were isolated by using a selective procedure based on pH indicator media. The mutant strains CR7098 and CR7099 failed to grow on or transport succinate, fumarate, or malate, but grew at wild-type rates on several other carbon sources. The C4-dicarboxylate transport system was inducible in strain 7012, but was expressed constitutively in four out of five succinate-positive revertants of strain CR7098. In the fifth CR7098 revertant (strain CR8008) the system was inducible. However, in contrast to strain 7012, strain CR8008 failed to use the C4-dicarboxylates in the presence of a second carbon source. Revertants of strain CR7099 were similar to strain 7012. Both strains CR7098 and CR7099 nodulated white and red clover at a rate similar to that of strain 7012, but nodules formed by the mutant strains were white and ineffective. Microscopic examination showed that the pattern of development of white clover nodules formed by strain CR7098 was similar to that observed with nodules formed by strain 7012, except that large amounts of starch accumulated in bacteroid-filled cells and senescence occurred earlier. Revertant strains were effective, except for strain CR8008, which formed ineffective nodules. The results show that a supply of C4-dicarboxylates to bacteroids is essential for nitrogen fixation in clover nodules. However, rhizobia within plant cells must also utilize other carbon sources to support growth and division.
Keywords: bacteroid energy source; succinate, fumarate, and malate; bacteroid development; microscopy; nitrogen fixation
Full text
PDF

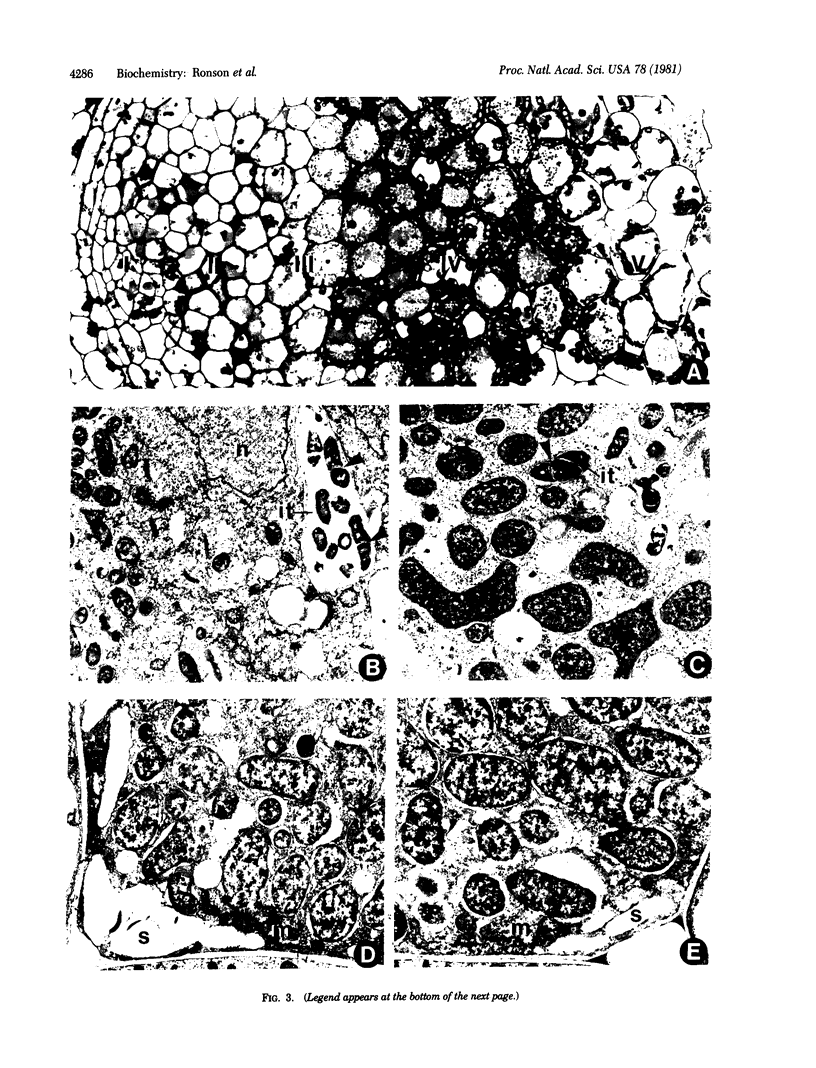


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubler R. E., Toscano W. A., Jr, Hartline R. A. Transport of succinate by Pseudomonas putida. Arch Biochem Biophys. 1974 Feb;160(2):422–429. doi: 10.1016/0003-9861(74)90416-0. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Fraenkel D. G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Succinate uptake and related proton movements in Escherichia coli K12. Biochem J. 1975 Dec;152(3):647–654. doi: 10.1042/bj1520647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Cameron M. J. Transport of C4-dicarboxylic acids in salmonella typhimurium. Arch Biochem Biophys. 1978 Sep;190(1):281–289. doi: 10.1016/0003-9861(78)90277-1. [DOI] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Bewick M. A. The molecular mechanisms of dicarboxylic acid transport in Escherichia coli K12. The role and orientation of the two membrane-bound dicarboxylate binding proteins. J Biol Chem. 1978 Nov 10;253(21):7826–7831. [PubMed] [Google Scholar]
- Moore J. V., Dixon B. Metastasis of a transplantable mammary tumour in rats treated with cyclophosphamide and/or irradiation. Br J Cancer. 1977 Aug;36(2):221–226. doi: 10.1038/bjc.1977.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P., Bullivant S., Grayston G. F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J Cell Sci. 1978 Apr;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]
- Scott D. B., Hennecke H., Lim S. T. The biosynthesis of nitrogenase MoFe protein polypeptides in free-living cultures of Rhizobium japonicum. Biochim Biophys Acta. 1979 Dec 17;565(2):365–378. doi: 10.1016/0005-2787(79)90212-0. [DOI] [PubMed] [Google Scholar]
- Stovall I., Cole M. Organic Acid Metabolism by Isolated Rhizobium japonicum Bacteroids. Plant Physiol. 1978 May;61(5):787–790. doi: 10.1104/pp.61.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. A micromethod for the purification and quantification of organic acids of the tricarboxylic acid cycle in plant tissues. Anal Biochem. 1979 May;95(1):311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]







