Abstract
The presence of immune memory at pathogen entry sites is a prerequisite for protection. Nevertheless, the mechanisms that warrant immunity at peripheral interfaces are not understood. Here we show that the non-classical MHC class I molecule, the thymus leukemia antigen (TL), induced on dendritic cells interacting with CD8αα on activated CD8αβ+T cells, mediated affinity-based selection of memory precursor cells. Furthermore, constitutive expression of TL on epithelial cells led to continued selection of mature CD8αβ memory T cells. The TL-CD8αα-driven memory process was essential for the generation of memory CD8αβ T cells in the intestine and accumulation of highly antigen sensitive CD8αβ memory T cells that form the first line of defense at the largest entry port for pathogens.
A hallmark of immune memory is that repeated infections are met with accelerated and enhanced protective immunity1. Furthermore, unlike naïve T lymphocytes, or central memory T cells (TCM), that reside in lymphoid tissues, some antigen-experienced T cells gain the capacity to persist long-term as effector memory T cells (TEM) in non-lymphoid tissues, such as the intestine2-5. TCM cells, which respond with a robust clonal expansion, are effective at protecting against infections by pathogens that replicate systemically6, but they likely are inadequate to prevent transmission of viruses, including the human immunodeficiency virus (HIV), or intracellular bacteria, which penetrate across mucosal epithelia3, 7. Effective resistance against transmission of such pathogens requires the presence of local antigen-specific TEM prior to re-challenge7. Therefore, strategies aimed at inducing a powerful protective immune response that also warrants the formation of pre-existing mucosal antigen-specific TEM are considered an essential goal of successful vaccinations.
Listeria monocytogenes (Lm), a Gram-positive intracellular pathogen of human and other mammals including mice, is a food-borne pathogen, which upon ingestion and uptake by phagocytic cells, such as monocytes and dendritic cells (DCs) disseminate from the intestine into the bloodstream and spread to various systemic tissues such as the liver8. In humans, ingested Lm may cause listeriosis because of its ability to also infect non-phagocytic cells such as the intestinal epithelial cells (IECs) through interaction of the molecule internalin expressed by the Lm and human E-cadherin expressed on the baso-latheral pole of the enterocytes9. Mice, by contrast, when infected orally do not develop listeriosis due to the inability of Lm internalin to interact with mouse E-cadherin. Instead, mice clear the infection of ingested bacteria with an effective CD8-dependent protective immune response, although bacteria that crossed the mucosal barrier can spread to the liver and other organs via the blood, as can occur in humans8. These observations have important implications for immunization strategies and indicate that the presence of local pre-existing mucosal immunity might be key for the induction of effective protective immunity against food-borne pathogens. Despite this however, most of the current knowledge of immune memory has been gained from model systems that use systemic immunization routes and memory generation in lymphoid tissues. Although such pre-existing immunity might be highly effective to combat pathogens that enter the body systemically, it is most likely inadequate to prevent entry and systemic spreading of pathogens, which invade via the mucosal route of the intestine. Mice can be infected by the oral route similarly to humans and because of the protective endogenous mucosal CD8-mediated immune response that is generated in mice in response to ingested bacteria, together with the emergence of genetically manipulated avirulent attenuated wild-type– and recombinant (ActA-) Lm strains as important vectors for vaccinations8, 10, 11, immunizations of mice with Lm introduced via the oral route represent an optimal approach to examine mechanisms and conditions that lead to the generation of effective pre-existing mucosal immune memory.
We showed previously that CD8 homodimers (CD8αα), induced on activated CD8αβ T cells, which maintain expression of CD8αβ mark those primary effector cells that preferentially differentiate to memory cells12. In mice, memory CD8αβ T cells that co-express CD8αα are greatly enriched in the epithelium of the intestine13. Thymus leukemia antigen (TL) is a high-affinity ligand for CD8αα14. TL is a non-classical, nonpolymorphic MHC class I molecule encoded on chromosome 17 in the H2-T region, a locus that has undergone genetic rearrangements to produce at least two functional alleles, H2-T3 in b-haplotype mouse strains, such as C57BL/6 and C3H/An, and H2-T18 found in d-haplotype mice such as BALB/c. Despite its name, TL is constitutively expressed on intestinal epithelial cells that are adjacent to the CD8αα+ T cells15,16. These findings suggest a role for epithelial TL in the accumulation of mucosal CD8αα+CD8αβ memory T cells, however the mechanisms that drive the CD8αα-dependent generation of mucosal immune memory remain unknown.
Using the oral Lm infection model to elicit a CD8-driven protective immune response initiated at the mucosal entry site, we define here an affinity-based selection mechanism controlled by TL expression, induced on antigen-presenting cells (APCs), that led to the survival and differentiation of high-affinity, CD8αα+CD8αβ memory precursor cells. Furthermore, constitutive expression of TL on the epithelium of the intestine continued to impose selection pressure, which contributes to the affinity maturation of the resident mucosal CD8αβ TEM.
