Abstract
The MiT family comprises four genes in mammals: Mitf, Tfe3, Tfeb, and Tfec, which encode transcription factors of the basic-helix-loop-helix/leucine zipper class. Mitf is well-known for its essential role in the development of melanocytes, however the functions of the other members of this family, and of interactions between them, are less well understood. We have now characterized the complete set of MiT genes from zebrafish, which totals six instead of four. The zebrafish genome contain two mitf (mitfa and mitfb), two tfe3 (tfe3a and tfe3b), and single tfeb and tfec genes; this distribution is shared with other teleosts. We present here the sequence and embryonic expression patterns for the zebrafish tfe3b, tfeb and tfec genes, and identify a new isoform of tfe3a. These findings will assist in elucidating the roles of the MiT gene family over the course of vertebrate evolution.
Keywords: zebrafish, Danio rerio, Mitf, transcription factor, gene duplication
INTRODUCTION
It has been suggested that over the course of metazoan evolution, organismal complexity has increased with the number of genes involved in transcriptional regulation rather than as a function of total gene number (Levine and Tjian, 2003). Sequence-specific DNA-binding proteins make up a subset of these regulators, and many families of such transcription factors have undergone expansion in multicellular organisms (Nowick and Stubbs, 2010). The potential for ever more complex regulation via networks of interacting transcription factors is especially evident for those proteins that function as dimers, such as those bearing helix-loop-helix (bHLH) domains, leucine zippers (bZIP), or both (bHLH-ZIP) (Amoutzias et al., 2008).
The MiT (microphthalmia/TFE) family comprises one small subfamily of basic helix-loop-helix/leucine zipper proteins that can form homodimers or heterodimers with each other but not with other bHLH or bHLH-ZIP proteins (Hemesath et al., 1994). Mitf was isolated as the gene responsible for the classic microphthalmia mouse mutant (Hodgkinson et al., 1993). Mitf is vital for the development of neural crest melanocytes as well as the pigmented retinal epithelium, and also plays an important role in osteoclasts and mast cells, at least in mammals (Steingrímsson et al., 2004). Tfe3 and Tfeb were isolated as genes encoding E-box binding proteins in B cells (Beckmann et al., 1990; Carr and Sharp, 1990), while Tfec was originally identified based on similarity to Tfe3 (Zhao et al., 1993). Tfeb is required for proper vascularization of the mouse placenta and inactivation of the gene results in embryonic lethality (Steingrímsson et al., 1998). Tfe3 and Tfec are dispensable for early development (Steingrimsson et al., 2002), however Tfe3 is involved in B cell activation (Merrell et al., 1997) and functions (with Mitf) in osteoclasts (Steingrimsson et al., 2002; Weilbaecher et al., 1998) and (with Tfeb) in T cells (Huan et al., 2006), while Tfec regulates gene expression in macrophages (Rehli et al., 2005; Zanocco-Marani et al., 2009). Tfe3 and Tfeb have also been linked to the regulation of genes involved in metabolism (Nakagawa et al., 2006; Settembre et al., 2011). Chromosomal translocations involving Tfe3 and Tfeb have been identified in a subset of renal carcinomas as well as other cancers (Davis and Fisher, 2007). Although expression of one isoform of Mitf is restricted to neural crest melanocytes , expression of other isoforms, and of other genes in the family, varies from tissue to tissue but is fairly broad (Kuiper et al., 2004; Steingrímsson et al., 2004).
The four-member MiT family of mammals likely arose via two rounds of whole genomic duplication (WGD) from a single ancestral gene (Simionato et al., 2007). Drosophila (Hallsson et al., 2004), C. elegans (Grove et al., 2009), and the basal chordate Ciona intestinalis (Satou et al., 2003) each contain single MiT genes. Zebrafish, as part of a vertebrate lineage, teleost fishes, thought to have undergone an additional WGD (Taylor et al., 2001), might be predicted to have more than four MiT genes reported in mammals. We previously identified duplicate mitf genes in the zebrafish (Lister et al., 2001). Here we describe the remainder of this family, which totals six members, and characterize the embryonic expression patterns of these genes.
RESULTS AND DISCUSSION
Isolation of additional members of the zebrafish MiT family
The identification of zebrafish orthologs of two of the four members of the MiT family has been previously reported; this included two MITF genes, mitfa and mitfb, as well as an ortholog of TFE3, tfe3a (Lister et al., 2001; Lister et al., 1999). Several molecular and bioinformatic approaches were taken to identify additional MiT family members encoded in the zebrafish genome, including degenerate PCR on cDNA and genomic DNA samples (data not shown), and mining of data from the zebrafish EST and genome sequencing projects. These efforts yielded an additional three genes, bringing the total to six. The same set of genes was identified in a bioinformatic survey of the basic helix-loop-helix transcription factor family of zebrafish (Wang et al., 2009), although this publication listed the size of the MiT subfamily as only five, treating the mitf duplicates as a single gene for reasons that are unclear.
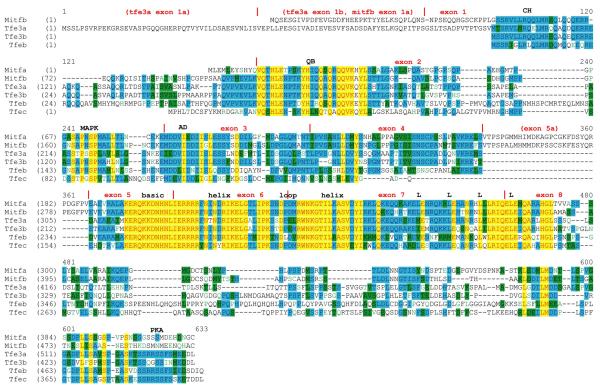
Figure 1 shows an alignment of all six zebrafish MiT proteins. The predicted sizes of the newly identified proteins are: Tfe3b, 445 amino acids; Tfeb, 493 amino acids; and Tfec, 396 amino acids. In the course of this work we uncovered an error in our originally published sequence for mitfb (Lister et al., 2001). The cDNA reported was actually chimeric; the 5′ end of mitfb was conjoined to a portion of an incompletely processed transcript of slc30a10, in the opposite orientation. (This also led to the determination of an incorrect map position for mitfb, see below.) The alignment shows the corrected sequence for Mitfb, with a predicted size of 500 amino acids. It should be noted that the expression pattern observed by RNA in situ hybridization with a probe corresponding to the corrected sequence of mitfb is identical to that originally described for this gene (Lister et al., 2001), which was obtained from a probe that contained a portion of slc30a10 (data not shown). Outside of the highly-conserved basic region and helix-loop-helix/leucine zipper domains, the predicted MiT proteins show the strongest similarity in the N-terminus, including charged/helix-forming (CH) and glutamine-rich/basic (QB) regions previously noted (Rehli et al., 1999), MAP kinase consensus phosphorylation site, and transcriptional activation domain (AD). In the C-terminus, a serine-rich region is conserved, although Mitfa and Mitfb lack the consensus cAMP-dependent protein kinase phosphorylation site (Ubersax and Ferrell, 2007). The zebrafish-specific exon 5a found in both mitfa and mitfb is not found in any of the other MiT genes. Interestingly, the four residues making up the leucine zipper are also not completely conserved: while the third and fourth positions are leucines in every zebrafish MiT protein, the first position is a leucine in only 3 of 6, and the second position in 5 of 6. The predicted leucine zipper of Tfeb contains leucine at only 2 of the 4 positions.
