Abstract
Currently used pharmaceutical nanocarriers, such as liposomes, micelles, and polymeric nanoparticles, demonstrate a broad variety of useful properties, such as longevity in the body; specific targeting to certain disease sites; enhanced intracellular penetration; contrast properties allowing for direct carrier visualization in vivo; stimili-sensitivity, and others. Some of those pharmaceutical carriers have already made their way into clinic, while others are still under preclinical development. In certain cases, the pharmaceutical nanocarriers combine several of the listed properties. Long-circulating immunoliposomes capable of prolonged residence in the blood and specific target recognition represent one of examples of this kind. The engineering of multifunctional pharmaceutical nanocarriers combining several useful properties in one particle can significantly enhance the efficacy of many therapeutic and diagnostic protocols. This paper considers the current status and possible future directions in the emerging area of multifunctional nanocarriers with primary attention on the combination of such properties as longevity, targetability, intracellular penetration, contrast loading, and stimuli sensitivity.
Keywords: Pharmaceutical nanocarriers, Drug targeting, Liposomes, Micelles, Cell-penetrating peptides, Stimuli-sensitive nanocarriers, Diagnostic imaging
I. INTRODUCTION
Various pharmaceutical carriers, including nanocarriers, such as nanospheres, nanocapsules, liposomes, micelles, cell ghosts, lipoproteins and many others are widely used for experimental and clinical delivery of therapeutic and diagnostic agents to enhance the in vivo efficiency of many drugs and drug administration protocols [1-3]. Various modifications of these carriers are often used to control their in vivo properties in a desirable fashion, for example, to increase the longevity and stability of the carrier in the circulation, achieve targeting effect specifically to pathological organ or tissue, impart sensitivity to certain stimuli characteristic of pathological area or applied from the outside of the body, provide visual information regarding carrier clearance and body distribution, etc. An increasing number of publications in the field of drug delivery systems (DDS) describe DDS that simultaneously demonstrate more that one useful function by combining, for example, longevity and targetability, targetability and stimuli-sensitivity, or longevity, targetability and contrast properties. Ideally, DDS could be engineered, which can simultaneously or sequentially demonstrate the following set of properties: (1) Stay (circulate) long in the body; (2) Specifically target the site of the disease; (3) Respond local stimuli characteristic of the pathological site, such as intrinsically abnormal pH values or temperature, or externally applied heat, magnetic field, or ultrasound, by releasing an entrapped drug or changing some other properties; (4) Provide an enhanced intracellular delivery of drugs and genes as required; (5) Carry a reporter (contrast) component supplying a real time information about the DDS biodistribution and target accumulation. Although a certain work in this direction is already done, see for example the reviews in [4-6], the development of DDS like this is still in its early stage.
II. LONGEVITY AND TARGETABILITY OF PHRMACEUTICAL NANOCARRIERS
Making long-circulating and targeted DDS is clearly the most developed approach. The longevity of drug carriers allows for maintaining a required level of a pharmaceutical agent in the blood for extended time intervals. In addition, long-circulating drug-containing nanocarriers can slowly accumulate (via the enhanced permeability and retention – EPR – effect, see [7, 8]) in pathological sites with affected and leaky vasculature (tumors, inflammations, infarcts), and facilitate drug delivery in those areas [7-9]. In addition, the prolonged circulation can help to achieve a better targeting effect for targeted (specific ligand-modified) drugs and drug carriers allowing for more time for their interaction with the target [10]. Naturally, long-circulating pharmaceuticals and pharmaceutical carriers represent an important and still growing area of biomedical research [10-15].
The most frequent way to impart the in vivo longevity to drug carriers is to modify their surface with certain synthetic polymers, such as poly(ethylene glycol) or PEG, as was initially suggested for liposomes [16-20]. Coating nanoparticles with PEG results in the formation of the polymeric layer over the particle surface, which is impermeable for other solutes even at relatively low polymer concentrations [21, 22] and sterically hinders the interaction and binding of blood components with their surface [18, 23-27], preventing thus drug carrier opsonization and capture by RES [28]. Currently, there exist many chemical approaches to synthesize activated derivatives of PEG and to couple these derivatives with a variety of drugs and drug carriers, see reviews in [29-31]. Although PEG is clearly the most popular for the preparation of long-circulating DDS, some other biocompatible, soluble, and hydrophilic polymers have also been suggested as steric protectors for pharmaceutical nanocarriers, such as single terminus lipid-modified poly(acryl amide) and poly(vinyl pyrrolidone) [26, 27], poly(acryloyl morpholine) [32-34], phospholipid(PE)-modified poly(2-methyl-2-oxazoline) or poly(2-ethyl-2-oxazoline) [35], phosphatidyl polyglycerols [36], and polyvinyl alcohol [37].
Although the PEGylation procedure seems to be the most developed to prepare long-circulating liposomes, there are many examples of absorbing/attaching PEG on the surface of various hydrophobic polymeric nanoparticles, which considerably influences their body residence time and biodistribution [38]. Long-circulating polymeric nanoparticles with insoluble core and water-soluble shell covalently linked to the core can also be prepared from block-copolymers of PEG and polylactide-glycolide (PEG-PLAGA) [39-41]. Grafting PEG onto the surface of gold particles via mercaptosilanes expectedly resulted in decreased protein adsorption onto modified particles and less platelet adhesion [42].
The most significant biological consequence of nanocarrier modification with PEG and similar polymers is sharp increase in its circulation time and decrease in their RES accumulation [11, 16, 22]. Various long-circulating nanocarriers have been shown to effectively accumulate in many tumors via the EPR effect [7-9, 43]. Long-circulating liposomes were prepared containing various anticancer agents, such as doxorubicin, arabinofuranosylcytosine, adriamycin, and vincristin [44-47]. PEG-liposome-incorporated doxorubicine (Doxil®) has already demonstrated very good clinical results [9, 48, 49].
The idea to add the property of the specific target recognition to the carrier’s ability to circulate long seems quite natural and was thoroughly investigated. Targeting of nanoparticular DDS with the aid of specific ligands selective to certain cell-surface components/receptors allows for the selective drug delivery to those namely cells. There are, however, some issues to be considered when designing such systems: (1) The ligand (antibody, another protein, peptide or carbohydrate) attached to the carrier surface may increase the rate of its uptake by the RES despite the presence of a sterically protecting grafts; see, for example [50]; (2) Ligand-bearing long-circulating nanocarriers could facilitate the development of an unwanted immune response (as was shown with the raise of anti-liposome antibodies), the extent of which depends on the type of the ligand (small peptides or Fv fragments are less immunogenic than a complete IgG molecule) and the liposome composition [51-53]; (3) The amount of ligand attached to the carrier may be critical to ensure successful binding with the target while maintaining the extended circulation of the carrier.
To achieve selective targeting by long-circulating PEGylated nanoparticulates, targeting ligands were attached to nanocarriers via the PEG spacer arm, so that the ligand is extended outside of the dense PEG coat, which allows for its unhindered binding to the target receptors (see the scheme on Figure 1). Ligands were attached to the activated water-exposed ends of liposome-grafted polymeric chain [54, 55]. For this purpose several types of end-group functionalized lipopolymers of general formula X-PEG-PE [29, 56], where X represents a reactive functional group-containing moiety. Most of the end-group functionalized PEG-lipids were synthesized from heterobifunctional PEG derivatives containing hydroxyl and carboxyl or amino groups. Another amphiphilic reactive PEG derivative, p-nitrophenylcarbonyl-PEG-PE (pNP-PEG-PE), was also introduced [55, 57, 58], which easily adsorbs on hydrophobic nanoparticles or incorporates into liposomes and micelles via its phospholipid residue, and readily binds amino group-containing compound via its water-exposed pNP group.
Figure 1.
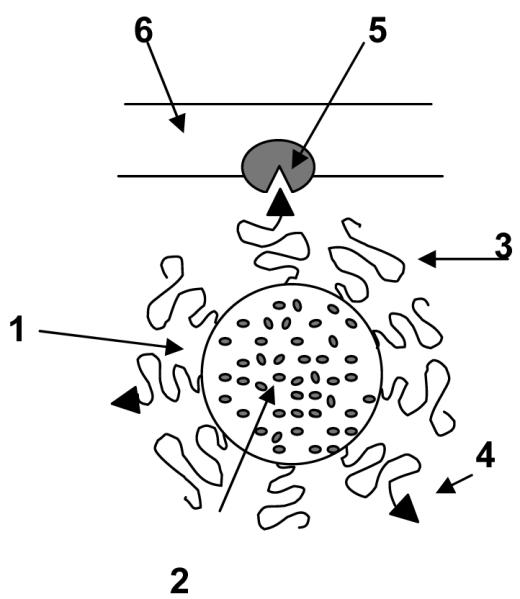
The schematic structure of long-circulating targeted nanocarrier: 1 – nanocarrier; 2 – drug; 3 – sterically protecting polymer (usually, PEG) grafted on the surface of the nanocarrier; 4 – targeting ligand (antibody, folate, transferring) chemically coupled with distant tips of some of the protecting polymer grafted chains; 5 – specific receptor targeted with the attached ligand; 6 – cell membrane.
Several strategies have been suggested to prepare targeted PEGylated nanocarriers, first of all, liposomes [56, 59]. The first approach involves the modification of preformed PEGylated nanocarriers, containing a certain number of reactive groups exposed into the aqueous surroundings. In the second approach, ligand-PEG-lipid conjugate is mixed with other liposome-forming components and then made into unilamellar vesicles [59-62]. According the third approach, a ligand modified with the reactive PEG-PE is post-inserted into preformed liposomes [59, 63].
The majority of research in this area relates to cancer targeting, which utilizes a variety of monoclonal antibodies. Thus, it was suggested to target HER2-overexpressing tumors using anti-HER2 long-circulating liposomes [51]. Antibody CC52 against rat colon adenocarcinoma CC531 attached to PEGylated liposomes provided specific accumulation of liposomes in rat model of metastatic CC531 [64]. A nucleosome-specific monoclonal antibody (mAb 2C5) capable of recognition of various tumor cells via the tumor cell surface-bound nucleosomes significantly improved Doxil® targeting to tumor cells and increased its cytotoxicity [65] both in vitro and in vivo in different test systems including intracranial human brain U-87 tumor xenograft in nude mice [66]. The same antibody was also used to effectively target long-circulating PEG-liposomes loaded with an agent for tumor photo-dynamic therapy (PDT) both to multiple cancer cells in vitro and to experimental tumors in vivo and provide a significantly enhanced tumor cell killing under the conditions of PDT [67].
Combination of immunoliposome and endosome-disruptive peptide improves the cytosolic delivery of the liposomal drug, increases cytotoxicity, and opens new approach to constructing targeted liposomal systems as shown with diphtheria toxin A chain incorporated together with pH-dependent fusogenic peptide diINF-7 into liposomes specific towards ovarian carcinoma [68].
Surface modification with antibodies was also applied to make targeted and long-circulating non-liposomal pharmaceutical nanocarriers, see [69] for review. Nanoparticles made of poly(lactic acid) were surface-modified with PEG and with anti-transferrin receptor monoclonal antibody to produce PEGylated immunoparticles with the size of about 120 nm and containing ca. 65 bound antibody molecules per single particle [70]. Mammalian cells (NIH3T3, 32D, Ba/F3, hybridoma 9E10) were surface-modified with distal terminus-activated oleyl-PEG, and various proteins (streptavidin, EGFP, and antibody) were successfully attached to the activated PEG termini [71] producing potentially interesting multifunctional (long-circulating and targeted) drug delivery system.
Similar combination of longevity and targetability can be also achieved by using some other specific ligands attached to long-circulating preparations. Thus, since transferrin (Tf) receptor (TfR) is overexpressed on the surface of many tumor cells, antibodies against TfR as well as Tf itself are among popular ligands for targeting various nanoparticular DDS including liposomes to tumors and inside tumor cells [72]. Recent studies involve the coupling of Tf to PEG on PEGylated liposomes in order to combine longevity and targetability [73]. Targeting tumors with folate-modified nanocarriers also represents a popular approach, since folate receptor (FR) expression is frequently overexpressed in many tumor cells [74-77]. Folate was attached to the surface of cyanoacrylate-based nanoparticles via activated PEG blocks [78]. Similarly, PEG-polycaprolactone-based particles were surface-modified with folate and, after loading with paclitaxel, demonstrated increased cytotoxicity [79]. Other specific ligands attached to long-circulating nanocarriers have also been used. Thus, hyaluronan-modified long-circulating liposomes loaded with mitomycin C are active against tumors overexpress hyaluronan receptors [80]. Vasoactive intestinal peptide (VIP) was attached to PEG-liposomes with radionuclides to target them to VIP-receptors of the tumor, which resulted in an enhanced breast cancer inhibition in rats [81]. PEG-liposomes were targeted by RGD peptides to integrins of tumor vasculature and, being loaded with doxorubicin, demonstrated increased efficiency against C26 colon carcinoma in mice [82].