Results
TL is not required for memory CD8αβ+T cells
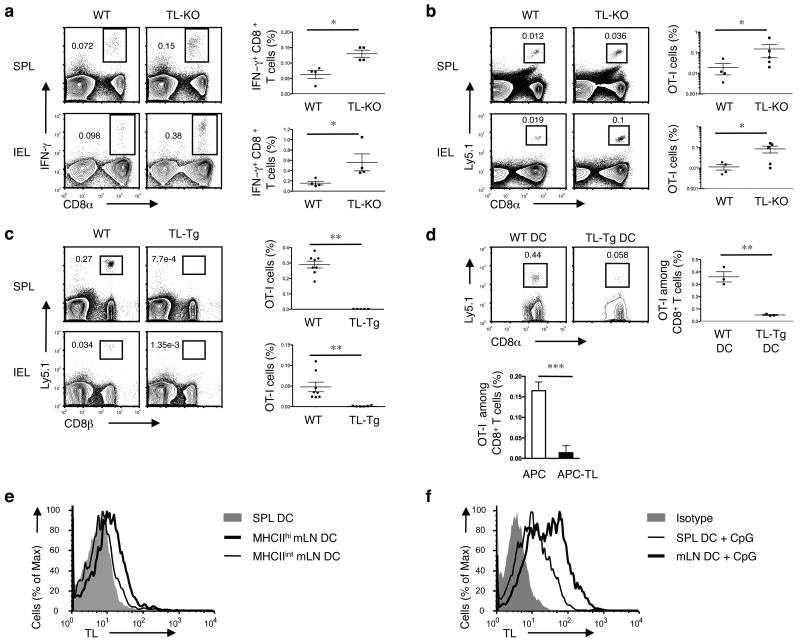
Considering the class I-like antigen presenting molecules encoded by the mouse genome, TL is distinguished because it has a particularly high affinity for CD8αα, due to unique amino acids substitutions at three positions in the membrane proximal α3 domain17. To assess if TL, the most likely physiologic ligand for CD8αα in vivo12, also plays a role in the generation of CD8αβ effector memory cells, we analyzed CD8αβ T cell memory differentiation in TL gene (H2-T3b) knock out mice18, referred here as TL- mice. The absence of TL did not impair, but rather enhanced, the generation of OVA-specific CD8αβ memory T cells in the spleen of TL- mice infected orally with Lm bacteria expressing OVA antigen (Lm-OVA) (Fig. 1a). A similar effect was observed in the epithelium of the intestine when intraepithelial lymphocytes (IELs) were analyzed (Fig. 1a). Likewise, naïve OVA peptide (OVAp) and H-2Kb specific monoclonal TCR transgenic OT-I T cells transferred to WT or TL- recipient mice that were subsequently orally infected with Lm-OVA also generated more OT-I TCR transgenic memory T cells in the absence of TL expression (Fig. 1b). These observations are consistent with previous published data using single chain MHC class I transgenic mice on a β2m-deficient background, which also indicated that in the absence of TL, normal or slightly enhanced memory formed in response to a viral infection19. All together, the data show that, whereas CD8αα promotes memory differentiation of CD8αβ effector T cells12, its high-affinity ligand TL, appears to inhibit this process.
Figure 1. TL negatively affects memory generation of CD8αβ+ T cells.
(a) WT or TL- mice were orally infected with 1 × 109 ActA- Lm-OVA. 30 days p.i., splenocytes (SPL) and IEL were isolated for IFN-γ intracellular staining and CD8α cell surface staining after ex vivo re-stimulation with OVA257-264 peptide. Graph depicts pooled data ± s.e.m.. (b) 5 × 104 naïve Ly5.1+ CD8+ OT-I cells were adoptively transferred into Ly5.2+ WT or TL- recipients. One day after transfer, mice were orally infected with 1 × 109 ActA- Lm-OVA. Donor OT-I cells were tracked in the spleen and IEL 2 months p.i. Graph depicts pooled data ± s.e.m.. (c) 1 × 106 naïve Ly5.1+ OT-I cells were transferred into WT or TL-Tg recipients. One day after transfer, mice were orally infected with 1 × 109 ActA- Lm-OVA. The donor OT-I cells in the spleens and IEL were tracked two months p.i. Graph depicts pooled data ± s.e.m.. (d) 5 × 104 Ly5.1+ naïve OT-I cells were transferred into C57BL/6 mice which were subsequently immunized i.v. with 5 × 105 OVAp-loaded DCs generated from bone marrow cells (BMDC) of WT or TL-Tg mice (top). Alternatively, OT-I cells were primed in vitro with TL negative APC or APC transfected with TL and then transferred to B6 recipients (bottom). Memory OT-I cells in the spleen were analyzed 2 months after DC immunization or after transfer of in vitro activated OT-I cells. Graph depicts pooled data ± s.e.m.. (e) Spleen or mLN DC were sorted based on the CD11c and MHC II expression and assessed for TL expression by flow cytometry. (f) Freshly isolated SPL or mLN DC were activated with CpG for 1 d and then analyzed for TL expression. * P < 0.05 and ** P < 0.001 (unpaired t-test). All data are representative of three independent experiments.
TL reduced the generation of memory CD8αβ+T cells
TL has a restricted pattern of expression20 that includes induction on APCs, such as DCs, in addition to expression on epithelial cells12. The increase in memory CD8αβ T cells seen in the absence of TL in TL- mice suggests that, under normal conditions, TL expression on subsets of priming DCs might negatively influence survival or differentiation of CD8αβ memory precursor cells. To test this possibility, we analyzed the effect of constitutive TL expression during priming using TL transgenic (TL-Tg) mice, which express an allelic form of TL (H2-T18d) under the control of the promoter from the MHC class I molecule, H-2Dd. In contrast to the outcome in TL-/hosts, transferred OT-I T cells which were primed in vivo in TL-Tg recipient mice that were orally infected with Lm-OVA failed to generate or sustain immune memory, either locally in the intestine or systemically, including in the spleen and liver (Fig. 1c and Supplementary Fig. 1). Moreover, OT-I T cells primed systemically in vivo using TL-Tg OVAp-loaded bone marrow (BM) DCs that were adoptively transferred failed to generate memory cells in the spleen of wild-type hosts (Fig. 1d). Similarly, OT-I cells initially primed in vitro by TL-Tg OVAp-expressing APCs, did not generate memory cells following adoptive transfer (Fig. 1d). These data indicate that TL expression on APCs interferes with the survival and memory programming of primary CD8αβ+ effector cells.
Under steady state conditions resting splenic DCs normally do not express detectable amounts of TL surface protein, although some induce it upon activation12. However, an analysis of different DC subsets indicated that in contrast to splenic DCs, a subset of mesenteric lymph node (mLN) DCs constitutively express low amounts of TL. This TL+ subset has the phenotype of mature migratory DCs (MHC class IIhi CD11c+ and CD103+CCR7+) (Supplementary Fig. 2a), which is also typical of those DCs that direct retinoic acid (RA)-based induction of gut homing receptors on the T cells they prime21, (Fig. 1e and Supplementary Fig. 2b). The expression of TL on these mucosal DCs was further upregulated during priming, and greatly enhanced in response to innate immune stimuli such as CpG oligodeoxynucleotides (Fig. 1f). These observations indicate that naïve T cells responding in vivo to gut-derived antigens are primed in the context of TL, expressed by the migratory DCs and greatly upregulated under inflammatory conditions.