Figure 1. Multiple sequence alignment of the zebrafish MiT family.
Alignment of amino acids sequences derived from ClustalW is shown. Red text on yellow background indicates highest similarity, followed by blue on cyan, black on green, and green on white. Vertical red lines indicate exon boundaries, and the basic region helix-loop-helix domain, and leucines comprising the leucine zipper are indicated. Abbreviations: CH, charged helical domain; QB, glutamine-rich, basic domain; MAPK, consensus MAP kinase phosphorylation site; AD, transcriptional activation domain; PKA, consensus cAMP-dependent protein kinase phosphorylation site.
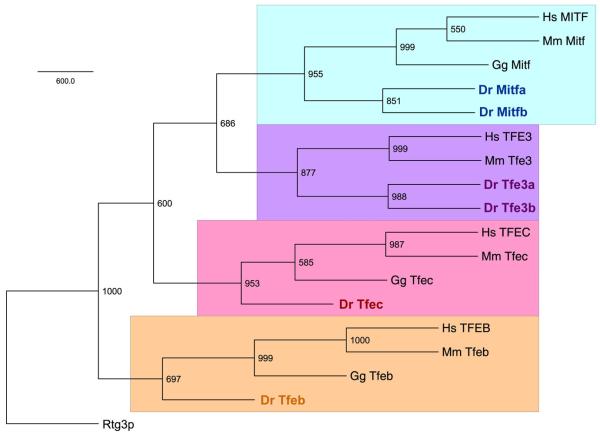
A phylogenetic tree of MiT proteins (using entire protein sequences) from human, mouse, chicken and zebrafish, confirms the assignments of the three newly identified genes as a second tfe3 (tfe3b; ZDB-GENE-010919-3), tfeb (ZDB-GENE-090807-3) and tfec (ZDB-GENE-041210-21)(Figure 2). The Mitf and Tfe3 proteins share the most similarity, with Tfeb being the most divergent member of the family, in agreement with a previous analysis of mammalian MiT proteins which used only the bHLH-ZIP domains (Rehli et al., 1999). To assess BLAST searches were made against other teleost genomes that have been or are in the process of being sequenced (http://www.ensembl.org/index.html ), including the pufferfishes Takifugu rubripes and Tetraodon nigroviridis, Japanese medaka, Oryzias latipes, and stickleback Gasterosteus aculeatus. These findings are summarized in Table 1. Except for medaka, for which only one tfe3 ortholog can be identified, all five teleost species contain two mitf genes, two tfe3 genes, and single tfeb and tfec genes, suggesting that tfeb and tfec duplicate genes may have been lost prior to the teleost radiation.
Figure 2. Phylogenetic tree of human, mouse, chicken, and zebrafish MiT proteins.
A majority rule consensus tree is shown with bootstrap values indicated at nodes. The four MiT clades are each highlighted a different color. Abbreviations: Hs, Homo sapiens; Mm, Mus musculus; Gg, Gallus gallus; Dr, Danio rerio.
Table 1. Teleost MiT genes.
| Species | Ensembl gene ID | map location | ortholog |
|---|---|---|---|
| Danio rerio | ENSDARG00000003732 | chr 6 | mitfa |
| ENSDARG00000076049 | chr 23 | mitfb | |
| ENSDARG00000004729 | chr 8 | tfe3a | |
| ENSDARG00000019457 | chr 11 | tfe3b | |
| ENSDARG00000010794 | chr 11 | tfeb | |
| ENSDARG00000008423 | chr 4 | tfec | |
| Oryzias latipes | ENSORLG00000003123 | chr 5 | mitfa |
| ENSORLG00000013461 | chr 7 | mitfb | |
| ENSORLG00000014134 | chr 5 | tfe3 | |
| ENSORLG00000015866 | chr 5 | tfeb | |
| ENSORLG00000010461 | chr 23 | tfec | |
| Takifugu rubripes | ENSTRUG00000001600 | scaffold 150 | mitfa |
| ENSTRUG00000012080 | scaffold 60 | mitfb | |
| ENSTRUG00000013198 | scaffold 79 | tfe3a | |
| ENSTRUG00000005584 | scaffold 215 | tfe3b | |
| ENSTRUG00000000653 | scaffold 79 | tfeb | |
| ENSTRUG00000016827 | scaffold 8 | tfec * | |
| ENSTRUG00000017315 | scaffold 2576 | tfec * | |
|
Tetraodon
nigroviridis |
ENSTNIG00000007360 | chr 11 | mitfa |
| ENSTNIG00000012564 | chr 9 | mitfb | |
| ENSTNIG00000007324 | chr 11 | tfe3a | |
| ENSTNIG00000019469 | unassigned | tfe3b | |
| ENSTNIG00000007281 | chr 11 | tfeb | |
| ENSTNIG00000018518 | chr 19 | tfec | |
|
Gasterosteus
aculeatus |
ENSGACG00000011875 | group XVII | mitfa |
| ENSGACG00000003425 | group XII | mitfb | |
| ENSGACG00000010355 | group XVII | tfe3a | |
| ENSGACG00000000794 | scaffold 141 | tfe3b | |
| ENSGACG00000000364 | scaffold 27 | tfeb | |
| ENSGACG00000018910 | group IV | tfec |
Coding regions of ENSTRUG00000016827 and ENSTRUG00000017315 are nearly identical at the nucleotide level and appear to represent the same gene.