III. LONG-CIRCULATING, TARGETED AND STIMULI-SENSITIVE NANOCARRIERS
Further development of the “multifunctional approach” involved the addition of certain stimuli-sensitive functions to long-circulating and targeted pharmaceutical nanocarriers. The idea was that certain stimuli intrinsically characteristic of the pathological zone or applied to this zone from the outside of the body could beneficially modify the properties of the drug-in-nanocarrier system, for example, providing enhanced or controlled drug release, improving cellular drug uptake, controlling intracellular drug fate or even allowing for certain physical action on the surrounding pathological tissue. The stimuli, which are currently utilized to modify the behavior of drug delivery systems inside the target are summarized in the Table 1. One can see that stimuli typical for a pathological tissues themselves include pH and redox conditions; temperature can serve as a local stimulus both within the tissue (inflammation is always accompanied with a local hyperthermia) and from the outside; ultrasound and (electro)magnetic field could be applied only “artificially” and mainly from the outside. For example, intratumoral pH value in solid tumors may drop to 6.5, i.e. one pH unit lower than in normal blood (7.4) because of hypoxia and massive cell death inside the tumor [83] [84], and drops still further inside cells, especially, inside endosomes (5.5 and even below) [85]. At the same time, intracellular concentration of glutathione (i.e. redox potential) in cancer cells is significantly (hundreds-fold) higher than normal extracellular level of glutathione [86]. All these stimuli have been successfully utilized for specific drug targeting (see Figure 2).
Table 1.
Stimuli that can be utilized to control the behavior and properties of drug delivery systems.
| Stimuli | Stimuli origin | |
|---|---|---|
| pH | internal - | decreased pH in pathological areas, such as tumors, infarcts, and inflammations, because of hypoxia and massive cell death; decreased pH in cell cytoplasm, endosomes, and lysosomes |
| redox potential | internal - | increased concentration of glutathione inside many pathological cells compared to its extracellular concentration |
| temperature | internal - | hyperthermia associated with inflammation |
| temperature | external – | can be caused inside target tissues by locally applied ultrasound or by locally applied high frequency causing the oscillation of target-accumulated magneto-sensitive nanoparticles with heat release |
| magnetic field | external – | magnetic field of different gradients and profiles applied to the body can concentrate magneto-sensitive DDS in required areas |
| Ultrasound | external – | sonication can be applied to the body to get a diagnostic signal from echogenic contrast agents and can also facilitate DDS penetration into cells and drug/gene release rom ultrasound-sensitive DDS |
Figure 2.
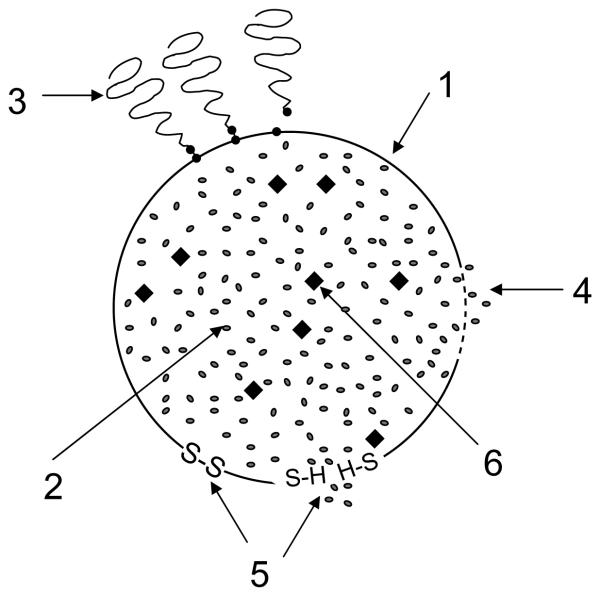
Schematic picture of different stimuli acting on the stimuli-sensitive nanocarrier and expected responses: 1 – nanocarrier; 2 – drug; 3 – protectine polymeric coating attached to the surface of the nanocarrier via pH-sensitive bonds could be detached and removed by the action of lowered pH in certain pathological areas (tumors) or inside cellular compartments (cytoplasm, endosome); 4 – temperature-sensitive coating or components of the carrier, which can be influenced by the heat (hyperthermia in certain pathological areas or the heat brought upon by an external source) to destabilize the carrier and allow for drug release; 5 – redox-sensitive coating or components of the carrier, which can be influenced by changing redox conditions (increased glutathione), for example by transforming –S-S-bonds into thiol groups, and allow for drug release; 6 – particles of magnetosensitive material (SPION), which can allow the whole nanocarrier to be transported to required site under the action of an external magnetic field.
pH-sensitive systems
Historically, pH-sensitivity was the first example of stimuli-sensitivity used to modify in a desired way drug/DDS behavior in the pathological areas with the decreased pH value, such as tumors, infarcts, inflammations. With this in mind, pH-sensitive/responsive components were incorporated/attached to nanocarriers. First studies relate to pH-sensitive liposomes, which have been made to destabilize and release the incorporated drug/DNA at lowered pH values. Such liposomes contained phospholipids like phosphatidylethanolamine variants with unsaturated acyl chains, capable of protonation or formation of non-bilayered structures at decreased pH and destabilizing liposomal or liposomal and endosomal membranes with the subsequent drug/DNA release from liposomes or from liposomes and endosomes [87-90].
Different methods of liposomal content delivery into the cytoplasm have been elaborated by adding the pH-sensitivity function to liposomal preparations, which can already bear some other functions, such as longevity and targetability [91, 92]. It was believed that such pH-sensitive carriers would destabilize the endosomal membrane when inside endosomes liberating the entrapped drug into the cytoplasm. For example, according to one of these methods, the liposome is made of pH-sensitive components and, after being endocytosed in the intact form, it fuses with the endovacuolar membrane under the action of lowered pH inside the endosome and destabilizes it, releasing its content into the cytoplasm [93]. Thus, namely endosomes become the gates from the outside into the cell cytoplasm [94]. Cellular drug delivery mediated by pH-sensitive liposomes is not a simple intracellular leakage from the lipid vesicle since the drug has to cross also the endosomal membrane [95]. The presence of fusogenic lipids in the liposome composition, such as unsaturated DOPE, is usually required to render pH-sensitivity to liposomes [96]. Multifunctional long-circulating PEGylated DOPE-containing pH-sensitive liposomes, although have a decreased pH-sensitivity, still effectively deliver their contents into cytoplasm (recent review in [97]). Antisense oligonucleotides were delivered into cells by anionic pH-sensitive PE-containing liposomes, which are stable in the blood, however, undergo phase transition at acidic endosomal pH and facilitating oligo release into cell cytoplasm (recent review in [98]). Serum stable, long-circulating PEGylated pH-sensitive liposomes were also prepared using, on the same liposome, the combination of PEG and pH-sensitive terminally alkylated copolymer of N-isopropylacrylamide and methacrylic [99]. Combination of liposome pH-sensitivity and specific ligand targeting for cytosolic drug delivery utilizing decreased endosomal pH values was described for both folate and Tf-targeted liposomes [100]. Additional modification of pH-sensitive liposomes with an antibody results in pH-sensitive immunoliposomes. A successful application of pH-sensitive immunoliposomes has been demonstrated for the delivery of a variety of molecules including fluorescent dyes, antitumor drugs, proteins and DNA [101]. In addition to membrane-destabilizing lipid components, there exists a large family of membrane-destabilizing anionic polymers that also can enhance the endosomal escape of various drugs and biomacromolecules [102]. This family includes various carboxylated polymers, copolymers of acrylic and methacrylic acids, copolymers of maleic acid, polymers and copolymers of N-isopropylacrylamide, which demonstrate lower critical solution (solubility/insolubility switch) at physiological temperatures and when precipitate, destabilize biomembranes they are interacting with [103]. Such polymers can be attached to the surface of drug/DNA-loaded nanocarriers allowing for endosome destabilization and cytoplasmic escape.
In case of polyplexes, which cannot directly destabilize the endosomal membrane, the mechanism of DNA escape from endosomes is associated with the ability of polymers, such as PEI, strongly protonate under the acidic pH inside endosome and create a charge gradient eventually provoking a water influx and endosomal swelling and disintegration [104]. In both cases, however, DNA-containing complexes when released into the cytosol, dissociate allowing for nuclear entry of free DNA. Nuclear translocation of the plasmid DNA is relatively inefficient because of the barrier function of the nuclear membrane and small size of nuclear pores (ca. 25 nm), in addition DNA degrades rather fast under the action of cytoplasmic nucleases [105], and only 0.1% of palsmids undergo nuclear translocation from the cytosol [106]. The attachment of nuclear localization sequences to plasmid DNA may enhance its nuclear translocation and transfection efficiency [107]. New approaches in using multifunctional carriers for DNA delivery include the application of bimetallic nanorods that can simultaneously bind compacted DNA plasmid and targeting ligands in a spatially defined manner [108].
Polymeric micelles can also demonstrate pH-sensitivity and ability to escape from endosomes. Thus, micelles prepared from PEG-poly(aspartate hydrazone adriamycin) easily release an active drug at lowered pH values typical for endosomes and facilitate its cytoplasmic delivery and toxicity against cancer cells [109]. Alternatively, micelles for intracellular delivery of antisense oligonucleotides (ODN) were prepared from ODN-PEG conjugates complexed with a cationic fusogenic peptide, KALA, and provided much higher intracellular delivery of the ODN, that could be achieved with free ODN [110]. One could also enhance an intracellular delivery of drug-loaded micelles by adding to their composition lipid components used in membrane-destabilizing Lipofectin®. The compensation of the negative charge of PEG-lipid micelles [111] by the addition of positively charged lipids to PEG-PE micelles could improve the uptake by cancer cells of drug-loaded mixed PEG-PE/positively charged lipid micelles. After the enhanced endocytosis, such micelles could escape from the endosomes and enter the cytoplasm of cancer cells. This approach was used to increase an intracellular delivery and, thus, the anticancer activity of the micellar paclitaxel by preparing paclitaxel-containing micelles from the mixture of PEG-PE and positively charged lipids [112]. Multifunctional polymeric micelles capable of pH-dependent dissociation and drug release when loaded with doxorubicin and supplemented with biotin as cancer cell-interacting ligand were also described in [113]. Paclitaxel was loaded into mixed micelles, which could undergo dissociation into unimers and drug liberation even above the CMC value because of the ionization of their components at certain pH values [114]. Poly-L-histidine-containing micelles also demonstrate pH-sensitivity because of its pH-dependent endosome-destabilizing property (via the imidazole residue) [115, 116]. Block copolymers containing 2-N-(morpholino)ethyl methacrylate (MEMA) component can also form pH-sensitive micelles [117].
pH-sensitive systems with detachable coatings
A special case of stimuli-sensitivity relates to long-circulating PEGylated pharmaceutical carriers. In this case, the chemistry is used, which allows for the detachment of protecting polymer (PEG) chains under the action of decreased pH value or increased temperature. The matter is that the stability of PEGylated nanocarriers may not always be favorable for drug delivery. In particular, if drug-containing nanocarriers accumulate inside the tumor, they may be unable to easily release the drug to kill the tumor cells. Likewise, if the carrier has to be taken up by a cell via an endocytic pathway, the presence of the PEG coat on its surface may preclude the contents from escaping the endosome and being delivered in the cytoplasm. In order to solve these problems, for example, in the case of long-circulating liposomes, the chemistry was developed to detach PEG from the lipid anchor in the desired conditions. Labile linkage that would degrade only in the acidic conditions characteristic of the endocytic vacuole or the acidotic tumor mass can be based on the diorto esters [118], vinyl esters [119], cystein-cleavable lipopolymers [120], double esters and hydrazones that are quite stable at pH around 7.5 but hydrolyzed relatively fast at pH values of 6 and below [118, 121, 122]. When the PEG brush is cleaved (for example, from the liposome surface), the membrane destabilization should occur, and the liposome contents would be delivered to its target (e.g., by escaping from the primary endosome into the cell cytoplasm).
Polymeric components with pH-sensitive (pH-cleavable) bonds are used to produce stimuli-responsive DDS that are stable in the circulation or in normal tissues, however, acquire the ability to degrade and release the entrapped drugs in body areas or cell compartments with lowered pH, such as tumors, infarcts, inflammation zones or cell cytoplasm or endosomes [97, 99, 123]. A variety of liposomes [124, 125] and polymeric micelles [115, 126, 127] have been described that include the components with acid-labile bonds as well as variety of drug conjugates capable of releasing such drugs as adriamycin [128], paclitaxel [129], doxorubicin [130], and DNA [131-133] in acidic cell compartments (endosomes) and pathological body areas under acidosis. Serum-stable, long-circulating PEGylated pH-sensitive liposomes were also prepared using the combination of PEG and pH-sensitive terminally alkylated copolymer of N-isopropylacrylamide and methacrylic [99] on the same liposome, since the attachment of the pH-sensitive polymer to the surface of liposomes might facilitate liposome destabilization and drug release in compartments with decreased pH values.
Combination of liposome pH-sensitivity and specific ligand targeting for cytosolic drug delivery utilizing decreased endosomal pH values was described for folate- and Tf-targeted liposomes [134-136].