TL induces Fas-mediated death of activated CD8αβT cells
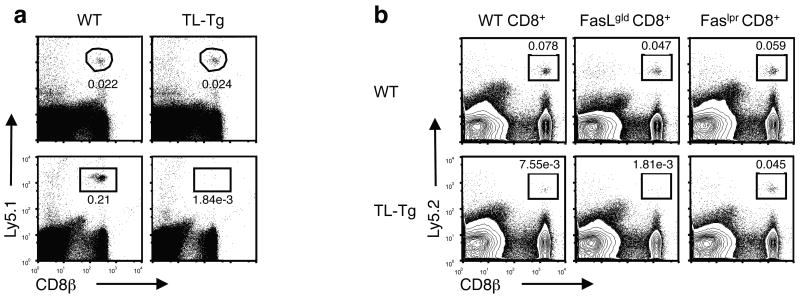
Although TL displays structural characteristics of MHC class I molecules, it does not function as a typical antigen-presenting molecule. The narrow distance between the α helices that form the boundaries of the antigen binding groove will not permit TL peptide binding and presentation22 and therefore TL fails to engage with the αβ TCR. Nevertheless the high degree of conserved sequence in the α3 domain allows TL to interact with the CD8αβ co-receptor, despite the exclusion of TL from the TCR activation complex14. A similar interaction of soluble HLA class I molecules with CD8αβ TCR co-receptor, separately from TCR ligation, was previously shown to lead to Fas-FasL-induced cell death23-26. To investigate if the TL interaction with CD8αβ on activated T cells might also lead to cell death, we measured the survival of naïve and antigen stimulated CD8αβ cells in the presence of constitutive TL expression in TL-Tg hosts. Whereas naïve donor cells survived similarly in wild-type or TL-Tg mice, activated CD8αβ+ OT-I cells survived only in wild-type but not in TL-Tg hosts (Fig. 2a), supporting the notion that TL-induced cell death (TICD) targets activated CD8αβ+ T cells. Activated Fas-deficient (Faslpr/lpr) CD8αβ+ donor T cells, however, were not deleted in TL-Tg recipient mice, providing evidence that, similar to the reported death by soluble HLA-G23, 24, TICD also involves the Fas–FasL-mediated death pathway (Fig. 2b).
Figure 2. TL mediates death of activated CD8αβ+ T cells.
(a) 1 × 106 naïve Ly5.1+ OT-I cells were transferred into WT or TL-Tg recipients and the donor cells in the spleens were tracked one month after transfer (top, n = 5 per group). 1 × 106 in vitro activated Ly5.1+ OT-I cells were transferred into WT or TL-Tg recipients and the donor cells in the spleen were tracked one month after transfer (bottom, n = 8 per group). (b) Naïve CD8 T cells sorted from WT, FasLgld or Faslpr mice were stimulated in vitro by anti-CD3/-CD28 beads for 3 d. 0.5 × 106 activated CD8 T cells were transferred into Ly5.1+ WT or TL-Tg recipients and the donor cells in the spleens were tracked 1 month after transfer. Representative data are shown of four mice analyzed in each group. Data are representative of two independent experiments (a, b).
Activation-induced CD8αα rescues CD8αβ effector T cells
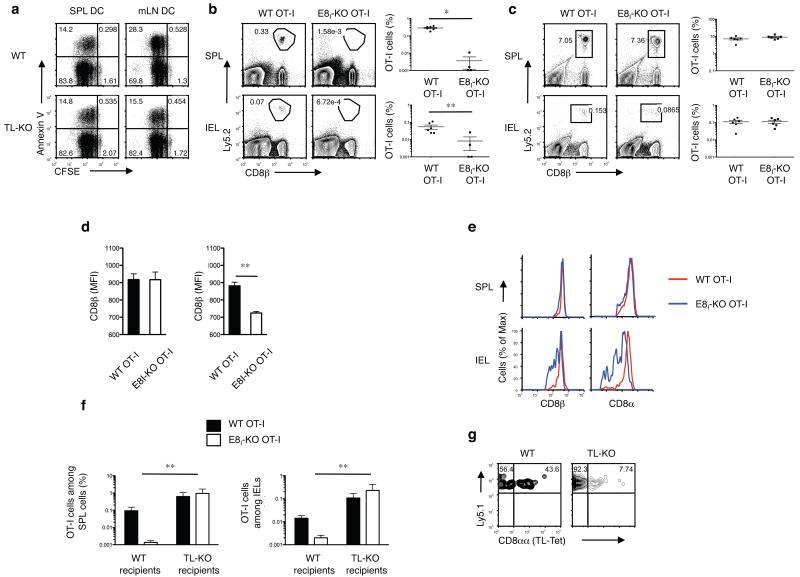
In contrast to CD8αβ, CD8αα does not function as a TCR co-receptor and similar to TL, CD8αα also does not engage in the TCR activation complex27, 28. However, whereas TL induces death of activated CD8αβ+ T cells, CD8αα, which exhibits a stronger affinity for TL compared to CD8αβ14, promotes the survival of CD8αβ effector cells12, suggesting that activation-induced CD8αα might interfere with TICD. To test this, we compared the survival and memory differentiation of CD8αβ effector T cells in the absence or presence of CD8αα. Due to a deletion of CD8α enhancer region I (Cd8a–E8I)29, OT-I CD8αβ donor cells on the E8I negative background (hereafter called ΔE8I OT-I CD8αβ+T cells do not induce detectable amounts of activation-dependent CD8αα 12. When analyzed in vitro, ΔE8I OT-I CD8αβ+T cells primed by OVA-loaded mLN DCs, which constitutively express TL (Fig. 1e), showed increased activation-induced death compared to their counterparts primed by splenic DCs (Fig. 3a). However, the increase in cell death induced by these mucosal APCs was not observed when mLN and splenic DCs isolated from TL- mice were compared (Fig. 3a).
Figure 3. Activation-induced CD8αα rescues CD8αβ primary effector T cells from TICD.