Chromosomal assignments for all six zebrafish MiT genes can be made in the most recent zebafish genome assembly, Zv9 (http://www.ensembl.org/Danio_rerio/Info/Index; Table 1). mitfa and tfe3a had previously been mapped to chromosomes 6 and 8 respectively (Lister et al., 2001; Lister et al., 1999). Zebrafish tfe3b and tfeb reside on chromosome 11 while tfec is found on chromosome 4. mitfb was previously localized to linkage group 13 by radiation hybrid mapping (Lister et al., 2001) with primers which were derived from the 3′, slc30a10 portion of the original clone, but the latest genome assembly places it on chromosome 23 instead. (slc30a10 indeed maps to chromosome 13; http://www.ensembl.org/Danio_rerio/Gene/Summary?g=ENSDARG00000034877;r=13:53636202-53640824;t=ENSDART00000052076).
Multiple MiT isoforms are expressed during zebrafish embryonic and larval stages
Because some of the sequences we derived originated from genomic DNA or were merely predicted in silico, we first used RT/PCR to determine if these genes were expressed during embryogenesis. RNA was isolated from newly-fertilized embryos as well as 72 hours post-fertilization (hpf) larvae, first-strand cDNA synthesized and PCR was performed with gene-specific primer pairs flanking at least one intron. Primers against tfe3a, which is already known to be expressed during embryogenesis (Lister et al., 2001) were used as a positive control. As seen in Figure 3A, expression of tfe3b, tfeb, and tfec could all be detected at 72 hpf. Two of the three new genes, tfe3b and tfeb, as well as tfe3a, could be detected as maternal transcripts.
Figure 3. MiT isoforms expressed during embryogenesis.
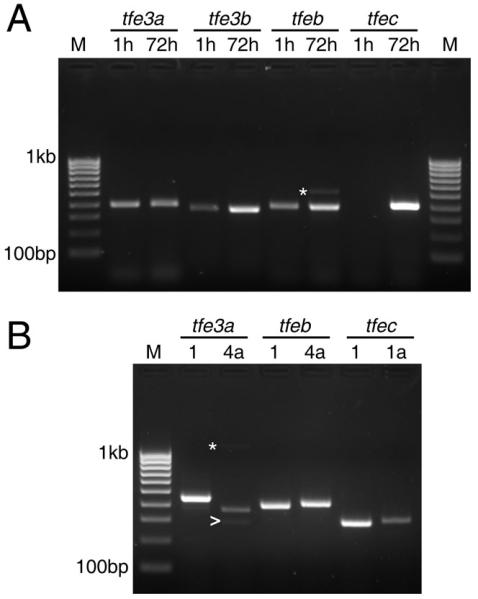
A. RT/PCR demonstrating expression of MiT genes at 1 and 72 hours post-fertilization. M, 100 basepair ladder markers. B. RT/PCR with isoform-specific primers at 72 hpf. Asterisks indicate non-specific products; arrow indicates tfe3a transcript lacking exon 6.
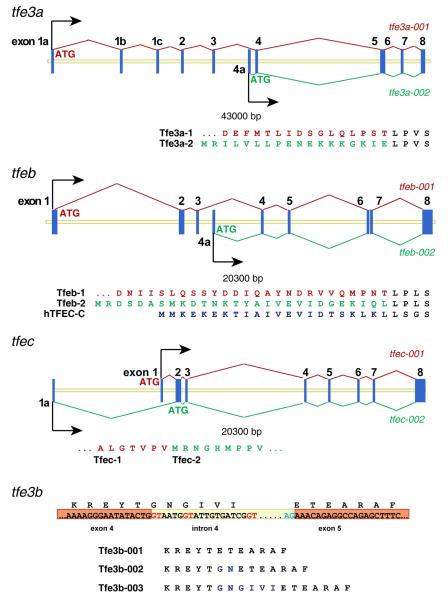
In various assemblies of the zebrafish genome, alternate first exons have been predicted for tfe3a, tfeb, and tfec. For zebrafish tfec, an alternate first exon is located 5.7 kilobases upstream of the first coding exon; transcripts beginning with this exon would begin translation with the first ATG in exon 2 to yield a polypeptide of 325 amino acids (Fig. 4). In the case of tfe3a and tfeb, the putative alternate first exon occurs between exons 3 and 4, and includes an initiation codon in the correct reading frame (Figure 4). The first exon of transcript tfe3a-002 would encode 18 novel amino acids, and give rise to an isoform with a total length of 292 amino acids, while the first exon of transcript tfeb-002 encodes 28 amino acids, for a protein with a total length of 327 amino acids. Interestingly, an alternative promoter and first exon has been described in the analogous position in the human TFEC gene (Kuiper et al., 2004); moreover, the polypeptides encoded by this exon in zebrafish tfeb and the human TFEC show significant similarity (Fig. 4). The shorter isoforms of Tfe3a and Tfeb would lack the conserved transcriptional activation domain encoded in exon 3 and might act to negatively regulate target gene expression (Roman et al., 1991; Zhao et al., 1993).
Figure 4. Structure of zebrafish MiT genes with alternative transcripts.
tfe3a and tfeb each produce a transcript from an alternate promoter/first exon located between exon 3 and 4. Coding sequence of the alternate exon is shown beneath, along with the longer isoform and, for Tfeb, the human TFEC-C isoform. For tfe3b, alternate splice donor sites (shown in red) produce mature transcripts encoding an additional 2 (Tfe3b-002) or 6 (Tfe3b-003) amino acids.
In the case of tfeb, support for the alternate isoform can be found in GenBank (Accession #CF265795) as an EST from a whole adult zebrafish library. To determine if this or other predicted isoforms are in fact expressed during embryogenesis we designed primers for their detection by PCR, along with primers specific to the isoforms for which there was already evidence from cDNA. All three alternate transcripts were detected in cDNA from 72 hpf larvae (Figure 3B). A tfe3a transcript starting from the alternate first exon but lacking exon 6 was detected at a low level (Fig. 3B, arrow); skipping this exon produces a frameshift that would lead to premature termination. Additionally, a tfec EST (I.M.A.G.E. clone #7226661) we obtained and sequenced was found to be full-length except for the absence of exon 7, which preserves the reading frame but encodes a protein missing the second helix and most of the adjacent loop and leucine zipper domains. To determine whether or not this was likely to represent an appreciable fraction of tfec transcripts, we conducted PCR with primers flanking the missing exon, however we were unable to detect a product representing the exon-skipping event at any stage tested (data not shown). We conclude that this clone represents a rare mis-splicing event rather than a transcript expressed during embryogenesis at significant levels.
