Abstract
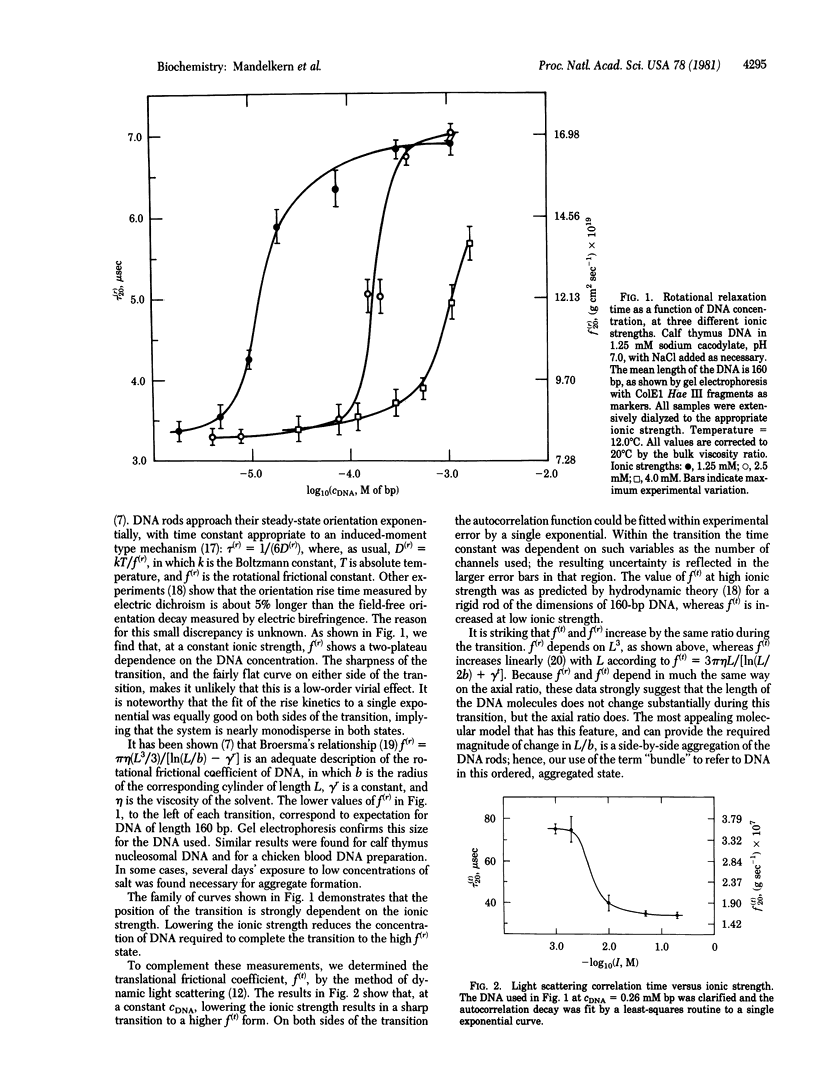
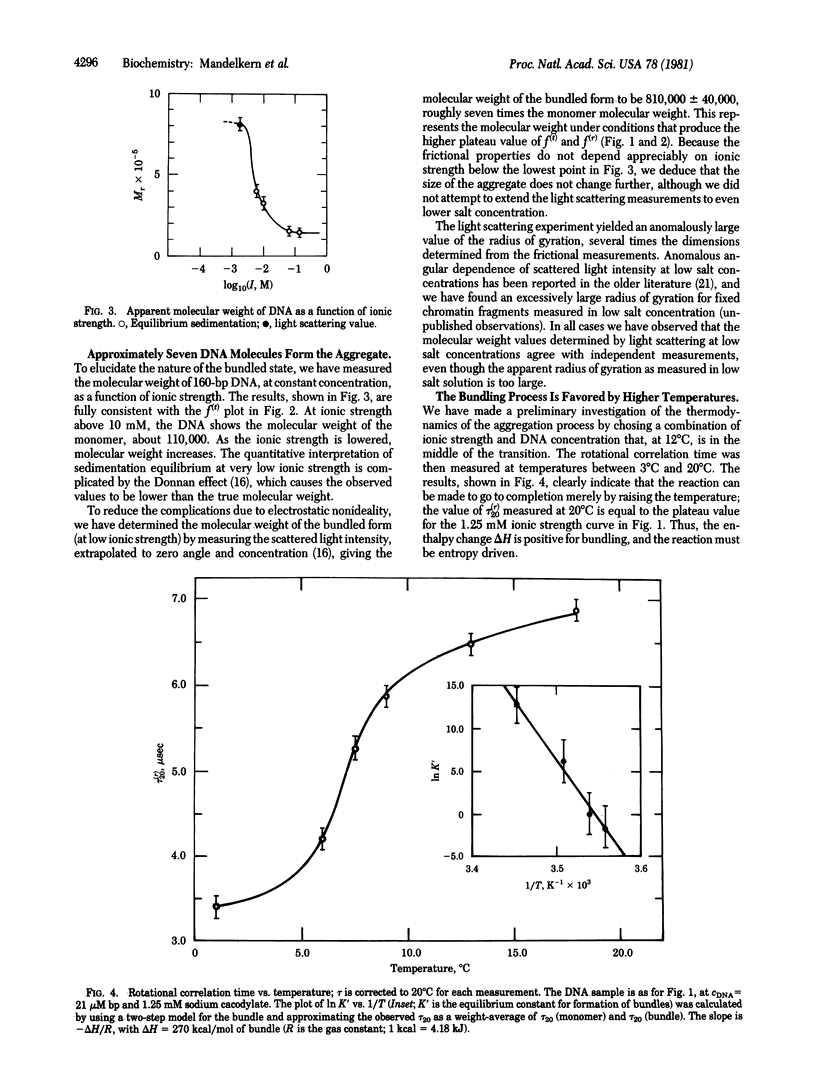
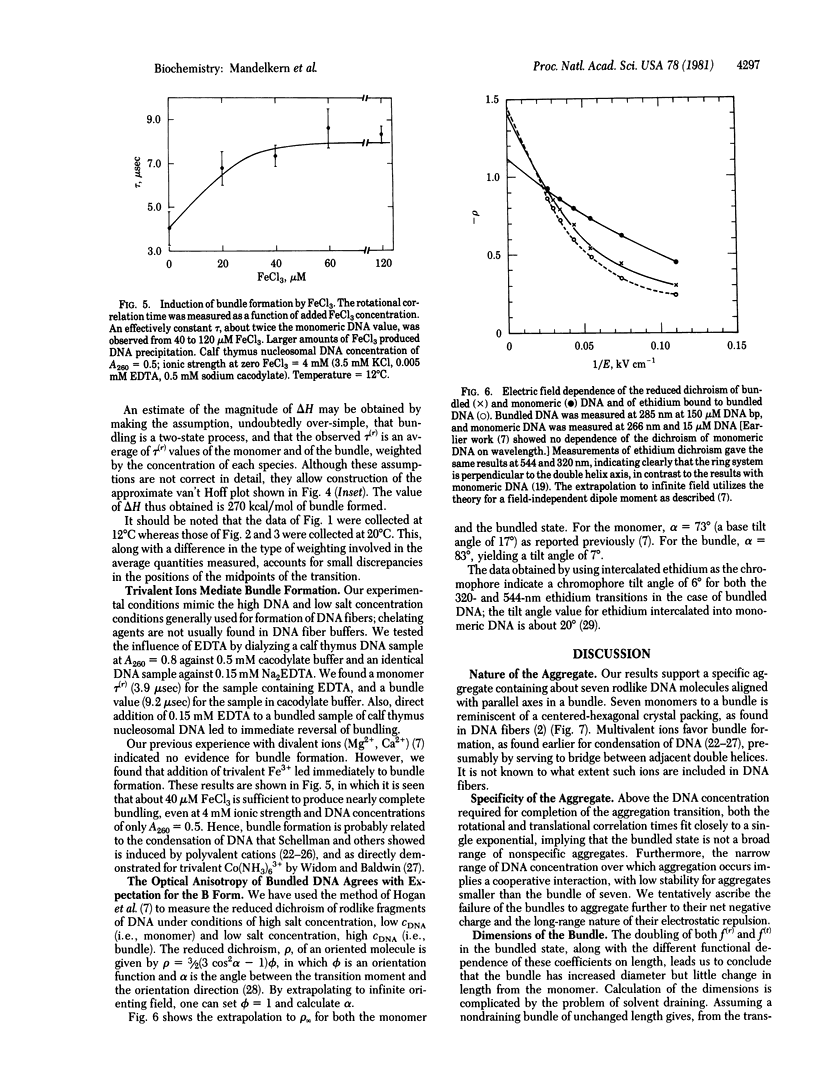
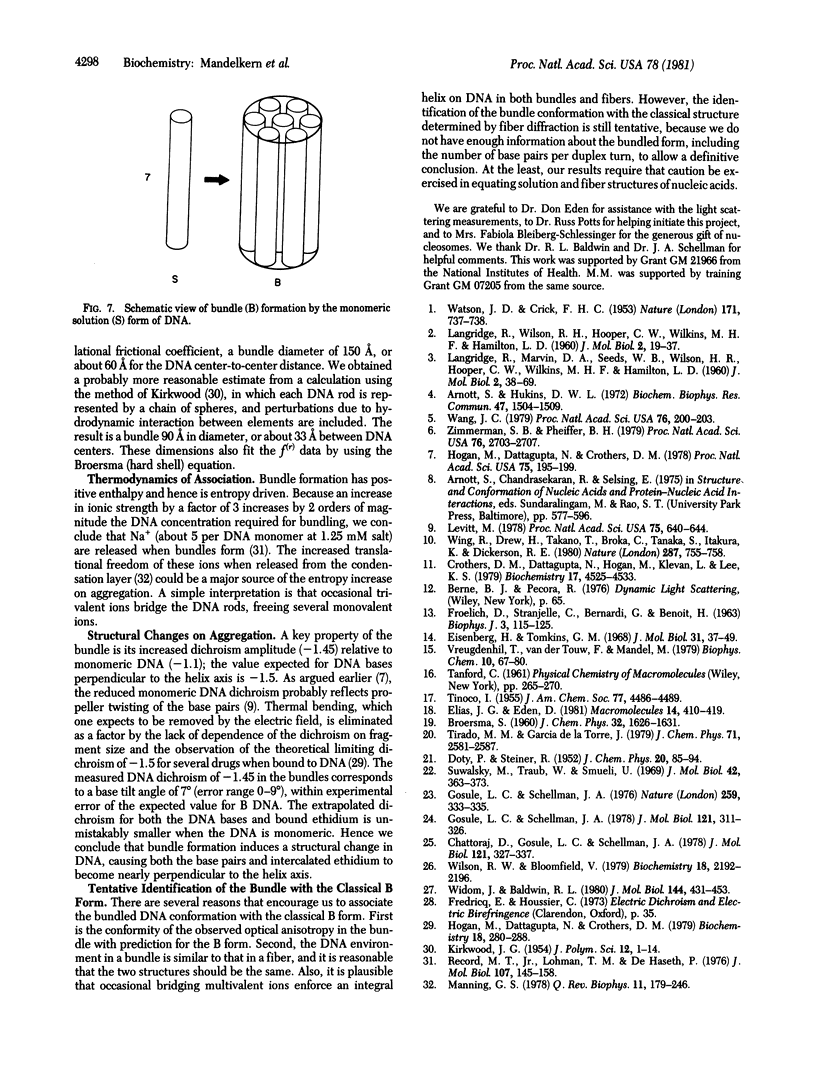
At low salt and high DNA concentration, rodlike DNA molecules form specific aggregates containing about seven parallel DNA molecules packed in a bundle. Bundle formation can be reversed by addition of small amounts of EDTA, and formation of the specific aggregates can be induced at higher salt concentration by addition of trivalent ions. In contrast to monomeric DNA, the aggregated species has the optical anisotropy expected for the B form as observed in fibers.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291x(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Dattagupta N., Hogan M., Klevan L., Lee K. S. Transient electric dichroism studies of nucleosomal particles. Biochemistry. 1978 Oct 17;17(21):4525–4533. doi: 10.1021/bi00614a026. [DOI] [PubMed] [Google Scholar]