(a) 2 × 105 CFSE-labeled naïve ΔE8I OT-I cells were cultured with 4 × 104 OVAp-pulsed DCs from SPL or mLN of WT or TL- mice that were previously orally infected with ActA- Lm-OVA. After 2 d culture, OT-I cells were harvested and analyzed for cell death by Annexin V staining. (b) 5 × 104 naïve Ly5.2+ WT or Ly5.2+ ΔE8I OT-I cells were adoptively transferred into Ly5.1 recipient mice. 1 d after transfer, mice were orally infected with 1 × 109 ActA- Lm-OVA. Donor OT-I cells were tracked in the spleen and IEL, 2 months p.i.. Graph depicts pooled data ± s.e.m.. (c) 5 × 104 naïve Ly5.2+ WT or Ly5.2+ ΔE8I OT-I cells were adoptively transferred into Ly5.1 recipient mice. 1 d after transfer, mice were intravenously infected with 2.5 × 105 ActA- Lm-OVA. Donor OT-I cells were tracked in the spleen and IEL, 2 months p.i.. Graph depicts pooled data ± s.e.m.. (d) As described in (b) and (c), CD8β expression (MFI) was measured on effector WT OT-I or ΔE8I OT-I cells 7 days post oral infection (left) or i.v. infection (right). (e) As described in (c), CD8β expression was measured on memory WT OT-I or ΔE8I OT-I cells 2 m post i.v. infection in the spleen and IEL (f, g) 5 × 104 naïve Ly5.1+Ly5.2+ WT OT-I and 5 × 104 naïve Ly5.2+ ΔE8I OT-I cells were co-transferred into Ly5.1+ WT or Ly5.1+ TL- mice. One day after transfer, the mice were orally infected with 1 × 109 ActA- Lm-OVA. Two months p.i., memory OT-I cells were tracked in the spleen and IEL. (f) Graph depicts pooled data ± s.e.m. (n = 5 per group). (g) Staining for CD8αα expression, using TL-tetramers, on gated memory WT OT-I cells in IEL of WT or TL- recipient mice 2m p.i. * P < 0.001 and ** P < 0.01 (unpaired t-test). Data are representative of three (a, b, c, e, f, g) and two (d) independent experiments.
Furthermore, similar to our previous published results showing impaired splenic memory generation by ΔE8I CD8αβ+T cells in response to intraperitonelial infection with lymphocytic choriomeningitis virus (LCMV)12, ΔE8I OT-I CD8αβ+ donor cells primed in vivo with Lm-OVA via the oral route, also failed to generate detectable memory cells in the spleen or the intestine of wild-type recipient mice (Fig. 3b). In contrast, a previous study using the same systemic LCMV immunization approach, ΔE8I CD8αβ+T cells did generate systemic memory30. However in that case, a substantial downregulation of CD8αβ was noticed during the initial priming phase, which was not seen on either wild-type CD8αβ T cells or the LCMV-primed ΔE8I CD8αβ+ T cells in our published study12. We considered the possibility that a difference in the strength of the LCMV viral stock utilized in the earlier study30 might have caused downregulation of CD8αβ and similar effects on survival as mediated by activation-induced CD8αα on normal wild-type CD8αβ T cells. To test this, we examined whether, in our current model, the memory response of ΔE8I OT-I CD8αβ+T cells was altered when mice were primed systemically with Lm-OVA introduced intravenously (i.v.), which induces a much more potent response compared to the oral route8. In contrast to the oral immunization, systemic priming of the ΔE8I OT-I CD8αβ+T cells with Lm-OVA resulted in a clear memory response in the spleen and in the intestine comparable to that of wild-type OT-I cells (Fig. 3c). Furthermore, similar to the previous study30, CD8αβ was severely down-regulated during the potent priming of ΔE8I OT-I CD8αβ+T cells activated via the i.v. route, whereas, such downregulation was not observed when ΔE8I OT-I CD8αβ+T cells were primed via the oral route (Fig. 3d). Although the activation-induced downregulation of CD8αβ could explain the survival of the ΔE8I OT-I CD8αβ+T cells during the systemic priming, it does not explain the long-term survival of these cells in the intestine where TL is constitutively expressed on the IECs. To further investigate this, we analyzed the CD8αβ expression of the ΔE8I OT-I CD8αβ+memory T cell subsets in the spleen and the intestine. Interestingly, whereas ΔE8I OT-I CD8αβ+ memory cells in the periphery expressed normal amounts of CD8αβ, memory ΔE8I OT-I CD8αβ+IELs that persist in the presence of TL expressed much less CD8αβ (Fig. 3e). These results indicate that the mechanism of TICD continuously and selectively shapes the repertoire of the memory cells that accumulate at the mucosal site of the intestine. In support of this hypothesis, in the absence of TL expression in the intestine of TL- hosts, similar to the systemic priming, there was no difference in the efficiency of memory formation in the spleen or the intestine when wild-type OT-I or ΔE8I OT-I CD8αβ+T cells were primed via the oral route (Fig. 3f). Furthermore, there was also no selective accumulation of CD8αα expressing wild-type OT-I CD8αβ IELs in the TL- hosts, indicating that TL on the epithelial cells in the intestine imposes a selective pressure to promote the local accumulation of CD8αα-expressing effector memory T cells (Fig. 3g). These results indicate that the requirement for activation-induced CD8αα on primary effector cells is in part determined by the presence of TL. The data are consistent with a role for activation-induced CD8αα in sequestering TL ligand away from the CD8αβ co-receptor, thereby avoiding TICD of the CD8αβ+ primary effector T cells.