An interesting example of novel pH-sensitive polymers is the pH-sensitive poly(β-amino ester), which rapidly dissolves at pH below 6.5, i.e. inside tumors, for example, and releases the drug incorporated into nanoparticles made of this polymers [137-140].
Dendrimeric systems derived from diaminobutane poly(propylene imine) with surface-attached PEG and loaded with various drugs demonstrated acid-sensitivity and were capable of releasing incorporated drugs when titrated with acids followed by the addition of sodium chloride solution [141]. Doxorubicin was attached to the synthetic dendritic polyester based on 2,2-bis(hydroxymethyl)propanoic acid via pH-sensitive linkages and was released from the carrier as pH value was lowered [142]. PEG-dendrimer combinations were used to build pH-sensitve micelles capable of releasing micelle-incorporated doxorubicin at acidic pH values [143].
The stimuli-sensitivity of PEG coats can also allow for the preparation of multifunctional drug delivery systems with temporarily “hidden” functions, which under normal circumstances, are “shielded” by the protective PEG coat, however become exposed after PEG detaches (see the next paragraph on Intracellular Drug Delivery). Such systems require that multiple functions attached to the surface of the nanocarrier should function in a certain coordinated way. For the above system the following requirements have to be met: (1) the life of the carrier in the circulation should be long enough to fit EPR effect or targeted delivery requirements (i.e. PEG coat mediating the longevity function or specific ligand mediating the targeting function should not be lost by the nanocarrier when in the circulation), and (2) the internalization of the carrier within the target cells should proceed fast not to allow for the carrier degradation and drug loss in the interstitial space (i.e. local stimuli-dependent removal of the protective function and the exposure of the temporarily hidden second function should proceed fast).
Temperature-sensitive systems
The idea of using temperature-sensitive nanocarriers naturally came from the fact that many pathological areas demonstrate distinct hyperthermia. Additionally, there exist various means to heat the required area in the body. The finding that a significantly greater fraction of the intravenously administered liposomes and other nanocarriers accumulated in the tumor mass upon heating to 42°C in human ovarian carcinoma xenograft model and a higher concentration and effectiveness was observed for doxorubicin delivered into tumors in temperature-sensitive liposomes clearly demonstrated the feasibility of this approach [144] [145].
Temperature-sensitive liposomes frequently include dipalmitoylphosphatidylcholine (DPPC) as the key component, since liposomes usually become leaky at a gel-to-liquid crystalline phase transition and this transition for DPPC takes place at 41°C [146]. Liposomes can also be made temperature-sensitive via the incorporation of grafting of certain polymers, which display a lower critical solution temperature (LCST) slightly above the physiological one [147, 148]. Because these polymers are soluble below LCST and precipitate when the temperature increases above the LCST, they can damage the liposomal membrane during precipitation and allow for drug release [149]. The most usual representative of this class of polymers is poly(N-isopropylacrylamide) (NIPAM) [150]. A review on thermo-responsive polymer-modified liposomes can be found in [147].
Similarly, polymeric micelles can be made temperature-sensitive by assembling them of amphiphilic co-polymers, in which one of the block demonstrate properties similar to NIPAM [151]. Exact properties of such micelles can be adjusted be chemival modifications of both, hydrophobic and hydrophilic blocks in such a way that the micelle can destabilize at temperatures above LCST and release the drug dissolved in its hydrophobic core [152].
Redox potential-sensitive systems
High redox potential difference, which exists between the reducing intracellular space and oxidizing extracellular space can also be utilized for the construction of stimuli-sensitive DDS [86]. With this in mind, drug or DNA can be loaded into the nanocarrier, whose structure is maintained under normal condition by disulfide bonds. As soon as those bonds are reduced to thiol groups due to the presence of high glutathione inside cells, the integrity of the carrier is compromised and drug or DNA can release. Thus, the authors of [153] have used polymers with positively charged and thiol groups incorporated into the polymer structure to complex DNA (via positive charge) and to form polymeric network (via disulfide bridges formed from groups). When reduced, disulfide bridges convert back to thiols, polymeric carrier disintegrates and facilitate DNA release. Intracellular delivery of plasmid DNA was also performed using thiolated gelatin nanoparticles [154, 155]. The transfection efficacy was also enhanced by using DNA condensed with thiolated polyethyleneimine [156]. Redox-responsive liposomes have also been prepared from standard phospholipids with the addition of a small quantity of a lipid, in which head and tail are linked by the disulfide bond [157]. Long-circulating redox-responsive liposomes with detachable PEG coat were described in [158].
Magnetically-sensitive systems
In still another approach, drug carriers, such as microcapsules, can be loaded not the drug alone, but also with magnetic nanoparticles allowing for manipulation of such capsules in magnetic field or with metallic nanoparticles, which can respond external electromagnetic field and control the rate of drug release by oscillating or heating the carrier [159]. In nanomedicine, iron oxide nanoparticles namely magnemite (γ-Fe2O3) or magnetite (Fe3O4), with particle size ca. 4-10 nm have drawn a special interest as clearly follows from several recent reviews on biomedical applications of these nanoparticles, see for example [160-163]. Due to their superparamagnetic properties and small size, they are referred to as superparamagnetic iron oxide nanoparticles (SPION). The use of SPION was suggested for drug targeting, bioseparation (cell sorting), magnetic resonance imaging, magnetic hyperthermia, magnetic transfection, etc. Several chemical methods can be used to prepare SPION. Most commonly used methods are controlled precipitation of iron salts in presence of an alkali [164, 165] or thermal decomposition method [166-168]. Irrespective of the method of synthesis, “plain” SPION are not stable at physiological conditions and aggregate because of the hydrophobic nature of these particles. In order to prevent aggregation or destabilization of SPION, it was suggested to coat the surface of these particles with certain compounds so that the particles can form homogenous suspensions into suitable solvents. Various substances, such as citric acid [169], dextran [170], poly(D,L-lactide-co-glycolide) [171], polyethyleneglycol-polycaprolactone block co-polymer [172], organic silanes [173], and unsaturated fatty acids [166], have been used. Some early attempts to incorporate SPION into polymeric micelles have also been described [174]; see also Figure 3.
Figure 3.
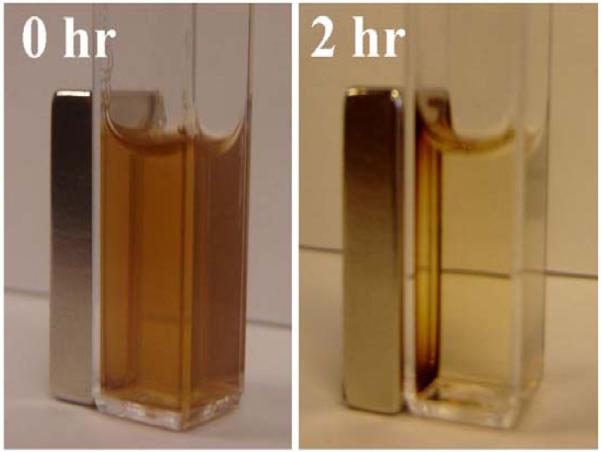
Magneto-sensitive nanoparticles. PEG-PE micelles loaded with SPION concentrate in the vicinity of externally applied magnet.
The concept of magnetic targeting or guiding magnetically susceptible particles towards the intended pathology site under the influence of external magnets has been received increased attention. If drugs molecules are somehow conjugated to such particles then such a system will offer increased therapeutic activity at lower doses and reduced undesired side-effect. Magnetic drug targeting (MDT) concept introduced by Widder et al. [175] in 1979 has recently received increased attention with advances in nanotechnology. Gang et al. [176] have demonstrated targeting of magnetic poly ε-caprolactone nanoparticles loaded with gemcitabine in pancreatic cancer xenograft mouse model using external magnets. Cinteza et al. have also reported co-loading polymeric micelles of diacylphospholipid-poly(ethylene glycol) with the photosensitizer drug 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a, and magnetic SPION for magnetic drug targeting in vitro. Alexiou et al. have used mitoxantrone-loaded SPION and targeted them to VX2 squamous cell carcinoma in rabbits by using external magnets [177, 178]. MDT has also been used to improve localized drug delivery to interstitial tumor targets. In particular, MDT is now being developed to improve drug delivery to tumor vessels. Thus, magnetite (MAG-C) was loaded into cationic liposomes together with etoposide and dacarbazine. It was noted that at lower concentrations MAG-C did not alter the efficiency of drug loading, but at higher concentrations (2.5mg/ml), drug incorporation decreased [179]. It is very important, that co-incorporation of SPION and drugs into the same nanocarriers only to a minimal extent influences the efficacy of drug loading [174].
Another interesting observation is that by increasing local temperatures by using SPIONs in an alternating magnetic field has the potential to either directly kill tumors or make them more susceptible in combination with radiation or chemotherapy. The temperature increase achieved in this way depends on the size, shape and accumulation of the nanoparticles in the intended site and on the applied alternating magnetic field.
Using magnetic fluid depots the concept of using magnetic hyperthermia has been illustrated by Johannsen et al. [180]. Wust et al. have carried out feasibility and tolerance studies for testing applicability of whole body magnetic field applicators and iron oxide nanoparticles in human patients. The study illustrated great potential of the SPION-based hyperthermia [181]. “Magnetic thermal ablation” approach was examined under in vivo animal conditions [182]. The method is based on tumor accumulation of SPION and the exposure of the tumor to an alternating magnetic field, whereby the tumor is eliminated by heat developed by oscillating SPION.
With increased understanding of the process at the macro molecular level and identifications of ligands and targets there have been a considerable progress in designing targeted systems. Boutry et al. have studied the targeting effect of E-selectin-specific ligand-modified SPION in mice with induced hepatitis and found that the SPIONs were retained extracellularly by the interaction with E-selectin over-expressed on the vascular endothelium [183]. Zhang et al. have designed αvβ3 integrin-angiogenisis-targeted SPION to noninvasively assess the angiogenic profile of tumors in vivo in nude mice grafted with subcutaneous tumor cells with different expression levels of αvβ3 integrin–positive vessels using 1.5 T MRI scanner [184].
Ultrasound-sensitive systems
The application of the external ultrasound to control drug delivery and release from nanocarriers is a relatively novel approach, although some publications on this subject go back to 1998, when acoustically active lipospheres have been described containing poaclitaxel [185]. The whole concept is based on the making DDS, which upon accumulation in required areas can be made leaky by the locally applied external ultrasound, and liberate incorporated drugs or genes. There already exist the whole set of promising data on drug and gene delivery by ultrasound-sensitive drug carriers, some of which are reviewed in [186-188]. Acoustically-active liposomes containing a small quantity of a certaing gas (air) or perfluorated hydrocarbon and initially developed as ultrasound contrast agent, can be loaded with various drugs and release these drugs after being damaged by applied ultrasound [189, 190]. Polymeric micelles have also been prepared, which can incorporate various drugs, such as doxorubicin, and release them after ultrasonication, which can also assist in delivering such DDS inside cells [191, 192]. Similar approach was also used for the local release of thrombolytic enzymes, such as tissue plasminogen activator, from echogenic liposomes in the area of clot formation [193]. In this case, specific binding of plasminogen activator with fibrin additionally facilitated drug accumulation in the target zone providing promising multifunctionality – contrast properties, targeting ability and thrombolytic drug release. Other examples of using ultrasound-sensitive carriers for targeting cardio-vascular pathologies are reviewed in [194]. When making DDS sensitive towards ultrasound, an important task appears of analyzing and estimating the destruction tresholds of echogenic carriers with clinical ultrasound [195]. Studies on ultrasound-sensitive formulations and the mechanisms controlling drug release from such formulations represent an important part of research on stimuli-sensitive nanocarriers [196, 197].
IV. INTRACELLULAR DRUG DELIVERY BY MULTIFUNCTIONAL NANOCARRIERS
Intracellular transport of biologically-active preparations including various large molecules (proteins, enzymes, antibodies) and even drug-loaded pharmaceutical nanocarriers, is one of the key problems in drug delivery. Many pharmaceutical agents need to be delivered intracellularly to exert their therapeutic action inside cytoplasm or onto nucleus or other specific organelles, such as lysosomes, mitochondria or endoplasmic reticulum. This group includes preparations for gene and antisense therapy, which have to reach cell nuclei; pro-apoptotic drugs, which target mitochondria; lysosomal enzymes, which have to reach lysosomal compartment; and some others. However, the cell membrane prevents various soluble small molecules as well as big molecules such as peptides, proteins and DNA from spontaneously entering cells unless there is an active transport mechanism as in case of some short peptides. Even if molecules/particles enter cell via the endocytic pathway, they become entrapped into endosomes and eventually end in lysosomes, where active degradation processes proceed under the action of the lysosomal enzymes. In addition, drugs inside cells still should find their way to specific organells where they are expected to utilize their therapeutic potential. This is especially important in case of gene delivery. Viral vectors for DNA delivery suffer from non-specificity and inherent risks of virus-induced complications. Non-viral delivery systems, first of all, cationic lipids/liposomes [198], also have certain drawbacks, such as same non-specificity, low efficiency, and cytotoxic reactions [199, 200], though new cationic lipid derivatives with decreased toxicity are currently under development [201]. Still, the traditional routes of internalization of DNA carriers by endocytosis or pinocytosis with subsequent degradation of the delivered DNA by lysosomal nucleases strongly limit the efficacy of transfection [202].