Finally, cloning and sequencing of tfe3b RT/PCR products indicated use of multiple alternate splice donor sites in exon 4 (Figure 4). These alternate splice forms encode proteins with an additional two or six amino acids. Interestingly, this is the same position where the zebrafish mitfs, mitfa and mitfb, contain an additional exon (Fig. 1) and the melanocyte specific M-isoform of mammalian Mitfs contains an alternatively spliced six amino acid stretch which influences DNA binding affinity (Hemesath et al., 1994).
Embryonic expression of tfe3b, tfeb and tfec
Whole mount RNA in situ hybridization was used to determine the spatial expression of the newly isolated MiT genes during the first three days of embryogenesis. Each displayed a spatially-restricted pattern, as described in detail below.
tfe3b
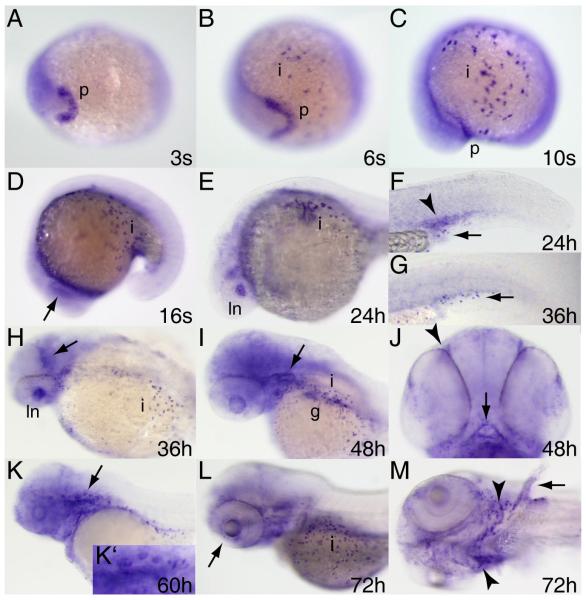
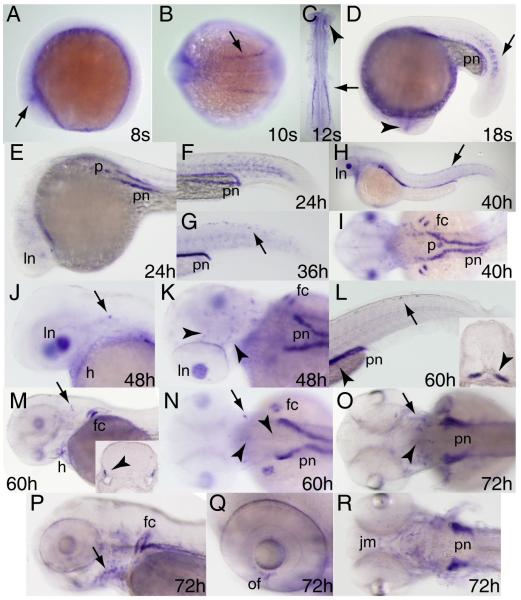
Low level tfe3b expression, perhaps derived from maternal transcripts, is present throughout the embryo at the beginning of gastrulation and persists through early somitogenesis (Fig. 5A-C and data not shown). First likely zygotic expression of tfe3b is detected in the polster at the 3-somite stage (Fig. 5A). By six somites tfe3b begins to be expressed in scattered cells of the epidermis over the yolk (Fig. 5B,C) that are suggestive of ionocytes (Janicke et al., 2010). Beginning around the 16-somite stage, tfe3b transcripts can be detected in the lens placode (Fig.5D), and by 24 hours post-fertilization in the lens itself (Fig.5E,H). tfe3b expression can be seen in the intermediate cell mass (ICM) at 24 hpf (Fig.5F) but this has largely subsided by 36 hpf (Fig.5G). Expression can be observed at the mid/hindbrain boundary at 36 hpf (Fig.5H). tfe3b is expressed diffusely through much of the head by 48 hpf, and more intensely in the otic epithelium, gut, and ionocytes (Fig.5I) as well as olfactory pits and mouth (Fig.5J). Expression continues at 60 hours in ear structures, notably the dorsal otic vesicle protrusions that will begin to establish the semicircular canals (Haddon and Lewis, 1996) (Fig.5K,K’). While lens expression of tfe3b has declined by 72 hpf, expression can be detected at the ciliary margin (Fig.6L, arrow). tfe3b expression in the pharyngeal arches at this stage (Fig.5L,M) is consistent with the identity of these cells as ionocytes associated with the developing gills (Varsamos et al., 2005). By 72 hpf tfe3b expression is also apparent in cartilage of the pectoral fins (Fig.5M).
Figure 5. Expression pattern of tfe3b during embryogenesis.
Whole mount RNA in situ hybridization was performed on embryos at the stages indicated. Expression begins in the polster (A,B) and is seen shortly thereafter in ionocytes (B,C). Expression is observed in the lens placode at the 16 somite stage (D, arrow), and thereafter (up to 36 hpf) in the lens itself (E,H). Expression in the intermediate cell mass (arrowhead, F) is seen at 24 hpf but has disappeared by 36 hpf (G; arrows indicate ionocytes). tfe3b is expressed at the mid-hindbrain boundary at 36 hpf (arrow, H) and in the otic epithelium (arrow, I) as well as gut tissue and ionocytes, and more diffusely through the head at 48 hpf. Expression is also seen in the olfactory pits (arrowhead, J) and mouth (arrow, J) at this stage. At 60 hpf, tfe3b is expressed in the epithelial protrusions of the dorsal otocyst (K, arrow; K’, higher magnification). L) Ciliary margin expression (arrow). M) Expression in ionocytes associated with the branchial arches (arrowheads) and in pectoral fin cartilage (arrow). All views lateral except A,B,J, ventral view; H,I, dorsolateral view; M, ventrolateral view. Abbreviations: p, polster; i, ionocytes; ln, lens; g, gut.