CD8αα marks the intensity of TCR activation
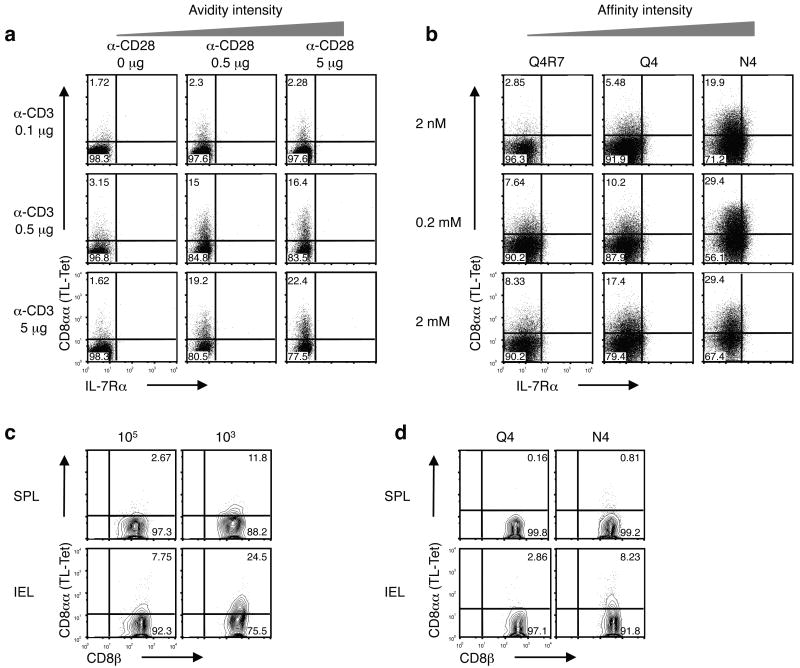
Due to the relatively high affinity interaction of CD8αα with TL, activation-induced expression of CD8αα can be distinguished from CD8αβ co-receptor expression using TL tetramers12. Activated CD8αβ T cells do not all induce CD8αα to the same extent and a variegated expression pattern of high (CD8ααhi) and low (CD8ααlo/-) expression is typically observed using TL tetramers. Together with the fact that the initial induction of CD8αα requires TCR stimulation, this finding suggests that there might be a close link between the intensity of TCR activation and the degree of CD8αα induction. In support of this notion, TL tetramer staining of polyclonal CD8αβ+ wild-type T lymphocytes activated in vitro with variable concentrations of soluble anti-CD3 and anti-CD28 showed graded increase in CD8αα expression with higher concentrations of CD3 plus CD28 mAbs (Fig. 4a). Furthermore, OT-I T cells stimulated with different altered peptide ligands (APLs) that bind equally well to the Kb class I molecule as the original OT-I ligand, SIINFEKL (N4), but which have different antigenic potencies31, also showed a tight association between the extent of CD8αα induction and the degree of TCR activation. Therefore the high affinity N4 ligand induced the highest amounts of CD8αα expression compared to the lower-affinity altered peptide ligands (Fig. 4b). Similar results of affinity-based induction of CD8αα were obtained in vivo when mice were analyzed that were infected orally with Lm-OVA after they received either a high- or a low-precursor frequency of naïve donor OT-I cells, which because of antigenic competition will model a low or high antigen dose, respectively (Fig. 4c). Additionally, we analyzed recipient mice adoptively transferred with equal amounts of OT-I precursor cells, but orally infected with bacteria expressing either the low-affinity Q4 (Lm-Q4)- or the high-affinity N4 (Lm-N4)-antigen. Mice receiving higher affinity N4 as compared to Q4 antigen generated more CD8αα-expressing CD8αβ T cells (Fig. 4d). In each of these approaches, the results consistently indicated that the extent of CD8αα induction represents a sensitive measurement for the intensity of the signal strength received through the activated TCR.
Figure 4. CD8αα expression correlates with the intensity of TCR activation.
(a) Total splenocytes were cultured in the presence of graded concentration of soluble anti-CD3 and anti-CD28. CD8αα expression, as measured by TL tetramer staining, was analyzed 3 d after in vitro culture. Representative data on gated CD8+ T cells are shown. IL-7Rα expression is also depicted. Three independent experiments were performed. (b) Naïve OT-I cells were cultured with artificial APC (MEC.B7) in the presence of graded concentration of OVA257-264 SIINFEKL (N4) or altered peptide ligands (Q4R7 and Q4). CD8αα expression was detected 2 d after in vitro culture. Three independent experiments were performed. (c) 1 x105 or 1 × 103 sorted naïve Ly5.1+ CD8+ OT-I cells were transferred into B6 recipient mice. 1 d after transfer, mice were orally infected with 1 × 109 ActA- Lm-OVA. 7 d p.i., CD8αα expression was analyzed on Ly5.1+ CD8+ OT-I cells from the spleen and IEL (representative data from a single mouse is shown, n = 4 mice per group). (d) 5 × 104 naïve Ly5.1+ CD8+ OT-I cells were transferred into WT recipient mice. 1 d after transfer, mice were orally infected with 2 × 108 WT Lm-Q4OVA or Lm-N4OVA. 7 d p.i., CD8αα expression was analyzed on donor OT-I cells from the spleen and IEL (representative data from a single mouse is shown, n = 5 mice per group). Data are representative of three (a, b) and two (c, d) independent experiments.
Similar to activation-induced CD8αα; IL-7 receptor (IL-7Rα) expression also has been proposed as a marker for memory precursor cells32. However, in contrast to CD8αα, IL-7R expression did not correlate with TCR activation, and instead, IL-7R was constitutively expressed on naïve cells and initially down regulated during activation at the time when CD8αα is first up regulated (Supplementary Fig. 3a). The reciprocal expression of CD8αα and IL-7R suggests different roles for these molecules in memory programming and/or survival. Consistent with this hypothesis, a mutation in the cytoplasmic IL-7Rα Y449XXM motif (IL-7Rα449F), known to impair the long-term survival of IL-7R-dependent CD8+ memory T cells33, did not interfere with the induction of CD8αα or the survival and generation of OT-I IL-7Rα449F memory T cells in response to an oral immunization with Lm-OVA (Supplementary Fig. 3b). These data indicate that the affinity-based selective programming of memory precursor cells does not depend on IL-7R signals.
Overall, the data indicate that in addition to being a memory precursor marker12, CD8αα expression also reports on the affinity or avidity of the antigen signal received by activated CD8αβ primary effector cells. The affinity-based induction of CD8αα together with the ability of CD8αα to sequester TL and avoid TICD therefore represents a mechanism to selectively preserve the most avid CD8αβ primary effector cells as part of the memory precursor pool.