The addition of the positive charge to the nanocarrier can significantly enhance its uptake by cells, and the use of cationic lipids and cationic polymers as transfection vectors for intracellular delivery of DNA was suggested about 20 years ago [202-204]. Currently this is a well-developed field (see one of the recent reviews in ref. [205]. Complexes between cationic lipids (such as Lipofectin®, an equimolar mixture of N-(1-(2,3-dioleyloxy)propyl)-N,N,N-trimethylammonium chloride – DOTMA and dioleoyl phosphatidylethanolamine - DOPE) and DNA (lipoplexes) and complexes between cationic polymers, such as polyethyleneimine (PEI) [206], and DNA (polyplexes) are formed because of strong electrostatic interactions between the positively charged carrier and negatively charged DNA. A slight net positive charged of already formed lipoplexes and polyplexes is believed to facilitate their interaction with negatively charged cells and improve transfection efficiency [207]. Endocytosis (including the receptor-mediated endocytosis) was repeatedly confirmed as the main mechanism of lipoplex/polyplex internalization by cells [208]. Of special importance is the fact that despite of endocytosis-mediated uptake lipolexes and polyplexes, DNA does not end in lysosomes but releases in the cytoplasm due to the destabilization of the endosomal membrane provoked by the positively charged lipid or polymeric component of the complexes. Since the application of stimuli-sensitive (pH-sensitive) pharmaceutical nanocarriers for intracellular was already discussed in the previous section, we will consider here other possibilities.
Relatively recent approach in intracellular drug delivery is based on the modification of drugs and drug carriers with certain proteins and peptides demonstrating a unique ability to penetrate into cells (“transduction” phenomenon). This function can be added on top of the longevity, targetability and stimuli-sensitivity of the pharmaceutical drug-loaded nanocarriers. Thus, the trans-activating transcriptional activator (TAT) protein from HIV-1 enters various cells when added to the surrounding media [209]. The same is true about several other cell-penetrating proteins and peptides (CPPs) [210]. For example, TAT peptide (TATp) includes a cluster of basic amino acids 47-57 (11-mer; Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg), which represents the minimal protein transduction domain (PTD) [211, 212]. The minimal PTD of Antp, called penetratin, is the 16-mer peptide (43-58 residues) [213]. Other CPPs that can be used for the modification of nanocarriers include VP22 ; transportan, a 27 amino acid-long chimeric CPP [214]; 18-mer amphipathic model peptide with the sequence KLALKLALKALKAALKLA [215]. Current data assume more than one mechanism for CPPs and CPP-mediated intracellular delivery of various molecules and particles. CPP-mediated intracellular delivery of large molecules and nanoparticles was proved to proceed via the energy-dependent macropinocytosis with subsequent enhanced escape from endosome into the cell cytoplasm [216], while individual CPPs or CPP-conjugated small molecules penetrate cells via electrostatic interactions and hydrogen bonding and do not seem to depend on the energy [217].
It was shown that CPPs could internalize nanosized particles into the cells [218]. Superparamagnetic iron oxide nanoparticles (SPION) conjugated with TATp and fluorescein isothiocyanate were taken up quickly by T cells, B cells and macrophages followed by migration of the conjugate primarily to the cytoplasm, which could be tracked readily by MRI [219]. A biocompatible dextran-coated SPION derivatized with TATp were internalized into lymphocytes by over 100-fold more efficiently than non-modified particles. The characterization on the number of TATp molecules required for an efficient delivery of magnetic nanoparticles revealed that higher numbers of TATp molecules (above 10 per single SPION) enhanced the intracellular accumulation of such particles by 100-fold [220]. The combination of longevity, magnetic properties and ability to penetrate inside cells results in pharmaceutical nanopreparations with new and unique properties including contrast properties allowing for MR visualization and tracing cells taking up such particles.
Even relatively large particles, such as liposomes, could be delivered into various cells by multiple TATp or other CPP molecules attached to their surface [57, 221, 222]. The translocation of TATp-liposomes (both plain and PEGylated) into cells required the direct interaction of the liposomal TATp with the cell surface [57, 223]. Complexes of TATp-liposomes with a plasmid (plasmid pEGFP-N1 encoding for the Green Fluorescence Protein, GFP) were used for successful in vitro transfection of various tumor and normal cells as well as for in vivo transfection of tumor cells in mice bearing Lewis lung carcinoma [224] (the combination of positive charge for DNA complexation and cell-penetrating functions). Antp and TATp coupled to small unilamellar liposomes were accumulated within tumor cells and dendritic cells more effectively than unmodified control liposomes [225]. Coupling of TATp to the outer surface of liposomes was also described that resulted in an enhanced binding and endocytosis of the liposomes in ovarian carcinoma cells [226]. Antp-liposomes have been also considered as a carrier system for an enhanced cell-specific delivery of liposome-entrapped molecules [225]. Octamer of arginin (R8) attached to the surface of siRNA-loaded liposomes provided their effective intracellular delivery and silencing of the targeted gene [227].
Cell-penetrating function could be beneficially combined with the stimuli-sensitivity discussed earlier. Thus, talking about the multifunctionality, one would like a nanoparticular DDS to be able to (1) specifically accumulate in the required organ or tissue, and then (2) penetrate inside target cells delivering its load (drug or DNA) intracellularly. Organ or tissue (tumor, infarct) accumulation could be achieved by the passive targeting via the enhanced permeability and retention (EPR) effect [7, 228] assisted by prolonged circulation of such nanocarrier (for example, as a result of its coating with protecting polymer such as PEG); or by the antibody-mediated active targeting [229, 230], while the intracellular delivery could be mediated by certain internalizable ligands (folate, transferrin) [77, 231] or by CPPs [232, 233]. Evidently, such DDS should simultaneously carry on its surface various active moieties, i.e. be multifunctional and possess the ability to “switch on” certain functions (such as intracellular penetration) only when necessary, for example under the action of local stimuli characteristic of the target pathological zone (first of all, increased temperature or lowered pH values characteristic of inflamed, ischemic, and neoplastic tissues). These “smart” DDS should be built in such a way that during the first phase of delivery, a non-specific cell-penetrating function is shielded by the function providing organ/tissue-specific delivery (sterically-protecting polymer or antibody). Upon accumulating in the target, protecting polymer or antibody attached to the surface of the DDS via the stimuli-sensitive bond should detach under the action of local pathological conditions (abnormal pH or temperature) and expose the previously hidden second function allowing for the subsequent delivery of the carrier and its cargo inside cells (see the general scheme inFigure 4A). This is especially important for CPP-bearing nanocarriers, since all CPPs are highly non-selective and can lead their cargo to any cells including many non-target ones.
Figure 4.
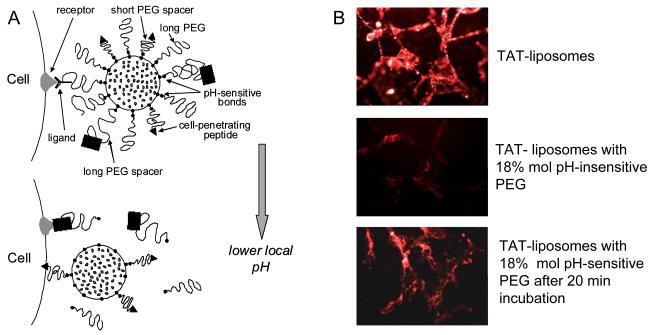
A – Schematic structure of a double-targeted “smart” nanocarrier with temporarily “hidden” function, for example cell-penetrating peptide, and “shielding” polymeric coat (with or without targeting antibody attached to it) providing longevity in the blood and specific target (tumor) accumulation and preventing the hidden function from the premature interaction with target cells. Polymeric chains are attached to the carrier surface via low pH-degradable bonds. After the accumulation in the tumor due to PEG (longevity) and/or antibody (specific targeting), pH-dependent de-shielding of the temporarily hidden cell-penetrating function allow for carrier penetration inside tumor cells. B – Interaction of “smart” TAT peptide-modified liposomes. Rhodamin-labeled TAT-liposomes are effectively taken by cells. The attachment of PEG-chains to the liposome surface (18% mol) sterically shields TAT finction and TAT-mediated liposome uptake is almost completely blocked. If, however, PEG is attached to the liposome surface via pH-sensitive bonds, its brief incubation at the lowered pH results in the elimination of PEG chains from the liposome surface, de-blocking TAT function and good TAT-mediated uptake of the liposomes by cells. Modified from [234-236].
We have recently suggested and prepared targeted long-circulating PEGylated liposomes and PEG-phosphatidylethanolamine (PEG-PE)-based micelles possessing several functionalities [234, 235]. First, such systems are capable of targeting a specific cell or organ by attaching the monoclonal antibody (infarct-specific antimyosin antibody 2G4 or cancer-specific anti-nucleosome antibody 2C5) to their surface. Second, these nanocarriers were additionally modified with TATp moieties attached to the surface of the nanocarrier via the short PEG spacer. PEG-PE used for liposome surface modification or for micelle preparation was made degradable by inserting the pH-sensitive hydrazone bond between PEG and PE (PEG-Hz-PE). Under normal pH values, TATp functions on the surface of nanocarriers were “shielded” by long protecting PEG chains (pH-degradable PEG2000-PE or PEG5000-PE) or by long pNP-PEG-PE moieties used to attach antibodies to the nanocarrier (non-pH-degradable PEG3400-PE or PEG5000-PE). At pH 7.5-8.0, both liposomes and micelles demonstrated high specific binding with antibody substrates, but very limited internalization by cells. However, upon brief incubation at lower pH values (pH 5.0-6.0) nanocarriers lose their protective PEG shell because of acidic hydrolysis of PEG-Hz-PE and are effectively internalized by cells via TATp moieties (Figure 4B).
In vivo, TATp-modified pGFP-loaded liposomal preparations have been administered intratumorarly in tumor-bearing mice and the efficacy of tumor cell transfection was followed after 72 h. The administration of pGFP-TATp-liposomes with non-pH-sensitive PEG coating has resulted in only minimal transfection of tumor cells because of steric hindrances for the liposome-to-cell interaction created by the PEG coat. Contrary, the administration of pGFP-TATp-liposomes with the low pH-detachable PEG resulted in the highly efficient transfection since the removal of PEG under the action of the decreased intratumoral pH leads to the exposure of the liposome-attached TATp residues, enhanced penetration of the liposomes inside tumor cells and effective intracellular delivery of the pGFP [236].
Interesting multifunctional envelope-type devices have been recently described for the cytoplasmic delivery of proteins, DNA and oligonucleotides [237]. Nanoparticles have been formed by the condensation of the substances to be delivered inside sells with lipid derivatives of CPPs, such as polyarginine, and were efficiently internalized be the cells and released their cargo into the cytosol.
V. MULTIFUNCTIONAL NANOCARRIERS FOR IMAGE-GUIDED DRUG DELIVERY AND DIAGNOSTICS
To use pharmaceutical nanocarriers for diagnostic/imaging purposes simultaneously with their therapeutic use and to allow for following their real-time biodistribution and target accumulation, the contrast reporter moieties can be added to multifunctionalized nanocarriers. Among nanocarriers for contrast agents, liposomes, micelles, and later dendrimers draw much attention. In case of liposomes, for example, two general approaches are used to prepare liposome-based contrasts for gamma- and MR-imaging, when heavy metal atoms are used as contrast moieties. The reporter metal could be chelated into a soluble chelator (such as diethylene triamine pentaacetic acid, DTPA) and than incorporated into the interior of a liposome [238]. Alternatively, DTPA or a similar chelating compound could be chemically modified with a hydrophobic group, which can anchor the chelating moiety onto the liposome surface during or after liposome preparation [239]. Different chelators and different hydrophobic anchors were tried for the preparation of 111In, 99mTc, Mn-, and Gd-loaded liposomes [240-247]. In the case of MR imaging, for a better MR signal, all reporter atoms should be freely exposed for interaction with water as in the case of membranotropic chelating agents - such as DTPA-stearylamine (DTPA-SA) [242] or DTPA-phosphatidyl ethanolamine (DTPA-PE) [239], which results in better relaxivity of the final preparation when compared with liposome-encapsulated paramagnetic ions [248-251]. The amphiphilic chelating probes (paramagnetic Gd-DTPA-PE and radioactive 111In-DTPA-SA) can also be incorporated into PEG(5 kDa)-PE micelles and used for in vivo MR and scintigraphy imaging [252].