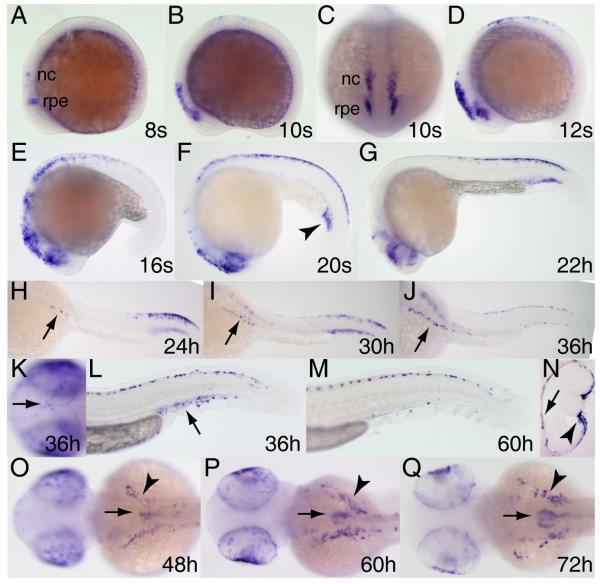
Figure 6. Expression pattern of tfeb during embryogenesis.
Whole mount RNA in situ hybridization was performed on embryos at the stages indicated. Expression is first observed in the eye (arrowheads in A,C,D), and shortly thereafter in the paraxial and lateral plate mesoderm (arrows in B-D). Lens expression increases between 24 and 48 hpf (E,J) and diminishes thereafter. tfeb expression is observed in the pronephros along most of its length from 18 somites (D, shown in transverse section in inset L, arrowhead) up to 72 hpf when it is concentrated in the proximal convoluted tubules (O,R). Expression in the presumptive pancreas is seen at 24 hpf (E) and 40 hpf (I) but declines thereafter (K,N). tfeb is expressed in cells along the dorsal midline (arrows in H,L), scattered cells associated with the otic capsule (arrows in J,M-O; inset in M shows transverse section through otic capsule, arrowhead indicates expressing cells) cells in the brain (arrowheads in K,N,O) and associated with the pharyngeal arches (arrow, P). Expression is also seen at 72 hpf in the optic fissure (of, Q) and jaw musculature (jm, R). A,D-H,J,L,M,P,Q, lateral views; B,I,N,O, dorsal views; K, dorsolateral view; R, ventral view. Abbreviations: pn, pronephros; ln, lens; p, pancreas; fc, fin cartilage; h, heart.
tfeb
tfeb can be detected as early as the 8 somite stage, first in small groups of cells at the posterior edge of the optic primordia (Fig.6A) and a short time later in paraxial and lateral plate mesoderm (Fig.6B,C). By the 18-somite stage tfeb expression can be seen in the pronephros and the medial portion of the posterior somites (Fig.6D,F). tfeb expression is observed in the pancreas beginning at 24 hpf (Fig.6E) but has declined in this tissue by 48 hours (Fig.6K). Expression is likewise observed in the heart between 24 and 60 hpf (Fig.5E,H,J,M) but declines thereafter; interestingly, sites of embryonic expression reported for a mouse Tfeb gene trap line included heart and kidney (McClive et al., 1998). Scattered tfeb expression along the dorsal midline is evident by 36hpf (Fig.6G,H) and can be seen in discrete cells, possibly iridophores (see below), at 60 hpf (Fig.6L). tfeb is expressed in the pectoral fin cartilage beginning around 40 hpf (Fig.6I). Small clusters of tfeb-expressing cells are apparent at the dorsal edge of the otic vesicle by 48 hpf (Fig.6J) and additional cells appear in the ear by 60 (Fig.6M,N) and 72 hpf (Fig.6O). Scattered tfeb-expressing cells are also observed deep in the brain along what may be commisural tracts (Fig.5K), and in the ventral hindbrain (Fig.5N,O); these cells may be macrophages or oligodendrocytes. Expression in the kidney continues to be robust at 60 hpf (L, inset; N), and by 72hpf the strongest expression of tfeb is observed in the pectoral fins and proximal convoluted tubules of the pronephros (Fig.5O,P,R). Expression can also be seen in the pharyngeal arches (Fig.6P), optic fissure (Fig.6Q) and jaw musculature (Fig.6R).
tfec
tfec is first seen in the posterior optic primordia and a few cranial neural crest cells at the 8-somite stage (Fig.7A). This expression expands and begins to include trunk neural crest between 10 and 12 somites (Fig.7B-D). Between 16 somites and 24 hours, tfec becomes expressed throughout the retinal pigment epithelium (RPE) and peak neural crest expression shifts from rostral to caudal domains (Fig.7E-G). Although Tfec expression in mammalian neural crest has not been reported, Tfec is expressed in mouse RPE (Rowan et al., 2004). Beginning at around the 20-somite stage expression can be seen in the intermediate cell mass; this expression can still be observed at 36 hpf (Fig.7L) but is mostly gone by 60 hpf (Fig.7M). Around 24 hpf, tfec-expressing cells begin to form clusters over the yolk on either side of the midline (Fig.7H-J); at the same time, the posterior dorsal expression domain begins to thin out and to shift anteriorly. This pattern is strongly suggestive of neural crest-derived iridophore pigment cells, which is further supported by continued expression in the bilateral patches over the yolk from 48 to 72 hpf (Fig.7O-Q, arrowheads). Scattered expressing cells can be seen on the dorsal surface of the head at 36 hpf (Fig.7K), another region from which iridophores will later arise. Expression of tfec in the eye persists through 72 hpf (Fig.7N-Q). At 48 hpf a cluster of tfec-expressing cells can be seen just right of the midline over the yolk (Fig.7O). Expression increases in this domain at 60 hpf (Fig.7P) and by 72 hpf these cells appear to be forming into the swim bladder (Fig.7Q).
Figure 7. Expression pattern of tfec during embryogenesis.