CD8αα also marks human high affinity CD8αβ T cells
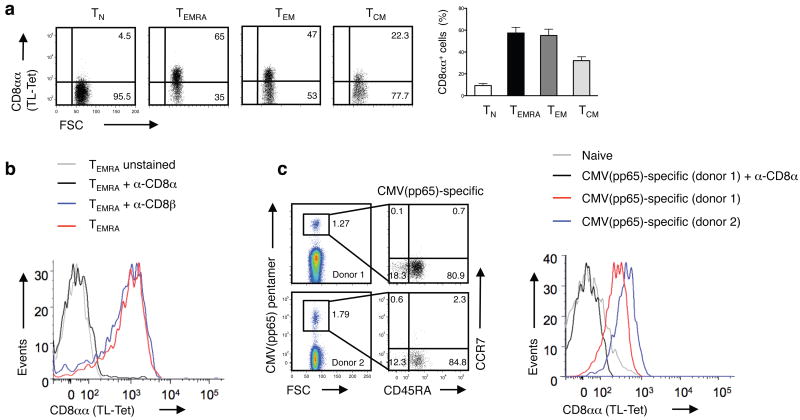
Previous evidence indicated that the mouse TL tetramers also detect expression of human CD8αα homodimers12,17. Consistent with activation-induced expression of CD8αα, human CD8αβ effector cells also stained with TL tetramers, whereas naïve T cells did not (Fig. 5a). TL tetramer staining could be blocked with an antibody specific for human CD8α, but not with anti-CD8β, confirming the specificity of the TL tetramer for human CD8αα (Fig. 5b and Supplementary Fig. 4). Furthermore, the subset of immunodominant, high-affinity, CMV-pp65-specific CD8αβ T cells34, isolated from cytomegalovirus (CMV)-sero-positive individuals, stained almost exclusively with TL tetramer, which could be blocked with anti-CD8α (Fig. 5c). These data indicate that CD8αα expression on human CD8αβ effector T cells also is associated with high-affinity effector cells.
Figure 5. CD8αα expression marks effector memory CD8αβ T cells in humans.
(a) Expression of CD8αα on polyclonal human naive (TN; CCR7+CD45RA+), recently activated effector-memory (TEMRA; CCR7–CD45RA+), effector-memory (TEM; CCR7–CD45RA–) and central-memory (TCM; CCR7+CD45RA–) CD8+ T cells was measured by TL-tetramer staining. The numbers indicate the percentage of TL-tetramerhi cells. Graph depicts pooled data ± s.e.m. on percentage of CD8αα expression on human peripheral blood CD8+ T cells (n = 9). The differences between TN and TEMRA, TEM or TCM were significant (P < 0.001, unpaired t-test). (b) TL-tetramer staining of human TEMRA CD8+ T cells is blocked by anti-CD8α but not anti-CD8β antibody. Data are representative of two independent experiments. (c) CMVpp65-specific CD8+ T cells display a TEM/TEMRA phenotype and persist at high frequency in humans. Data from two representative donors from a total of six persons are shown. The TL-tetramer staining was absent on naive CD8+ T cells and was blocked by an anti-CD8α.
RA and TGF-β promote affinity-based CD8αα expression
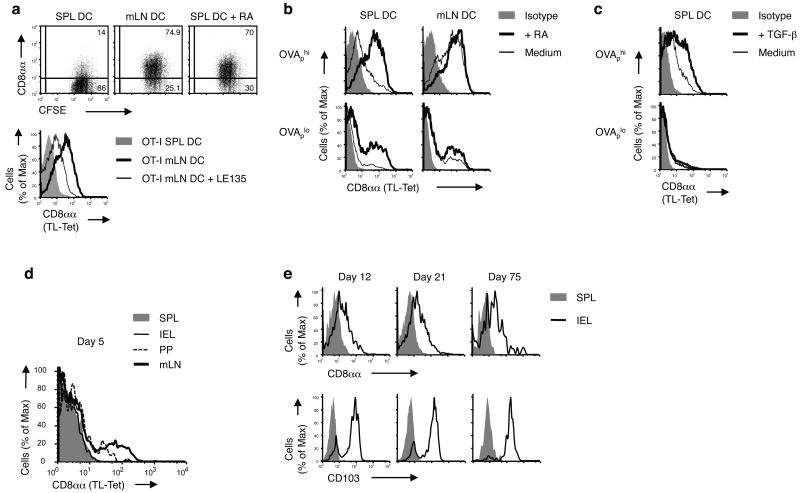
CD8αα-expressing CD8αβ T cells are highly enriched in the epithelium of the intestine, suggesting that a selective process based on CD8αα expression might drive this localized accumulation. Consistent with this notion, when primed in vitro CD8αα induction was stronger using mLN DCs compared to splenic DCs (Fig. 6a,b). In the presence of exogenous RA, normally released by mLN DCs during priming21, splenic DCs also mediated strong induction of CD8αα on the OT-I T cells they primed (Fig. 6a,b). Conversely, a retinoic acid receptor (RAR) inhibitor reduced CD8αα expression on T cells primed by mLN DCs (Fig. 6a). Likewise, TGF-β known to be an important modulator of mucosal T cell differentiation also increased CD8αα induction on activated CD8αβ primary effector cells (Fig. 6c). Both, RA- and TGF-β-mediated enhanced induction of CD8αα were observed, however, only under strong antigen activation conditions, suggesting that they influence CD8 effector differentiation by further promoting the selective marking of high-affinity effector cells by CD8αα (Fig. 6b, c).
Figure 6. Retinoic acid promotes the affinity-based accumulation of CD8αα+ CD8αβ T cells in the intestine.