To still further increase liposome load with diagnostic moieties, polychelating amphiphilic polymers (PAP) were synthesized consisting of the main chain with multiple side chelating groups capable of firm binding many reporter metal atoms and hydrophobic terminal group allowing for polymer adsorption onto hydrophobic nanoparticles or incorporation into hydrophobic domains of liposomes or micelles [253]. Such surface modification of nanocarriers allows for sharp increase in the number of bound reporter metal atoms per particle and image signal intensity. In case of MR, metal atoms chelated into polymer side groups are directly exposed to the water environment that enhances the relaxivity of the paramagnetic ions and leads to the corresponding enhancement of the vesicle contrast properties [244, 254, 255]. Such PAP-nanoparticles were used for in vivo MR imaging of lymphatic system components with Gd-loaded nanocarriers. Liposomes and micelles have been studied as delivery vehicles to the lymphatic [256, 257]. Liposomes loaded with chelated paramagnetic ions (Gd, Dy, Mn, Fe) could serve as MRI contrast agents mostly for the visualization of the macrophage-rich tissues such as organs of the reticuloendothelial system [258]. The overall performance of Gd-PAP-liposomes or -micelles could be further improved in case of the co-incorporation of amphiphilic PEG onto the liposome membrane or micelle surface, which can be explained by increased relaxivity of PEG-Gd-liposomes because of the presence of increased amount of PEG-associated water protons in the close vicinity of chelated Gd ions [259, 260]. Multifunctional approach certainly is important here, since in addition to the enhanced relaxivity, the coating of liposome surface with PEG polymer can help in avoiding the contrast agent uptake in the site of injection by resident phagocytic cells. In case of multifunctional nanocarriers additionally loaded with a drug, the presence of a contrast moiety allows for the real-time control of drug accumulation in the target. All said is also true other nanoparticulate carriers including polymeric micelles [261, 262]. Both PAP-bearing liposomes and micelles additionally containing PEG on their surface can also serve as long-circulating contrast agents for the blood pool gamma- or MR-imaging. Gd-PAP-PEG-liposomes additionally modified with the cancer-specific monoclonal antibody demonstrated fast and specific tumor accumulation and could serve as effective contrast agents for tumor MRI [263]
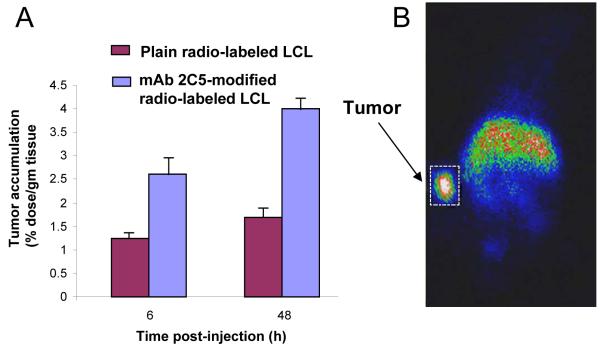
The combination of drug loading, longevity, targetability, and contrast properties results in multifunctional nanopharmaceuticals of new generation. Thus, long-circulating PEGylated liposomes loaded with doxorubicin and additionally decorated with a tumor-specific antibody and contrast moieties [264-266] demonstrated an increased therapeutic activity in vivo, and their target accumulation coud be easily followed by gamma-scintigraphy (see Figure 5) or MRI. Multifunctional nanocarriers for image-guided drug delivery, which combine therapeutic and imaging agents merged in one preparation have also been described in [267] and [191], the last one of these studies combining the ultrasonic tumor imaging with targeted therapy by doxorubicin.
Figure 5.
Combination of the longevity, targetability, and contrast function. Radiolabeled (111In) long-circulating (PEG) liposomes (LCL) modified with a tumor-targeted ligand (cancer-specific monoclonal antibody 2C5) demonstrate an enhanced tumor accumulation – A. This can be used for the fast and specific tumor visualization by gamma-scintigraphy (mice) – B. Modified from [264].
Summing up, the development of a broad variety of multifunctional and stimuli-sensitive pharmaceutical nanocarriers represents now an important area of DDS research, and eventually could allow for combined therapeutic and diagnostic systems with dramatically enhanced efficacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- [1].Torchilin VP, editor. Nanoparticualtes as Pharmaceutical carriers. Imperial College Press; London, UK: 2006. [Google Scholar]
- [2].Domb AJ, Tabata Y, Kumar M.N.V. Ravi, Farber S, editors. Nanoparticles for Pharmaceutical Applications. American Scientific Publishers; Stevenson Ranch, CA: 2007. [Google Scholar]
- [3].Thassu D, Deleers M, Pathak Y, editors. Nanoparticulate Drug Delivery Systems. Informa Healthcare USA; New York, NY: 2007. [Google Scholar]
- [4].Bernkop-Schnurch A, Walker G. Multifunctional matrices for oral peptide delivery. Crit. Rev. Ther. Drug Carrier Syst. 2001;18:459–501. [PubMed] [Google Scholar]
- [5].van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006;3:205–216. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- [6].Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- [7].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [8].Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- [9].Gabizon AA. Liposome circulation time and tumor targeting: Implications for cancer chemotherapy. Adv. Drug Deliv. Rev. 1995;16:285–294. [Google Scholar]
- [10].Torchilin VP. How do polymers prolong circulation times of liposomes. J. Liposome Res. 1996;9:99–116. [Google Scholar]
- [11].Torchilin VP. Polymer-coated long-circulating microparticulate pharmaceuticals. J. Microencapsul. 1998;15:1–19. doi: 10.3109/02652049809006831. [DOI] [PubMed] [Google Scholar]
- [12].Lasic DD, Martin FJ, editors. Stealth liposomes. CRC Press; Boca Raton: 1995. [Google Scholar]
- [13].Cohen S, Bernstein H, editors. Microparticulate systems for the delivery of proteins and vaccines. Marcel Dekker; New York: 1996. [Google Scholar]
- [14].Trubetskoy VS, Torchilin VP. Use of polyoxyethylene-lipid conjugates as long-circulating carriers for delivery of therapeutic and diagnostoc agents. Adv. Drug Deliv. Rev. 1995;16:311–320. [Google Scholar]
- [15].Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- [16].Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- [17].Maruyama K, Yuda T, Okamoto A, Ishikura C, Kojima S, Iwatsuru M. Effect of molecular weight in amphipathic polyethyleneglycol on prolonging the circulation time of large unilamellar liposomes. Chem. Pharm. Bull. (Tokyo) 1991;39:1620–1622. doi: 10.1248/cpb.39.1620. [DOI] [PubMed] [Google Scholar]
- [18].Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles. Biochim. Biophys. Acta. 1991;1062:77–82. doi: 10.1016/0005-2736(91)90337-8. [DOI] [PubMed] [Google Scholar]
- [19].Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- [20].Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gabizon A, Papahadjopoulos D. The role of surface charge and hydrophilic groups on liposome clearance in vivo. Biochim. Biophys. Acta. 1992;1103:94–100. doi: 10.1016/0005-2736(92)90061-p. [DOI] [PubMed] [Google Scholar]
- [22].Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Jr., Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- [23].Woodle MC. Surface-modified liposomes: assessment and characterization for increased stability and prolonged blood circulation. Chem. Phys. Lipids. 1993;64:249–262. doi: 10.1016/0009-3084(93)90069-f. [DOI] [PubMed] [Google Scholar]
- [24].Allen TM. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1994;13:285–309. [Google Scholar]
- [25].Chonn A, Semple SC, Cullis PR. Separation of large unilamellar liposomes from blood components by a spin column procedure: towards identifying plasma proteins which mediate liposome clearance in vivo. Biochim. Biophys. Acta. 1991;1070:215–222. doi: 10.1016/0005-2736(91)90167-7. [DOI] [PubMed] [Google Scholar]
- [26].Chonn A, Semple SC, Cullis PR. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J. Biol. Chem. 1992;267:18759–18765. [PubMed] [Google Scholar]
- [27].Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta. 1991;1070:187–192. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- [28].Senior JH. Fate and behavior of liposomes in vivo: a review of controlling factors. Crit. Rev. Ther. Drug Carrier Syst. 1987;3:123–193. [PubMed] [Google Scholar]
- [29].Zalipsky S. Chemistry of polyethylene glycol conjugates with biologically active molecules. Adv. Drug Deliv. Rev. 1995;16:157–182. [Google Scholar]
- [30].Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–417. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- [31].Torchilin VP. Strategies and means for drug targeting: an overview. In: Muzykantov V, Torchilin VP, editors. Biomedical aspects of drug targeting. Kluwer Academic Pub.; Boston: 2002. pp. 3–26. [Google Scholar]
- [32].Monfardini C, Schiavon O, Caliceti P, Morpurgo M, Harris JM, Veronese FM. A branched monomethoxypoly(ethylene glycol) for protein modification. Bioconjug. Chem. 1995;6:62–69. doi: 10.1021/bc00031a006. [DOI] [PubMed] [Google Scholar]
- [33].Ranucci E, Spagnoli G, Sartore L, Ferutti P. Synthesis and molecular weight characterization of low molecular weight end-functionalized poly(4-acryloymorpholine) Macromol. Chem. Phys. 1994;195:3469–3479. [Google Scholar]
- [34].Sartore L, Ranucci E, Ferutti P, Caliceti P, Schiavon O, Veronese FM. Low molecular weight end-functionalized poly(N-vinylpyrrolidone) for the modifications of polypeptide aminogroups. J. Bioact. Compact. Polym. 1994;9:411–427. [Google Scholar]
- [35].Woodle MC, Engbers CM, Zalipsky S. New amphipatic polymer-lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjug. Chem. 1994;5:493–496. doi: 10.1021/bc00030a001. [DOI] [PubMed] [Google Scholar]
- [36].Maruyama K, Okuizumi S, Ishida O, Yamauchi H, Kikuchi H, Iwatsuru M. Phosphatidyl polyglycerols prolong liposome circulation in vivo. Int. J. Pharm. 1994;111:103–107. [Google Scholar]
- [37].Takeuchi H, Kojima H, Toyoda T, Yamamoto H, Hino T, Kawashima Y. Prolonged circulation time of doxorubicin-loaded liposomes coated with a modified polyvinyl alcohol after intravenous injection in rats. Eur. J. Pharm. Biopharm. 1999;48:123–129. doi: 10.1016/s0939-6411(99)00029-6. [DOI] [PubMed] [Google Scholar]
- [38].Porter CJ, Moghimi SM, Illum L, Davis SS. The polyoxyethylene/polyoxypropylene block co-polymer poloxamer-407 selectively redirects intravenously injected microspheres to sinusoidal endothelial cells of rabbit bone marrow. FEBS Lett. 1992;305:62–66. doi: 10.1016/0014-5793(92)80655-z. [DOI] [PubMed] [Google Scholar]
- [39].Krause HJ, Schwartz A, Rohdewald P. Polylactic acid nanoparticles, a colloidal drug delivery system for lipophilic drugs. Int. J. Pharm. 1985;27:145–155. [Google Scholar]
- [40].Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- [41].Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang F, Kang ET, Neoh KG, Huang W. Modification of gold surface by grafting of poly(ethylene glycol) for reduction in protein adsorption and platelet adhesion. J. Biomater. Sci. Polym. Ed. 2001;12:515–531. doi: 10.1163/156856201300194252. [DOI] [PubMed] [Google Scholar]
- [43].Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. U. S. A. 1988;85:6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang SK, Stauffer PR, Hong K, Guo JW, Phillips TL, Huang A, Papahadjopoulos D. Liposomes and hyperthermia in mice: increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994;54:2186–2191. [PubMed] [Google Scholar]
- [45].Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- [46].Boman NL, Masin D, Mayer LD, Cullis PR, Bally MB. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994;54:2830–2833. [PubMed] [Google Scholar]
- [47].Allen TM, Mehra T, Hansen C, Chin YC. Stealth liposomes: an improved sustained release system for 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1992;52:2431–2439. [PubMed] [Google Scholar]
- [48].Rose PG. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist. 2005;10:205–214. doi: 10.1634/theoncologist.10-3-205. [DOI] [PubMed] [Google Scholar]
- [49].Ewer MS, Martin FJ, Henderson C, Shapiro CL, Benjamin RS, Gabizon AA. Cardiac safety of liposomal anthracyclines. Semin. Oncol. 2004;31:161–181. doi: 10.1053/j.seminoncol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [50].Klibanov AL. Antibody-mediated targeting of PEG-coated liposomes. In: Woodle MC, Storm G, editors. Long circulating liposomes: old drugs, new therapeutics. Springer; Berlin: 1998. p. 269. [Google Scholar]
- [51].Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. J. Control. Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- [52].Harding JA, Engbers CM, Newman MS, Goldstein NI, Zalipsky S. Immunogenicity and pharmacokinetic attributes of poly(ethylene glycol)-grafted immunoliposomes. Biochim. Biophys. Acta. 1997;1327:181–192. doi: 10.1016/s0005-2736(97)00056-4. [DOI] [PubMed] [Google Scholar]
- [53].Benhar I, Padlan EA, Jung SH, Lee B, Pastan I. Rapid humanization of the Fv of monoclonal antibody B3 by using framework exchange of the recombinant immunotoxin B3(Fv)-PE38. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12051–12055. doi: 10.1073/pnas.91.25.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Blume G, Cevc G, Crommelin MD, Bakker-Woudenberg IA, Kluft C, Storm G. Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim. Biophys. Acta. 1993;1149:180–184. doi: 10.1016/0005-2736(93)90039-3. [DOI] [PubMed] [Google Scholar]