Whole mount RNA in situ hybridization was performed on embryos at the stages indicated. Expression begins in the posterior eye region/presumptive retinal pigment epithelium and a few neural crest cells at the 8-somite stage (A) and expands during somitogenesis (B-F). tfec-expressing cells populate bilateral patches over the yolk beginning at 24 hpf (arrows, H-J), increasing in number and extent through 72 hpf (arrowheads, O-Q), and are also found on top of the head (arrow, K) and dorso- and ventromedially in the trunk and tail (L,M), all positions characteristic of iridophores. Expression in the intermediate cell mass is seen by the 20 somite stage (arrowhead, F), has weakened by 36 hpf (arrowhead, L) and is gone by 60 hpf (M). Expression in the retinal pigment epithelium (arrow) and ciliary margin (arrowhead) is observed in transverse section of the eye at 60 hpf (N, dorsal is to the top). Beginning at 48 hpf, expression is observed in the developing swim bladder (arrows, O-Q). Lateral views A,B,D-G,L,M; frontal view, C; dorsolateral views, H-J; dorsal views K,O-Q. Abbreviations: nc, neural crest; rpe, (presumptive) retinal pigment epithelium.
Summary and Conclusions
We have shown here that the zebrafish MiT family consists of six genes compared to the four of mammals; duplicate mitf and tfe3 genes have been retained, but not tfeb or tfec. Surveys of avian genomes have suggested that they contain only two or three MiT genes (Liu et al., 2011). Single Mitf family members have been found in the demosponge Amphimedon queenslandica, the lancelet Branchiostoma floridae, and the tunicate Ciona intestinalis (Satou et al., 2003; Simionato et al., 2007). What function this single gene serves in any of these organisms is not known; what new functions the expanded gene family may have acquired in more complex metazoans, and specifically how the six MiT genes may contribute to phenotypic diversity in teleost fishes, remain only partially answered questions.
The genes of the zebrafish MiT family display embryonic expression patterns that are surprisingly tissue-restricted and complex given the broad expression reported for their mammalian orthologs (Kuiper et al., 2004; Steingrímsson et al., 2004). While expression of some of the zebrafish MiT genes is clearly conserved with other organisms (e.g. mitfa in melanogenic cells, (Lister et al., 1999); tfec in RPE and tfeb in heart and kidney, this study), other aspects of expression do not appear to be conserved: for example, with the possible exception of tfe3a (Lister et al., 2001), expression of MiT genes does not appear to persist in hematopoietic tissues, although it may be that MiT gene expression is restricted to definitive hematopoiesis (de Jong and Zon, 2005). Likewise, the first osteoclasts do not appear in zebrafish until approximately 12 days post-fertilization (Hammond and Schulte-Merker, 2009), much later than our analysis here.
In several tissues, the expression of two or more genes appears to overlap and therefore the potential for regulation by heterodimeric complexes exist (Amoutzias et al., 2008). These include the intermediate cell mass (tfe3a, tfe3b, tfeb, tfec); retinal pigment epithelium (mitfa, mitfb, tfec), lens (tfe3a, tfeb), and ciliary margin (mitfb, tfec, tfe3b, tfeb) of the eye; ear, pectoral fins and pharyngeal arches (tfe3b, tfeb); and a subset of neural crest cells (tfeb, tfec). Much more extensive analysis with fluorescent in situ hybridization will be required to verify co-localization within any of these tissues. Such analysis, along with the generation of mutations and gene knockdown reagents in each member of the family, and overexpression of wild-type and dominant-interfering MiT proteins, will comprise the next steps in unraveling the functions of this set of genes.
EXPERIMENTAL PROCEDURES
Animal use
All procedures were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Embryos were obtained from natural matings of adults from the AB strain, or AB/WIK hybrids and staged according to Kimmel et al., 1995. Embryos were incubated in the presence of 0.003% N-phenylthiourea (PTU; Sigma P7629) to prevent melanin synthesis.
Cloning and phylogenetic analysis
A fragment of tfe3b was originally identified by degenerate PCR using primers and protocol previously described (Lister et al., 1999). A partial tfeb cDNA was first identified as an EST (fc83b12) and was subsequently extended by 5′SMART-RACE (Clontech) with the gene-specific nested primers B5RACE: 5′-TGG AGG TGG TTC TCC TCA GGG CTG C-3′, and B5NRACE: 5′-GGA GTT GCT GGG GAG GCC GTG G-3′. A fragment of tfec exon 8 was identified by performing PCR on genomic DNA with the degenerate primers F4: 5′- CCA GGA GCT GGA GAT NCA RGC NMG-3′ and R3: 5′-GGG GGA CAC GGA GGA CAR NAR NGG RTC -3′, designed using CODEHOP (Rose et al., 1998), and was extended by 5′SMART-RACE with the gene-specific nested primers C5RACE: 5′-GAG GTG AGA TGA CAG TTC GAC TGT GCC CA-3′, and C5NRACE: 5′-ATG CAG TCA TAC TGG GCA AAC CAT GAG CA-3′. PCR products were subcloned and sequenced, and used in BLAST searches against genome sequencing data as it became available. A full-length tfe3b clone to be used as a riboprobe template (see below) was obtained by RT/PCR of 72 hpf first-strand cDNA with the following primers: forward, 5′-GAA TTC CAC CAT GTC TTC GAG AGT GTT GCT C-3′; reverse, 5′-ACT AGT CTA ACA AGC ATG CTG GTT CTC CTC C-3′, followed by TOPO TA cloning (Invitrogen). The tfec cDNA missing exon 7 (I.M.A.G.E. clone #7226661, obtained from Open Biosystems) was excised from pME18S-FL3 with EcoRI and Spe I and cloned into pBluescript SK- (Stratagene).
Nucleotide sequences have been submitted to GenBank with the following accession numbers: tfe3a isoform 2, JN105111; tfe3b isoform 1 JN105115; tfe3b isoform 2, JN105116; tfe3b isoform 3, JN105117; tfeb isoform 1, JN105112; tfeb isoform 2, JN105113; tfec isoform 2 JN105114; mitfb (corrected complete coding sequence), JN105118. The accession number for tfec isoform 1 is NM_001030105.
The zebrafish MiT family proteins were aligned using ClustalW in the VectorNTI AlignX package (Invitrogen). Zebrafish MiTs were also aligned with mouse, human and chicken MiT proteins using ClustalW. The accession number for the sequences used were: human MITF isoform 1, NP_937802; mouse Mitf isoform 2, NP_032627; chicken Mitf, BAA25648; human TFE3, NP_006512; mouse Tfe3, NP_766060; human TFEB, NP_001161299; mouse Tfeb isoform a, NP_035679; chicken Tfeb, NP_001026093; human TFEC, NP_036384; mouse Tfec, NP_112475; chicken Tfec, Q5XFQ6; zebrafish Mitfa, NP_570998; zebrafish Tfe3a, NP_571923; zebrafish Tfec, NP_001025276; yeast Rtg3p, NP_009447. The resulting msf file was used to build a phylogenetic tree with 1000 bootstraps using the protdist, neighbor, and consense programs of PHYLIP 3.69 (Felsenstein, 2005). The tree was drawn using FigTree 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree).