- Eisenberg H., Tomkins G. M. Molecular weight of the subunits, oligomeric and associated forms of bovine liver glutamate dehydrogenase. J Mol Biol. 1968 Jan 14;31(1):37–49. doi: 10.1016/0022-2836(68)90052-1. [DOI] [PubMed] [Google Scholar]
- FROELICH D., STRAZIELLE C., BERNARDI G., BENOIT H. Low-angle light scattering of deoxyribonucleic acid. Biophys J. 1963 Mar;3:115–125. doi: 10.1016/s0006-3495(63)86808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism of rod-like DNA molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):195–199. doi: 10.1073/pnas.75.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism studies of the structure of the DNA complex with intercalated drugs. Biochemistry. 1979 Jan 23;18(2):280–288. doi: 10.1021/bi00569a007. [DOI] [PubMed] [Google Scholar]
- Levitt M. How many base-pairs per turn does DNA have in solution and in chromatin? Some theoretical calculations. Proc Natl Acad Sci U S A. 1978 Feb;75(2):640–644. doi: 10.1073/pnas.75.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Suwalsky M., Traub W., Shmueli U., Subirana J. A. An X-ray study of the interaction of DNA with spermine. J Mol Biol. 1969 Jun 14;42(2):363–373. doi: 10.1016/0022-2836(69)90049-7. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil T., van der Touw F., Mandel M. Electric permittivity and dielectric dispersion of low-molecular weight DNA at low ionic strength. Biophys Chem. 1979 Jul;10(1):67–80. doi: 10.1016/0301-4622(79)80007-1. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Helical repeat of DNA in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Helical parameters of DNA do not change when DNA fibers are wetted: X-ray diffraction study. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2703–2707. doi: 10.1073/pnas.76.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]