- [55].Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim. Biophys. Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- [56].Zalipsky S, Gittelman J, Mullah N, Qazen MM, Harding JA. Biologically active ligand-bearing polymer-grafted liposomes. In: Gregoriadis G, editor. Targeting of drugs 6: strategies for stealth therapeutic systems. Plenum Press; New York: 1998. pp. 131–139. [Google Scholar]
- [57].Torchilin VP, Rammohan R, Weissig V, Khaw BA, Klibanov A, Samokhin GP. PEG-Immunoliposomes: attachment of monoclonal antibody to distal ends of PEG chains via p-Nitrophenylcarbonyl groups. 27th International Symposium on Controlled Release of Bioactive Materials; Paris: Controlled Release Society, Inc; 2000. pp. 217–218. [Google Scholar]
- [58].Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zalipsky S, Mullah N, Harding JA, Gittelman J, Guo L, DeFrees SA. Poly(ethylene glycol)-grafted liposomes with oligopeptide or oligosaccharide ligands appended to the termini of the polymer chains. Bioconjug. Chem. 1997;8:111–118. doi: 10.1021/bc9600832. [DOI] [PubMed] [Google Scholar]
- [60].Wong JY, Kuhl TL, Israelachvili JN, Mullah N, Zalipsky S. Direct measurement of a tethered ligand-receptor interaction potential. Science. 1997;275:820–822. doi: 10.1126/science.275.5301.820. [DOI] [PubMed] [Google Scholar]
- [61].Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, Zalipsky S. Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: in vitro studies. Bioconjug. Chem. 1999;10:289–298. doi: 10.1021/bc9801124. [DOI] [PubMed] [Google Scholar]
- [62].DeFrees SA, Phillips L, Guo L, Zalipsky S. Sialyl Lewis x liposomes as a multivalent ligand and inhibitor of E-selectinmediated cellular adhesion. J. Am. Chem. Soc. 1996;118:6101–6104. [Google Scholar]
- [63].Yoshioka H. Surface modification of haemoglobin-containing liposomes with polyethylene glycol prevents liposome aggregation in blood plasma. Biomaterials. 1991;12:861–864. doi: 10.1016/0142-9612(91)90075-l. [DOI] [PubMed] [Google Scholar]
- [64].Kamps JA, Koning GA, Velinova MJ, Morselt HW, Wilkens M, Gorter A, Donga J, Scherphof GL. Uptake of long-circulating immunoliposomes, directed against colon adenocarcinoma cells, by liver metastases of colon cancer. J. Drug Target. 2000;8:235–245. doi: 10.3109/10611860008997902. [DOI] [PubMed] [Google Scholar]
- [65].Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J. Control. Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [66].Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol. Immunother. 2007;56:1215–1223. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Roby A, Erdogan S, Torchilin VP. Enhanced In Vivo Antitumor Efficacy of Poorly Soluble PDT Agent, Meso-Tetraphenylporphine, in PEG-PE-Based Tumor-Targeted Immunomicelles. Cancer Biol. Ther. 2007;6 doi: 10.4161/cbt.6.7.4345. [DOI] [PubMed] [Google Scholar]
- [68].Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J. Biol. Chem. 2002;277:27135–27143. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- [69].Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [70].Olivier JC, Huertas R, Lee HJ, Calon F, Pardridge WM. Synthesis of pegylated immunonanoparticles. Pharm. Res. 2002;19:1137–1143. doi: 10.1023/a:1019842024814. [DOI] [PubMed] [Google Scholar]
- [71].Kato K, Itoh C, Yasukouchi T, Nagamune T. Rapid protein anchoring into the membranes of Mammalian cells using oleyl chain and poly(ethylene glycol) derivatives. Biotechnol. Prog. 2004;20:897–904. doi: 10.1021/bp0342093. [DOI] [PubMed] [Google Scholar]
- [72].Hatakeyama H, Akita H, Maruyama K, Suhara T, Harashima H. Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. Int. J. Pharm. 2004;281:25–33. doi: 10.1016/j.ijpharm.2004.05.025. [DOI] [PubMed] [Google Scholar]
- [73].Ishida O, Maruyama K, Tanahashi H, Iwatsuru M, Sasaki K, Eriguchi M, Yanagie H. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm. Res. 2001;18:1042–1048. doi: 10.1023/a:1010960900254. [DOI] [PubMed] [Google Scholar]
- [74].Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5572–5576. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994;269:3198–3204. [PubMed] [Google Scholar]
- [76].Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- [77].Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv. Drug Deliv. Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [78].Stella B, Arpicco S, Peracchia MT, Desmaele D, Hoebeke J, Renoir M, D’Angelo J, Cattel L, Couvreur P. Design of folic acid-conjugated nanoparticles for drug targeting. J. Pharm. Sci. 2000;89:1452–1464. doi: 10.1002/1520-6017(200011)89:11<1452::aid-jps8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [79].Park EK, Lee SB, Lee YM. Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials. 2005;26:1053–1061. doi: 10.1016/j.biomaterials.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [80].Peer D, Margalit R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int. J. Cancer. 2004;108:780–789. doi: 10.1002/ijc.11615. [DOI] [PubMed] [Google Scholar]
- [81].Dagar S, Krishnadas A, Rubinstein I, Blend MJ, Onyuksel H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: in vivo studies. J. Control. Release. 2003;91:123–133. doi: 10.1016/s0168-3659(03)00242-6. [DOI] [PubMed] [Google Scholar]
- [82].Schiffelers RM, Koning GA, ten Hagen TL, Fens MH, Schraa AJ, Janssen AP, Kok RJ, Molema G, Storm G. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release. 2003;91:115–122. doi: 10.1016/s0168-3659(03)00240-2. [DOI] [PubMed] [Google Scholar]
- [83].Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer. Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- [84].Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- [85].Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- [86].Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- [87].Wang CY, Huang L. Highly efficient DNA delivery mediated by pH-sensitive immunoliposomes. Biochemistry (Mosc) 1989;28:9508–9514. doi: 10.1021/bi00450a039. [DOI] [PubMed] [Google Scholar]
- [88].Litzinger DC, Huang L. Phosphatidylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochim. Biophys. Acta. 1992;1113:201–227. doi: 10.1016/0304-4157(92)90039-d. [DOI] [PubMed] [Google Scholar]
- [89].Couvreur P, Fattal E, Malvy C. pH-sensitive liposomes: an intellegent system for the delivery of antisense oligonucleotides. J. Liposome Res. 1997;7:1–18. [Google Scholar]
- [90].Connor J, Huang L. pH-sensitive immunoliposomes as an efficient and target-specific carrier for antitumor drugs. Cancer Res. 1986;46:3431–3435. [PubMed] [Google Scholar]
- [91].Straubinger RM, Duzgunes N, Papahadjopoulos D. pH-sensitive liposomes mediate cytoplasmic delivery of encapsulated macromolecules. FEBS Lett. 1985;179:148–154. doi: 10.1016/0014-5793(85)80210-6. [DOI] [PubMed] [Google Scholar]
- [92].Torchilin VP, editor. Immobilized enzymes in medicine. Springer-Verlag; Berlin ; New York: 1991. [Google Scholar]
- [93].Torchilin VP, Zhou F, Huang L. pH-sensitive liposomes. J Liposome Res. 1993;3:201–255. [Google Scholar]
- [94].Sheff D. Endosomes as a route for drug delivery in the real world. Adv. Drug Deliv. Rev. 2004;56:927–930. doi: 10.1016/j.addr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [95].Asokan A, Cho MJ. Cytosolic delivery of macromolecules. II. Mechanistic studies with pH-sensitive morpholine lipids. Biochim. Biophys. Acta. 2003;1611:151–160. doi: 10.1016/s0005-2736(03)00050-6. [DOI] [PubMed] [Google Scholar]
- [96].Shalaev EY, Steponkus PL. Phase diagram of 1,2-dioleoylphosphatidylethanolamine (DOPE):water system at subzero temperatures and at low water contents. Biochim. Biophys. Acta. 1999;1419:229–247. doi: 10.1016/s0005-2736(99)00068-1. [DOI] [PubMed] [Google Scholar]
- [97].Simoes S, Moreira JN, Fonseca C, Duzgunes N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004;56:947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- [98].Fattal E, Couvreur P, Dubernet C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv. Drug Deliv. Rev. 2004;56:931–946. doi: 10.1016/j.addr.2003.10.037. [DOI] [PubMed] [Google Scholar]
- [99].Roux E, Passirani C, Scheffold S, Benoit JP, Leroux JC. Serum-stable and long-circulating, PEGylated, pH-sensitive liposomes. J. Control. Release. 2004;94:447–451. doi: 10.1016/j.jconrel.2003.10.024. [DOI] [PubMed] [Google Scholar]
- [100].Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol. Cancer Ther. 2002;1:337–346. [PubMed] [Google Scholar]
- [101].Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- [102].Yessine MA, Leroux JC. Membrane-destabilizing polyanions: interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv. Drug Deliv. Rev. 2004;56:999–1021. doi: 10.1016/j.addr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- [103].Yessine MA, Lafleur M, Meier C, Petereit HU, Leroux JC. Characterization of the membrane-destabilizing properties of different pH-sensitive methacrylic acid copolymers. Biochim. Biophys. Acta. 2003;1613:28–38. doi: 10.1016/s0005-2736(03)00137-8. [DOI] [PubMed] [Google Scholar]
- [104].Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pollard H, Toumaniantz G, Amos JL, Avet-Loiseau H, Guihard G, Behr JP, Escande D. Ca2+-sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J. Gene Med. 2001;3:153–164. doi: 10.1002/jgm.160. [DOI] [PubMed] [Google Scholar]
- [106].Pollard H, Remy JS, Loussouarn G, Demolombe S, Behr JP, Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J. Biol. Chem. 1998;273:7507–7511. doi: 10.1074/jbc.273.13.7507. [DOI] [PubMed] [Google Scholar]
- [107].Branden LJ, Mohamed AJ, Smith CI. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat. Biotechnol. 1999;17:784–787. doi: 10.1038/11726. [DOI] [PubMed] [Google Scholar]
- [108].Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nature materials. 2003;2:668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- [109].Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- [110].Jeong JH, Kim SW, Park TG. Novel intracellular delivery system of antisense oligonucleotide by self-assembled hybrid micelles composed of DNA/PEG conjugate and cationic fusogenic peptide. Bioconjug. Chem. 2003;14:473–479. doi: 10.1021/bc025632k. [DOI] [PubMed] [Google Scholar]
- [111].Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J. Control. Release. 2004;94:187–193. doi: 10.1016/j.jconrel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [112].Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target. 2005;13:73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- [114].Shim WS, Kim SW, Choi EK, Park HJ, Kim JS, Lee DS. Novel pH sensitive block copolymer micelles for solvent free drug loading. Macromol. Biosci. 2006;6:179–186. doi: 10.1002/mabi.200500182. [DOI] [PubMed] [Google Scholar]
- [115].Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- [116].Lee E, Na K, Bae Y. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- [117].Liu S, Armes SP. Synthesis and aqueous solution behavior of a pH-responsive schizophrenic diblock copolymer. Langmuir. 2003;19:4432–4438. [Google Scholar]
- [118].Guo X, Szoka FC., Jr. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG-diortho ester-lipid conjugate. Bioconjug. Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- [119].Boomer JA, Thompson DH. Synthesis of acid-labile diplasmenyl lipids for drug and gene delivery applications. Chem. Phys. Lipids. 1999;99:145–153. doi: 10.1016/s0009-3084(99)00033-x. [DOI] [PubMed] [Google Scholar]
- [120].Zalipsky S, Qazen M, Walker JA, 2nd, Mullah N, Quinn YP, Huang SK. Bioconjug. Chem. Vol. 10. 1999. New detachable poly(ethylene glycol) conjugates: cysteine-cleavable lipopolymers regenerating natural phospholipid, diacyl phosphatidylethanolamine; pp. 703–707. [DOI] [PubMed] [Google Scholar]
- [121].Kratz F, Beyer U, Schutte MT. Drug-polymer conjugates containing acid-cleavable bonds. Crit. Rev. Ther. Drug Carrier Syst. 1999;16:245–288. doi: 10.1615/critrevtherdrugcarriersyst.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- [122].Zhang JX, Zalipsky S, Mullah N, Pechar M, Allen TM. Pharmaco attributes of dioleoylphosphatidylethanolamine/cholesterylhemisuccinate liposomes containing different types of cleavable lipopolymers. Pharmacol. Res. 2004;49:185–198. doi: 10.1016/j.phrs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [123].Roux E, Francis M, Winnik FM, Leroux JC. Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs. Int. J. Pharm. 2002;242:25–36. doi: 10.1016/s0378-5173(02)00183-7. [DOI] [PubMed] [Google Scholar]
- [124].Leroux J, Roux E, Le Garrec D, Hong K, Drummond DC. N-isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J. Control. Release. 2001;72:71–84. doi: 10.1016/s0168-3659(01)00263-2. [DOI] [PubMed] [Google Scholar]
- [125].Roux E, Stomp R, Giasson S, Pezolet M, Moreau P, Leroux JC. Steric stabilization of liposomes by pH-responsive N-isopropylacrylamide copolymer. J. Pharm. Sci. 2002;91:1795–1802. doi: 10.1002/jps.10172. [DOI] [PubMed] [Google Scholar]
- [126].Sudimack JJ, Guo W, Tjarks W, Lee RJ. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochim. Biophys. Acta. 2002;1564:31–37. doi: 10.1016/s0005-2736(02)00399-1. [DOI] [PubMed] [Google Scholar]