BLAST searches were made against other teleost genomes available through Ensembl (http://www.ensembl.org/index.html): Takifugu rubripes (assembly 4.0, June 2005), Tetraodon nigroviridis (assembly 7, April 2003), Oryzias latipes (MEDAKA1, October 2005), Gasterosteus aculeatus (BROAD S1, February 2006).
RT/PCR
For expression analysis by RT/PCR, RNA was prepared from 25 embryos at each timepoint using Trizol (Invitrogen) and reverse-transcribed with oligo-dT (Bioline) and AccuScript High Fidelity RT (Stratagene). Primers were designed with the assistance of Primer3, v.0.4.0 (http://frodo.wi.mit.edu/primer3/) and are listed in Table 2. Agarose gel images were captured using an AlphaImager (Alpha Innotech).
Table 2. Additional oligonucleotides used in this study.
| tfe3a 3′ forward | 5′-CGC ATT CAG GAA CTG GAA AT-3′ |
| tfe3a 3′ reverse | 5′-TTT TGA GGC TCC TGG AGA AA-3′ |
| tfe3b 3′ forward | 5′-AAA TAC AAG CAC GCC ACC AT-3′ |
| tfe3b 3′ reverse | 5′-GAG AAG AGC ACA TCC GAA CC-3′ |
| tfeb 3′ forward | 5′-TAA ACG CAT GCA GAA GGA TG-3′ |
| tfeb 3′ reverse | 5′-TCG CAC AAG TCC AGA GAC TG-3′ |
| tfec 3′ forward | 5′-GAG CAG CAA CAC GCT CGT G-3′ |
| tfec 3′ reverse | 5′-CCG CTG GTG CTC CTC CTT-3′ |
| tfe3a1a 5′ forward | 5′-AGC CCG CTT GCT GTG CTC-3′ |
| tfe3a4a 5′ forward | 5′-GAG GAT TCT GGT TTT ACT GC-3′ |
| tfe3a 5′ reverse | 5′-TGC AGC TTG CGG ATG TAG TCG-3′ |
| tfeb1 5′ forward | 5′-CAC GAG AAC GAG ATG GAT GA-3′ |
| tfeb4a 5′ forward | 5′-GGA GAG GTG GAG GGA GAA CG-3′ |
| tfeb 5′ reverse | 5′-CCA CAG AGG CCC TCA GAA TA-3′ |
| tfec1 5′ forward | 5′-CCG ACT GTA GTT TCT ACA AGA TGA GA-3′ |
| tfec1a 5′ forward | 5′-ATG AGC CGC TGC TGT GTG GT-3′ |
| tfec 5′ reverse | 5′-CTC ACT GTC TTG ATT GTT GGC-3′ |
In situ hybridization
Digoxigenin-labeled RNA probes were generated from the following plasmid templates: pCR4-tfe3b (nt 1-1356), linearized with Not I and transcribed with T3 RNA polymerase; pCR-tfeb 5′RACE (nt 63-1103), linearized with SpeI and transcribed with T7 RNA polymerase; pBS-tfec (nt 122-749, 898-1410), linearized with EcoRI and transcribed with T3 RNA polymerase. In situ hybridization was carried out as described previously (C. Thisse & B. Thisse, 2008). After clearing, samples were equilibrated in 50% glycerol/1x PBS for photography on a SZX12 dissecting stereomicroscope with DP70 camera (Olympus). For histology, already-stained specimens were equilibrated in 30% sucrose in 1x PBS, embedded in Tissue-Tek O.C.T. (Sakura Finetek, Torrance, CA) and cryosectioned at 18 μM. Sections were mounted in 1x PBS and photographed with a Spot RT CCD camera (Diagnostic Instruments) on a Nikon ECLIPSE E800M microscope. All images were processed using Photoshop CS (Adobe).
ACKNOWLEDGMENTS
We wish to thank Khaled Alsayegh, Jamie Lahvic, and Tahseen Rabbani for early work on this project, and Tom Carney for information regarding ionocytes. Wild-type AB and WIK strain zebrafish used in this study were obtained from the Zebrafish International Resource Center (ZIRC), which is supported by grant P40 RR012546 from the NIH-NCRR. Microscopy and histology were performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463-02).