(a) OT-I cells were stimulated by OVAp-loaded DCs from SPL or mLN of WT mice with or without 100 nM RA (dot plots) or with LE135 (histogram) in vitro for 3 d and CD8αα expression was analyzed. Data are representative of five independent experiments. (b,c) OT-I cells were stimulated by SPL or mLN DCs pulsed with OVAp (high, 1 nM; low, 0.01 nM) in the presence or absence of 100 nM RA (b) or 5 ng/ml TGF-β (c) in vitro for 3 d and CD8αα expression was analyzed. Data are representative of more than five independent experiments. (d,e) 0.5 × 106 CD8+ OT-I cells isolated from naïve Ly5.1+ OT-I+ Rag-/- mice were adoptively transferred into B6 recipient mice. 1 d after transfer, mice were orally infected by 0.5 × 109 ActA- Lm-OVA. CD8αα expression was measured on gated donor OT-I cells from the SPL, mLN, PP and IEL, 5 d p.i. (d). CD8αα and CD103 expression is shown on gated donor OT-I cells from the spleen and IEL on days 12, 21 and 75 p.i. (e). Representative data from two to three mice per group are shown. At least three independent experiments were performed.
The enhanced expression of TL on the mucosal migratory DCs known to release RA and TGF-β (Fig. 1e, f), together with the increased CD8αα induction on high-affinity effectors in the presence of RA as well as TGF-β, indicate that the selective rescue of high-affinity memory precursor cells is geared toward effector cells that home to the gut. In agreement with this notion, CD8αα-expressing OT-I cells were first detectable at mucosal induction sites, such as mLNs and Peyer's patches, early after oral antigen exposure (Fig. 6d) and gradually accumulated during the contraction and memory phase within the pool of αEβ7 integrin (CD103+) TEM in the gut epithelium, but not in the CD103- memory cells that persist systemically (Fig. 6e). Thus, indicating that the mucosal memory cells are selected for based on the quality of their TCR whereas peripheral memory cells are not.
TL on the IECs continues to select mature CD8αβ TEM
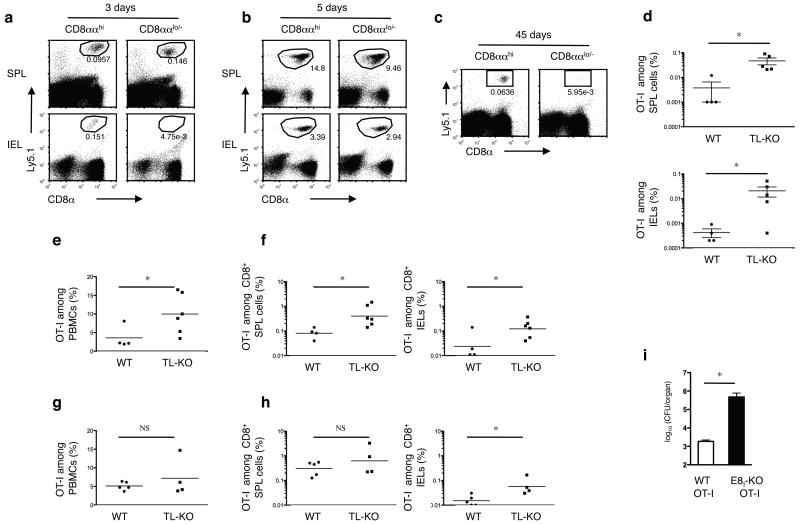
The constitutive TL expression on intestinal epithelial cells15,16, suggests that TL might continuously shape the resident mucosal memory CD8αβ+ T cell population even after rechallenge. To investigate this possibility, we examined the fate of primary and secondary CD8ααhi- or CD8ααlo/--CD8αβ effector T cells in vivo. OT-I cells initially primed in vitro in the absence of TL, using TL negative APCs, were sorted into CD8ααhi- and CD8ααlo/--primary effector T cells and adoptively transferred to wild-type recipient mice. Both subsets of primary effector cells displayed a similar short-term homing capacity (Supplementary Fig. 5a), and both effector T cell types responded alike when tested in vitro for interferon-γ production (Supplementary Fig. 5b) or in vivo for cytotoxicity (Supplementary Fig. 5c). However, in response to an oral re-challenge with Lm-OVA, primary memory OT-I T cells derived from the CD8ααhi precursors expanded in the spleen and in the intestine of the wild-type recipient mice, whereas memory cells from the CD8ααlo/- effector pool were only detectable in the host spleen, but not in the intestine (Fig. 7a). These data indicate that only CD8ααhi primary memory cells can persist long-term as mucosal TEM in proximity of TL constitutively expressed on the epithelial cells. Upon re-challenge, activated memory cells in lymphoid tissues expand and migrate as secondary effector cells to non-lymphoid tissues, such as the epithelium of the gut35. In agreement with this, comparable numbers of effector cells derived from either CD8ααhi or CD8ααlo/- primary effector OT-I cells were present in the intestine 5 days after recall (Fig. 7b). Nevertheless, when analyzed 45 days later, progeny of CD8ααhi effector cells were present as secondary memory cells in both the spleen and the intestine, whereas the CD8ααlo/- secondary effector cells did not remain as secondary TEM in the intestine (Fig. 7c). In contrast, in vitro primed CD8ααlo/-CD8αβ+ OT-I effector cells accumulated efficiently as TEM within the gut epithelium of TL- recipient mice (Fig. 7d), indicating that the constitutive expression of TL continues to mediate selective pressure that prevents the accumulation of CD8ααlo/- primary and secondary effector cells as mucosal TEM. These results also specify that CD8ααlo/- effector cells are not intrinsically incapable of converting to mucosal TEM, but that under normal physiological conditions, only CD8ααhi primary effector cells form long-lived mucosal TEM in proximity to TL constitutively expressed on the epithelial cells.
Figure 7. Constitutive expression of TL on intestinal epithelial cells mediates selection of mature memory CD8αβ T cells.