- [127].Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- [128].Jones MC, Ranger M, Leroux JC. pH-sensitive unimolecular polymeric micelles: synthesis of a novel drug carrier. Bioconjug. Chem. 2003;14:774–781. doi: 10.1021/bc020041f. [DOI] [PubMed] [Google Scholar]
- [129].Suzawa T, Nagamura S, Saito H, Ohta S, Hanai N, Kanazawa J, Okabe M, Yamasaki M. Enhanced tumor cell selectivity of adriamycin-monoclonal antibody conjugate via a poly(ethylene glycol)-based cleavable linker. J. Control. Release. 2002;79:229–242. doi: 10.1016/s0168-3659(01)00554-5. [DOI] [PubMed] [Google Scholar]
- [130].Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J. Control. Release. 2003;86:223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- [131].Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J. Control. Release. 2002;82:17–27. doi: 10.1016/s0168-3659(02)00088-3. [DOI] [PubMed] [Google Scholar]
- [132].Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjug. Chem. 2001;12:906–910. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- [133].Venugopalan P, Jain S, Sankar S, Singh P, Rawat A, Vyas SP. pH-sensitive liposomes: mechanism of triggered release to drug and gene delivery prospects. Pharmazie. 2002;57:659–671. [PubMed] [Google Scholar]
- [134].Turk MJ, Reddy JA, Chmielewski JA, Low PS. Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs. Biochim. Biophys. Acta. 2002;1559:56–68. doi: 10.1016/s0005-2736(01)00441-2. [DOI] [PubMed] [Google Scholar]
- [135].Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, Kamiya H, Harashima H. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry (Mosc) 2004;43:5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- [136].Shi G, Guo W, Stephenson SM, Lee RJ. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release. 2002;80:309–319. doi: 10.1016/s0168-3659(02)00017-2. [DOI] [PubMed] [Google Scholar]
- [137].Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm. Res. 2005;22:2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. in vitro evaluations. Mol. Pharmaceutics. 2005;2:357–366. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm. 2005;293:261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- [140].Devalapally H, Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;59:477–484. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- [141].Paleos CM, Tsiourvas D, Sideratou Z, Tziveleka L. Acid- and salt-triggered multifunctional poly(propylene imine) dendrimer as a prospective drug delivery system. Biomacromolecules. 2004;5:524–529. doi: 10.1021/bm030068h. [DOI] [PubMed] [Google Scholar]
- [142].Ihre R, De Jesu O.L. Padilla, Szoka FC, Frechet JM. Polyester dendritic systems for drug delivery applications: design, synthesis, and characterization. Bioconjug. Chem. 2002;13:443–452. doi: 10.1021/bc010102u. [DOI] [PubMed] [Google Scholar]
- [143].Gillies ER, Jonsson TB, Fréchet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J. Am. Chem. Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- [144].Meyer DE, Kong GA, Dewhirst MW, Chilkoti AA. Drug targeting using thermally responsive polymers and local hyperthermia. J. Controll Release. 2001;74:213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- [145].Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperth. 2006;22:205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- [146].Yatvin MB, Weinstein JN, Dennis WH, R. B. Design of liposomes for enhanced local release of drugs by hyperthermia. Sci. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- [147].Kono K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001;53:307–319. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- [148].Schild HG. Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 1992;17:163–249. [Google Scholar]
- [149].Kono K, Nakai R, Morimoto K, Takagishi T. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim. Biophys. Acta (BBA) - Biomembranes. 1999;1416:239–250. doi: 10.1016/s0005-2736(98)00226-0. [DOI] [PubMed] [Google Scholar]
- [150].Kono K, Yoshino K, Takagishi T. Effect of poly(ethylene glycol) grafts on temperature-sensitivity of thermosensitive polymer-modified liposomes. J. Control. Release. 2002;80:321–332. doi: 10.1016/s0168-3659(02)00018-4. [DOI] [PubMed] [Google Scholar]
- [151].Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 1995;374:240–242. [Google Scholar]
- [152].Chung YMJE, Okano T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J. Control. Release. 2000;65:93–103. doi: 10.1016/s0168-3659(99)00242-4. [DOI] [PubMed] [Google Scholar]
- [153].Cavallaro G, Campisi M, Licciardi M, Ogris M, Giammona G. Reversibly stable thiopolyplexes for intracellular delivery of genes. J. Control. Release. 2006;115:322–334. doi: 10.1016/j.jconrel.2006.07.027. [DOI] [PubMed] [Google Scholar]
- [154].Kommareddy S, Amiji M. Preparation and evaluation of thiol-modified gelatin nanoparticles for intracellular DNA delivery in response to glutathione. Bioconjug. Chem. 2005;16:1423–1432. doi: 10.1021/bc050146t. [DOI] [PubMed] [Google Scholar]
- [155].Kommareddy S, Amiji M. Poly(ethylene glycol)-modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomed. 2007;3:32–42. doi: 10.1016/j.nano.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Carlisle ETRC, Briggs SS, Preece JA, Ulbrich K, Seymour LW. Polymer-coated polyethylenimine/DNA complexes designed for triggered activation by intracellular reduction. J. Gene Med. 2004;6:337–344. doi: 10.1002/jgm.525. [DOI] [PubMed] [Google Scholar]
- [157].Kevin RW, Otto S. Reversible vovalent chemistry in drug delivery. Cur. Drug Discov. Tech. 2005;2:123–160. doi: 10.2174/1570163054866882. [DOI] [PubMed] [Google Scholar]
- [158].Kirpotin D, Hong KL, Mullah N, Papahadjopoulos D, Zalipsky S. Liposomes with detachable polymer coating: Destabilization and fusion of dioleoylphosphatidylethanolamine vesicles triggered by cleavage of surface-grafted poly(ethylene glycol) FEBS Lett. 1996;388:115–118. doi: 10.1016/0014-5793(96)00521-2. [DOI] [PubMed] [Google Scholar]
- [159].Sukhorukov GB, Rogach AL, Garstka M, Springer S, Parak WJ, Munoz-Javier A, Kreft O, Skirtach AG, Susha AS, Ramaye Y, Palankar R, Winterhalter M. Multifunctionalized polymer microcapsules: novel tools for biological and pharmacological applications. Small. 2007;3:944–955. doi: 10.1002/smll.200600622. [DOI] [PubMed] [Google Scholar]
- [160].Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine (London, England) 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- [161].Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [162].Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- [163].Shinkai M, Ito A. Functional magnetic particles for medical application. Adv. Biochem. Eng. Biotechnol. 2004;91:191–220. doi: 10.1007/b94212. [DOI] [PubMed] [Google Scholar]
- [164].Shen T, Weissleder R, Papisov M, Bogdanov A, Jr., Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn. Reson. Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- [165].Kellar KE, Fujii DK, Gunther WH, Briley-Saebo K, Spiller M, Koenig SH. ‘NC100150’, a preparation of iron oxide nanoparticles ideal for positive-contrast MR angiography. MAGMA. 1999;8:207–213. doi: 10.1007/BF02594600. [DOI] [PubMed] [Google Scholar]
- [166].Hyeon T, Lee SS, Park J, Chung Y, Na HB. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001;123:12798–12801. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- [167].Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- [168].Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- [169].Wagner S, Schnorr J, Pilgrimm H, Hamm B, Taupitz M. Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest. Radiol. 2002;37:167–177. doi: 10.1097/00004424-200204000-00002. [DOI] [PubMed] [Google Scholar]
- [170].Anzai Y, Prince MR. Iron oxide-enhanced MR lymphography: the evaluation of cervical lymph node metastases in head and neck cancer. J. Magn. Reson. Imaging. 1997;7:75–81. doi: 10.1002/jmri.1880070111. [DOI] [PubMed] [Google Scholar]
- [171].Okassa L. Ngaboni, Marchais H, Douziech-Eyrolles L, Cohen-Jonathan S, Souce M, Dubois P, Chourpa I. Development and characterization of sub-micron poly(D,L-lactide-co-glycolide) particles loaded with magnetite/maghemite nanoparticles. Int. J. Pharm. 2005;302:187–196. doi: 10.1016/j.ijpharm.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [172].Gou ML, Qian ZY, Wang H, Tang YB, Huang MJ, Kan B, Wen YJ, Dai M, Li XY, Gong CY, Tu MJ. Preparation and characterization of magnetic poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) microspheres. J. Mater. Sci. Mater. Med. 2007;19:1033–41. doi: 10.1007/s10856-007-3230-3. [DOI] [PubMed] [Google Scholar]
- [173].Zhang C, Wangler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Kruger R, Huss R, Seliger C, Semmler W, Kiessling F. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23:1427–1434. doi: 10.1021/la061879k. [DOI] [PubMed] [Google Scholar]
- [174].Cinteza LO, Ohulchanskyy TY, Sahoo Y, Bergey EJ, Pandey RK, Prasad PN. Diacyllipid micelle-based nanocarrier for magnetically guided delivery of drugs in photodynamic therapy. Mol. Pharm. 2006;3:415–423. doi: 10.1021/mp060015p. [DOI] [PubMed] [Google Scholar]
- [175].Widder KJ, Senyel AE, Scarpelli GD. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 1978;158:141–146. doi: 10.3181/00379727-158-40158. [DOI] [PubMed] [Google Scholar]
- [176].Gang J, Park SB, Hyung W, Choi EH, Wen J, Kim HS, Shul YG, Haam S, Song SY. Magnetic poly epsilon-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model. J. Drug Target. 2007;15:445–453. doi: 10.1080/10611860701453901. [DOI] [PubMed] [Google Scholar]
- [177].Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lubbe AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60:6641–6648. [PubMed] [Google Scholar]
- [178].Alexiou C, Jurgons R, Schmid RJ, Bergemann C, Henke J, Erhardt W, Huenges E, Parak F. Magnetic drug targeting--biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J. Drug Target. 2003;11:139–149. doi: 10.1080/1061186031000150791. [DOI] [PubMed] [Google Scholar]
- [179].Dandamudi S, Campbell RB. The drug loading, cytotoxicty and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials. 2007;28:4673–4683. doi: 10.1016/j.biomaterials.2007.07.024. [DOI] [PubMed] [Google Scholar]
- [180].Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int. J. Hyperthermia. 2005;21:637–647. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- [181].Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy--feasibility, tolerance and achieved temperatures. Int. J. Hyperthermia. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- [182].Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest. Radiol. 2002;37:580–586. doi: 10.1097/00004424-200210000-00008. [DOI] [PubMed] [Google Scholar]
- [183].Boutry S, Laurent S, Elst LV, Muller RN. Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media Mol. Imaging. 2006;1:15–22. doi: 10.1002/cmmi.87. [DOI] [PubMed] [Google Scholar]
- [184].Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- [185].Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest. Radiol. 1998;33:886–892. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- [186].Unger EC, Hersh E, Vannan M, McCreery T. Gene delivery using ultrasound contrast agents. Echocardiography. 2001;18:355–361. doi: 10.1046/j.1540-8175.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- [187].Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv. Drug Deliv. Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- [188].Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J. Control. Release. 2006;114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- [189].Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim. Biophys. Acta. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [190].Tartis MS, McCallan J, Lum AF, LaBell R, Stieger SM, Matsunaga TO, Ferrara KW. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med. Biol. 2006;32:1771–1780. doi: 10.1016/j.ultrasmedbio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [191].Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- [192].Husseini GA, Rosa M.A. Diaz de la, Gabuji T, Zeng Y, Christensen DA, Pitt WG. Release of doxorubicin from unstabilized and stabilized micelles under the action of ultrasound. J. Nanosci. Nanotechnol. 2007;7:1028–1033. doi: 10.1166/jnn.2007.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Tiukinhoy-Laing SD, Buchanan K, Parikh D, Huang S, MacDonald RC, McPherson DD, Klegerman ME. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J. Drug Target. 2007;15:109–114. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- [194].Tsutsui JM, Grayburn PA, Xie F, Porter TR. Drug and gene delivery and enhancement of thrombolysis using ultrasound and microbubbles. Cardiol. Clin. 2004;22:299–312. vii. doi: 10.1016/j.ccl.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [195].Smith DA, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med. Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- [196].Fang JY, Hung CF, Liao MH, Chien CC. A study of the formulation design of acoustically active lipospheres as carriers for drug delivery. Eur. J. Pharm. Biopharm. 2007;67:67–75. doi: 10.1016/j.ejpb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [197].Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, Kost J, Barenholz Y. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23:4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- [198].Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- [199].Scheule RK, St George JA, Bagley RG, Marshall J, Kaplan JM, Akita GY, Wang KX, Lee ER, Harris DJ, Jiang C, Yew NS, Smith AE, Cheng SH. Basis of pulmonary toxicity associated with cationic lipid-mediated gene transfer to the mammalian lung. Hum. Gene Ther. 1997;8:689–707. doi: 10.1089/hum.1997.8.6-689. [DOI] [PubMed] [Google Scholar]