REFERENCES
- Amoutzias GD, Robertson DL, Van de Peer Y, Oliver SG. Choose your partners: dimerization in eukaryotic transcription factors. Trends Biochem Sci. 2008;33:220–9. doi: 10.1016/j.tibs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Su LK, Kadesch T. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev. 1990;4:167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- Carr CS, Sharp PA. A helix-loop-helix protein related to the immunoglobulin E box-binding proteins. Mol Cell Biol. 1990;10:4384–8. doi: 10.1128/mcb.10.8.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, Fisher DE. MiT transcription factor associated malignancies in man. Cell Cycle. 2007;6:1724–9. doi: 10.4161/cc.6.14.4484. [DOI] [PubMed] [Google Scholar]
- Grove CA, De Masi F, Barrasa MI, Newburger DE, Alkema MJ, Bulyk ML, Walhout AJ. A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell. 2009;138:314–27. doi: 10.1016/j.cell.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–28. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hallsson JH, Haflidadóttir BS, Stivers C, Odenwald W, Arnheiter H, Pignoni F, Steingrímsson E. The basic helix-loop-helix leucine zipper transcription factor Mitf is conserved in Drosophila and functions in eye development. Genetics. 2004;167:233–41. doi: 10.1534/genetics.167.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CL, Schulte-Merker S. Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development. 2009;136:3991–4000. doi: 10.1242/dev.042150. [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Steingrímsson E, McGill G, Hansen MJ, Vaught J, Hodgkinson CA, Arnheiter H, Copeland NG, Jenkins NA, Fisher DE. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8:2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Huan C, Kelly ML, Steele R, Shapira I, Gottesman SRS, Roman CAJ. Transcription factors TFE3 and TFEB are critical for CD40 ligand expression and thymus-dependent humoral immunity. Nat Immunol. 2006;7:1082–91. doi: 10.1038/ni1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke M, Renisch B, Hammerschmidt M. Zebrafish grainyhead-like1 is a common marker of different non-keratinocyte epidermal cell lineages, which segregate from each other in a Foxi3-dependent manner. Int J Dev Biol. 2010;54:837–50. doi: 10.1387/ijdb.092877mj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kuiper RP, Schepens M, Thijssen J, Schoenmakers EFPM, van Kessel AG. Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res. 2004;32:2315–22. doi: 10.1093/nar/gkh571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–51. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- Lister JA, Close J, Raible DW. Duplicate mitf genes in zebrafish: complementary expression and conservation of melanogenic potential. Dev Biol. 2001;237:333–44. doi: 10.1006/dbio.2001.0379. [DOI] [PubMed] [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. Nacre Encodes a Zebrafish Microphthalmia-Related Protein That regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–67. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- Liu W-Y, Zhao C-J. Genome-wide identification and analysis of the chicken basic helix-loop-helix factors. Comp Funct Genomics. 2010;2010:682095. doi: 10.1155/2010/682095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhao C. Molecular phylogenetic analysis of zebra finch basic helix-loop-helix transcription factors. Biochem Genet. 2011;49:226–41. doi: 10.1007/s10528-010-9401-9. [DOI] [PubMed] [Google Scholar]
- McClive P, Pall G, Newton K, Lee M, Mullins J, Forrester L. Gene trap integrations expressed in the developing heart: insertion site affects splicing of the PT1-ATG vector. Dev Dyn. 1998;212:267–76. doi: 10.1002/(SICI)1097-0177(199806)212:2<267::AID-AJA11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Merrell K, Wells S, Henderson A, Gorman J, Alt F, Stall A, Calame K. The absence of the transcription activator TFE3 impairs activation of B cells in vivo. Mol Cell Biol. 1997;17:3335–44. doi: 10.1128/mcb.17.6.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Shimano H, Yoshikawa T, Ide T, Tamura M, Furusawa M, Yamamoto T, Inoue N, Matsuzaka T, Takahashi A, Hasty AH, Suzuki H, Sone H, Toyoshima H, Yahagi N, Yamada N. TFE3 transcriptionally activates hepatic IRS-2, participates in insulin signaling and ameliorates diabetes. Nat Med. 2006;12:107–13. doi: 10.1038/nm1334. [DOI] [PubMed] [Google Scholar]
- Nowick K, Stubbs L. Lineage-specific transcription factors and the evolution of gene regulatory networks. Brief Funct Genomics. 2010;9:65–78. doi: 10.1093/bfgp/elp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehli M, Den Elzen N, Cassady AI, Ostrowski MC, Hume DA. Cloning and characterization of the murine genes for bHLH-ZIP transcription factors TFEC and TFEB reveal a common gene organization for all MiT subfamily members. Genomics. 1999;56:111–20. doi: 10.1006/geno.1998.5588. [DOI] [PubMed] [Google Scholar]
- Rehli M, Sulzbacher S, Pape S, Ravasi T, Wells CA, Heinz S, Söllner L, El Chartouni C, Krause SW, Steingrímsson E, Hume DA, Andreesen R. Transcription factor Tfec contributes to the IL-4-inducible expression of a small group of genes in mouse macrophages including the granulocyte colony-stimulating. J Immunol. 2005;174:7111–22. doi: 10.4049/jimmunol.174.11.7111. [DOI] [PubMed] [Google Scholar]
- Roman C, Cohn L, Calame K. A dominant negative form of transcription activator mTFE3 created by differential splicing. Science. 1991;254:94–7. doi: 10.1126/science.1840705. [DOI] [PubMed] [Google Scholar]
- Rose TM, Schultz ER, Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res. 1998;26:1628–35. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Chen CMA, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–52. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Levine M, Kohara Y, Rokhsar D, Satoh N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. I. Genes for bHLH transcription factors. Dev Genes Evol. 2003;213:213–21. doi: 10.1007/s00427-003-0319-7. [DOI] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Arencibia MG, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011 May 26; doi: 10.1126/science.1204592. 2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato E, Ledent V, Richards G, Thomas-Chollier M, Kerner P, Coornaert D, Degnan BM, Vervoort M. Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evol Biol. 2007;7:33. doi: 10.1186/1471-2148-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson E, Tessarollo L, Pathak B, Hou L, Arnheiter H, Copeland NG, Jenkins NA. Mitf and Tfe3, two members of the Mitf-Tfe family of bHLH-Zip transcription factors, have important but functionally redundant roles in osteoclast development. Proc Natl Acad Sci USA. 2002;99:4477–82. doi: 10.1073/pnas.072071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson E, Tessarollo L, Reid SW, Jenkins NA, Copeland NG. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development. 1998;125:4607–16. doi: 10.1242/dev.125.23.4607. [DOI] [PubMed] [Google Scholar]
- Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–79. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE., Jr. Mechanisms of specificity in protein phosphorylation. Nat rev Mol Cell Biol. 2007;8:530–41. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- Varsamos S, Nebel C, Charmantier G. Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A Physiol. 2005;141:401–29. doi: 10.1016/j.cbpb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen K, Yao Q, Zheng X, Yang Z. Phylogenetic analysis of zebrafish basic helix-loop-helix transcription factors. J Mol Evol. 2009;68:629–40. doi: 10.1007/s00239-009-9232-7. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Hershey CL, Takemoto CM, Horstmann M, Hemesath TJ, Tashjian AH, Fisher DE. Age-resolving osteopetrosis: a rat model implicating microphthalmia and the related transcription factor TFE3. J Exp Med. 1998;187:775–85. doi: 10.1084/jem.187.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanocco-Marani T, Vignudelli T, Parenti S, Gemelli C, Condorelli F, Martello A, Selmi T, Grande A, Ferrari S. TFE3 transcription factor regulates the expression of MAFB during macrophage differentiation. Exp Cell Res. 2009;315:1798–808. doi: 10.1016/j.yexcr.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhao Q, Zhou X, Mattei MG, de Crombrugghe B. TFEC, a basic helix-loop-helix protein, forms heterodimers with TFE3 and inhibits TFE3-dependent transcription activation. Mol Cell Biol. 1993;13:4505–12. doi: 10.1128/mcb.13.8.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]