- [200].Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- [201].Tang F, Hughes JA. Use of dithiodiglycolic acid as a tether for cationic lipids decreases the cytotoxicity and increases transgene expression of plasmid DNA in vitro. Bioconjug. Chem. 1999;10:791–796. doi: 10.1021/bc990016i. [DOI] [PubMed] [Google Scholar]
- [202].Xu Y, Szoka FC., Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry (Mosc) 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- [203].Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- [204].Duzgunes N, Felgner PL. Intracellular delivery of nucleic acids and transcription factors by cationic liposomes. Methods Enzymol. 1993;221:303–306. doi: 10.1016/0076-6879(93)21025-4. [DOI] [PubMed] [Google Scholar]
- [205].Elouahabi A, Ruysschaert JM. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [206].Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- [207].Sakurai F, Inoue R, Nishino Y, Okuda A, Matsumoto O, Taga T, Yamashita F, Takakura Y, Hashida M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J. Control. Release. 2000;66:255–269. doi: 10.1016/s0168-3659(99)00280-1. [DOI] [PubMed] [Google Scholar]
- [208].Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS Pharm. Sci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- [210].Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim. Biophys. Acta. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- [211].Loret EP, Vives E, Ho PS, Rochat H, Van Rietschoten J, Johnson WC., Jr. Activating region of HIV-1 Tat protein: vacuum UV circular dichroism and energy minimization. Biochemistry (Mosc) 1991;30:6013–6023. doi: 10.1021/bi00238a027. [DOI] [PubMed] [Google Scholar]
- [212].Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- [213].Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- [214].Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- [215].Oehlke J, Scheller A, Wiesner B, Krause E, Beyermann M, Klauschenz E, Melzig M, Bienert M. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- [216].Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- [217].Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- [218].Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug. Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- [219].Kaufman CL, Williams M, Ryle LM, Smith TL, Tanner M, Ho C. Superparamagnetic iron oxide particles transactivator protein-fluorescein isothiocyanate particle labeling for in vivo magnetic resonance imaging detection of cell migration: uptake and durability. Transplantation. 2003;76:1043–1046. doi: 10.1097/01.TP.0000090164.42732.47. [DOI] [PubMed] [Google Scholar]
- [220].Zhao M, Kircher MF, Josephson L, Weissleder R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjug. Chem. 2002;13:840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]
- [221].Tseng YL, Liu JJ, Hong RL. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy study. Mol. Pharmacol. 2002;62:864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- [222].Gorodetsky R, Levdansky L, Vexler A, Shimeliovich I, Kassis I, Ben-Moshe M, Magdassi S, Marx G. Liposome transduction into cells enhanced by haptotactic peptides (Haptides) homologous to fibrinogen C-termini. J. Control. Release. 2004;95:477–488. doi: 10.1016/j.jconrel.2003.12.023. [DOI] [PubMed] [Google Scholar]
- [223].Levchenko TS, Rammohan R, Volodina N, Torchilin VP. Tat peptide-mediated intracellular delivery of liposomes. Methods Enzymol. 2003;372:339–349. doi: 10.1016/s0076-6879(03)72019-9. [DOI] [PubMed] [Google Scholar]
- [224].Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Marty C, Meylan C, Schott H, Ballmer-Hofer K, Schwendener RA. Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes. Cell Mol. Life Sci. 2004;61:1785–1794. doi: 10.1007/s00018-004-4166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Fretz MM, Koning GA, Mastrobattista E, Jiskoot W, Storm G. OVCAR-3 cells internalize TAT-peptide modified liposomes by endocytosis. Biochim. Biophys. Acta. 2004;1665:48–56. doi: 10.1016/j.bbamem.2004.06.022. [DOI] [PubMed] [Google Scholar]
- [227].Zhang C, Tang N, Liu X, Liang W, Xu W, Torchilin VP. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J. Control. Release. 2006;112:229–239. doi: 10.1016/j.jconrel.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Palmer TN, Caride VJ, Caldecourt MA, Twickler J, Abdullah V. The mechanism of liposome accumulation in infarction. Biochim. Biophys. Acta. 1984;797:363–368. doi: 10.1016/0304-4165(84)90258-7. [DOI] [PubMed] [Google Scholar]
- [229].Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorganic & medicinal chemistry. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- [230].Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PubMed] [Google Scholar]
- [231].Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv. Drug Deliv. Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [232].Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv. Drug Deliv. Rev . 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [233].Lochmann D, Jauk E, Zimmer A. Drug delivery of oligonucleotides by peptides. Eur. J. Pharm. Biopharm. 2004;58:237–251. doi: 10.1016/j.ejpb.2004.03.031. [DOI] [PubMed] [Google Scholar]
- [234].Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug. Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug. Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Kale A, Torchilin V. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified PEGylated liposomes. J Drug Target. 2007 doi: 10.1080/10611860701498203. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Suzuki R, Yamada Y, Harashima H. Efficient cytoplasmic protein delivery by means of a multifunctional envelope-type nano device. Biol. Pharm. Bull. 2007;30:758–762. doi: 10.1248/bpb.30.758. [DOI] [PubMed] [Google Scholar]
- [238].Tilcock C, Unger E, Cullis P, MacDougall P. Liposomal Gd-DTPA: preparation and characterization of relaxivity. Radiology. 1989;171:77–80. doi: 10.1148/radiology.171.1.2928549. [DOI] [PubMed] [Google Scholar]
- [239].Kabalka GW, Davis MA, Holmberg E, Maruyama K, Huang L. Gadolinium-labeled liposomes containing amphiphilic Gd-DTPA derivatives of varying chain length: targeted MRI contrast enhancement agents for the liver. Magn. Reson. Imaging. 1991;9:373–377. doi: 10.1016/0730-725x(91)90425-l. [DOI] [PubMed] [Google Scholar]
- [240].Phillips WT, Goins B. Targeted delivery of imaging agents by liposomes. In: Torchilin VP, editor. Handbook of targeted delivery of imaging agents. CRC Press; Boca Raton: 1995. pp. 149–173. [Google Scholar]
- [241].Tilcock C. Liposomal paramagnetic magnetic resonance contrast agents. In: Gregoriadis G, editor. Liposome technology. vol. 2. CRC Press; Boca Raton, Fla.: 1993. pp. 65–87. [Google Scholar]
- [242].Schwendener RA, Wuthrich R, Duewell S, Wehrli E, von Schulthess GK. A pharmacokinetic and MRI study of unilamellar gadolinium-, manganese-, and iron-DTPA-stearate liposomes as organ-specific contrast agents. Invest. Radiol. 1990;25:922–932. doi: 10.1097/00004424-199008000-00009. [DOI] [PubMed] [Google Scholar]
- [243].Torchilin VP, Trubetskoy VS. In vivo visualizing of organs and tissues with liposomes. J. Liposome Res. 1995;5:795–812. [Google Scholar]
- [244].Torchilin VP. Surface-modified liposomes in gamma- and MR-imaging. Adv. Drug Deliv. Rev. 1997;24:301–313. [Google Scholar]
- [245].Kabalka GW, Davis MA, Moss TH, Buonocore E, Hubner K, Holmberg E, Maruyama K, Huang L. Gadolinium-labeled liposomes containing various amphiphilic Gd-DTPA derivatives: targeted MRI contrast enhancement agents for the liver. Magn. Reson. Med. 1991;19:406–415. doi: 10.1002/mrm.1910190231. [DOI] [PubMed] [Google Scholar]
- [246].Grant CW, Karlik S, Florio E. A liposomal MRI contrast agent: phosphatidylethanolamine-DTPA. Magn. Reson. Med. 1989;11:236–243. doi: 10.1002/mrm.1910110211. [DOI] [PubMed] [Google Scholar]
- [247].Glogard C, Stensrud G, Hovland R, Fossheim SL, Klaveness J. Liposomes as carriers of amphiphilic gadolinium chelates: the effect of membrane composition on incorporation efficacy and in vitro relaxivity. Int. J. Pharm. 2002;233:131–140. doi: 10.1016/s0378-5173(01)00935-8. [DOI] [PubMed] [Google Scholar]
- [248].Unger E, Cardenas D, Zerella A, Fajardo LL, Tilcock C. Biodistribution and clearance of liposomal gadolinium-DTPA. Invest. Radiol. 1990;25:638–644. doi: 10.1097/00004424-199006000-00004. [DOI] [PubMed] [Google Scholar]
- [249].Barsky D, Putz B, Schulten K, Magin RL. Theory of paramagnetic contrast agents in liposome systems. Magn. Reson. Med. 1992;24:1–13. doi: 10.1002/mrm.1910240102. [DOI] [PubMed] [Google Scholar]
- [250].Putz B, Barsky D, Schulten K. Mechanisms of liposomal contrast agents in magnetic resonance imaging. J. Liposome Res. 1994;4:771–808. [Google Scholar]
- [251].Schwendener RA. Liposomes as carriers for paramagnetic gadolinium chelates as organ specific contrast agents for magnetic resonance imaging (MRI) J. Liposome Res. 1994;4:837–855. [Google Scholar]
- [252].Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- [253].Torchilin VP. Polymeric contrast agents for medical imaging. Curr. Pharm. Biotechnol. 2000;1:183–215. doi: 10.2174/1389201003378960. [DOI] [PubMed] [Google Scholar]
- [254].Trubetskoy VS, Torchilin VP. New approaches in the chemical design of Gd-containing liposomes for use in magnetic resonance imaging of lymph nodes. J. Liposome Res. 1994;4:961–980. [Google Scholar]
- [255].Torchilin VP. Novel polymers in microparticulate diagnostic agents. Chem. Tech. 1999;29:27–34. [Google Scholar]
- [256].Trubetskoy VS, Torchilin VP. Polyethyleneglycol based micelles as carriers of therapeutic and diagnostic agents. S.T.P. Pharma Sciences. 1996;6:79–86. [Google Scholar]
- [257].Torchilin VP, Trubetskoy VS, Wolf GL. Magnetic resonance imaging of lymph nodes with GD-containing liposomes. In: Torchilin VP, editor. Handbook of targeted delivery of imaging agents. CRC Press; Boca Raton: 1995. pp. 403–413. [DOI] [PubMed] [Google Scholar]
- [258].Unger EC, Winokur T, MacDougall P, Rosenblum J, Clair M, Gatenby R, Tilcock C. Hepatic metastases: liposomal Gd-DTPA-enhanced MR imaging. Radiology. 1989;171:81–85. doi: 10.1148/radiology.171.1.2928550. [DOI] [PubMed] [Google Scholar]
- [259].Torchilin VP, Trubetskoy VS, Narula J, Khaw BA. PEG-modified liposomes for gamma- and magentic resonance imaging. In: Lasic DD, Martin FJ, editors. Stealth liposomes. CRC Press; Boca Raton: 1995. pp. 225–231. [Google Scholar]
- [260].Trubetskoy VS, Cannillo JA, Milshtein A, Wolf GL, Torchilin VP. Controlled delivery of Gd-containing liposomes to lymph nodes: surface modification may enhance MRI contrast properties. Magn. Reson. Imaging. 1995;13:31–37. doi: 10.1016/0730-725x(94)00083-f. [DOI] [PubMed] [Google Scholar]
- [261].Torchilin VP. Pharmacokinetic considerations in the development of labeled liposomes and micelles for diagnostic imaging. Q. J. Nucl. Med. 1997;41:141–153. [PubMed] [Google Scholar]
- [262].Trubetskoy VS, Frank-Kamenetsky MD, Whiteman KR, Wolf GL, Torchilin VP. Stable polymeric micelles: lymphangiographic contrast media for gamma scintigraphy and magnetic resonance imaging. Acad. Radiol. 1996;3:232–238. doi: 10.1016/s1076-6332(96)80448-x. [DOI] [PubMed] [Google Scholar]
- [263].Erdogan S, Roby A, Torchilin VP. Enhancet tumor magnetic resonance imaging with gadolinium-loaded polyhelating polymer-containing tumor-targeted liposomes. J. Magnetic Resonance Imaging. 2007 doi: 10.1002/jmri.21202. in press. [DOI] [PubMed] [Google Scholar]
- [264].Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:1196–1205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- [265].Erdogan S, Roby A, Torchilin VP. Enhanced tumor visualization by gamma-scintigraphy with 111-In-labeled polychelating-polymer-containing immunoliposomes. Mol. Pharm. 2006;3:525–530. doi: 10.1021/mp060055t. [DOI] [PubMed] [Google Scholar]
- [266].Elbayoumi TA, Pabba S, Roby A, Torchilin VP. Antinucleosome antibody-modified liposomes and lipid-core micelles for tumor-targeted delivery of therapeutic and diagnostic agents. J. Liposome Res. 2007;17:1–14. doi: 10.1080/08982100601186474. [DOI] [PubMed] [Google Scholar]
- [267].Koning GA, Krijger GC. Targeted multifunctional lipid-based nanocarriers for image-guided drug delivery. Anticancer Agents Med. Chem. 2007;7:425–440. doi: 10.2174/187152007781058613. [DOI] [PubMed] [Google Scholar]