(a,b) Naïve Ly5.1+ CD8+OT-I cells were cultured in the presence of APC (MEC.B7.SigOVA). After 2 days' culture, CD8ααhi and CD8ααlo/- OT-I cells were sorted and cultured for 3 more days in vitro. Then 0.5 × 106 CD8ααhi or CD8ααlo/- cells were adoptively transferred into B6 recipients. One month after transfer, mice were orally infected with 5 × 108 ActA- Lm-OVA. Donor Ly5.1+ OT-I cells were tracked in the spleen and IEL 3 d (a) and 5 d (b) p.i.. Representative data from 3-4 mice in each group are shown. At least five independent experiments were performed. (c) As shown in (a), secondary OT-I memory cells were assessed in the IEL 45 d p.i.. Representative data from three to four mice in each group are shown. At least three independent experiments were performed. (d) Sorted in vitro activated Ly5.1+ CD8ααlo/- OT-I cells were cultured for 3 d and 0.5 × 106 primary effector cells were transferred into WT or TL-recipients. One month after transfer, mice were orally infected with 5 × 108 ActA- Lm-OVA. 4 months p.i., memory OT-I cells were tracked in the spleens and IEL. Pooled data ± s.e.m. are shown. At least two independent experiments were performed. (e, f) 5 × 104 naïve CD8+ OT-I cells were transferred into Ly5.1+ WT or Ly5.1+ TL- recipient mice. 1 d after transfer, mice were orally infected with Lm-Q4OVA. Effector OT-I cells in the peripheral blood (7 d p.i.) and memory OT-I cells (2 m p.i.) in the spleen and IEL were analyzed. Pooled data ± s.e.m. are shown. (g, h) 5 × 104 naïve CD8+ OT-I cells were transferred into Ly5.1+ WT or Ly5.1+ TL- recipient mice. 1 d after transfer, mice were intravenously infected with Lm-Q4OVA. Effector OT-I cells in the peripheral blood (7 d p.i.) and memory OT-I cells (2 m p.i.) in the spleen and IEL were analyzed. Pooled data ± s.e.m. are shown. Data are representative of three independent experiments(e, f, g, h). (i) Ly5.1 mice adoptively transferred with 5 × 104 naïve WT or ΔE8I OT-I cells were orally immunized with 1 × 109 ActA- Lm-OVA. Two months after immunization, mice were re-challenged orally with 1 × 1010 WT Lm-OVA. Bacterial loads in the livers were assessed day 3 p.i.. Pooled data ± s.e.m. are shown (n = 6). Representative data are shown of three independent experiments. * P < 0.05; NS: not significant (unpaired t-test).
TL mediates affinity maturation of memory CD8αβ T cells
A previous study, using low-affinity APLs for in vivo priming, suggested that affinity maturation of memory T cells was the result of enhanced expansion and delayed contraction of the high-affinity responding effector cells31. Our results here suggest that, in addition, the TL-mediated selective survival of CD8αα-expressing high-affinity memory cells might also contribute to ensure affinity maturation of memory populations at the mucosal border. To provide direct evidence for this hypothesis, we used the same APL approach31 to examine memory generation in vivo in the presence or absence of TL. Naïve OT-I cells were adoptively transferred to either wild-type or TL- recipient mice that were subsequently orally infected with bacteria expressing the low affinity APL, Q4 (Lm-Q4), which does not effectively induce CD8αα on primed OT-I effector cells (Fig. 4d). Fewer OT-I effector cells were detectable in the blood of wild-type mice compared to TL- animals when analyzed 7 days after oral immunization with Lm-Q4 (Fig. 7e), suggesting that TL induced on the priming mucosal APCs controls in part the expansion of the primary effector pool. In the intestine, where TL is constitutively expressed by the IECs, the selective impact of TL was even more pronounced. OT-I memory IELs generated in response to the low affinity peptide Q4 were barely detectable in wild-type animals whereas they were readily present in the TL- mice (Fig. 7f). These results indicate that the continuous expression of TL on the epithelium of the intestine selectively eliminates low-affinity cells from the pool of mature mucosal TEM. Together with the increase in mucosal memory in the TL- mice, also the systemic memory pool was increased (Fig. 7f), suggesting that in addition to the TL-mediated affinity selection of mature memory cells in the intestine, TL induced on mucosal APCs during priming might provide a first affinity- selection step by eliminating low-affinity or -avidity primary effector cells form the memory precursor pool. To further test this idea, we immunized wild-type or TL-/recipient mice with Lm-Q4 via the i.v. route. Under these conditions, TL expression during priming had only a minimal effect on the effector- or memory phase of peripheral T cells, which was comparable between the TL- and wild-type recipient mice (Figs. 7g and 7h). In sharp contrast however, the accumulation of TEM at the mucosal site, where TL is constitutively expressed, remained under the TL selective pressure (Fig. 7h). These results indicated that T cells primed at the mucosal priming site undergo an initial selection step mediated by TL induced on the migratory DCs, whereas the constitutive expression of TL on the mucosal epithelium provides for additional and constant selection pressure that drives continuous affinity maturation of the mature memory pool that accumulates long-term at the mucosal interface of the intestine. (Supplementary Fig. 6)
To evaluate the importance of pre-existing TL-CD8αα-selected mucosal memory T cells residing at this entry port, we examined the resistance against an oral infection with Lm in mice with or without affinity-selected memory cells. To this end, mice transferred with either wild-type or ΔE8I OT-I cells were orally immunized with an attenuated strain of Lm-OVA (ActA- Lm-OVA) (109 cfu). The mice were subsequently re-challenged orally with a higher dose (1010 cfu) of wild-type Lm-OVA and analyzed for systemic spreading of the pathogen. In contrast to the efficient resistance observed in mice which received the wild-type OT-I precursor cells, which generated memory cells in the intestine and elsewhere, pre-immunized mice that initially received the ΔE8I OT-I precursor cells failed to generate affinity selected memory and they showed significantly less resistance to prevent systemic spreading of the orally introduced pathogen (Fig. 7i). These results underscore the importance of the selective immune memory differentiation process as a critical mechanism for the efficient generation of pre-existing immunity at critical mucosal interfaces that form the main entry sites for invading pathogens.
Discussion
The data presented here define a fundamentally new concept for our understanding of immune memory differentiation and in particular mucosal immunity. The data demonstrate that an affinity-based selective process operates in vivo that preserves the optimal effector cells to become long-lived memory T cells that form pre-existing and heightened protective immunity. This mechanism is especially geared to generate high-affinity TEM that line the mucosal barrier of the intestine, where most pathogens enter the body.
The results also demonstrate a functional role TL, a non-classical MHC class I-like molecule, in mediating TICD of CD8αβ effector T cells that fail to induce CD8αα. Because TL is a much stronger ligand for CD8αα as compared to CD8αβ, CD8αα likely can sequester TL away from CD8αβ and prevent TICD. The induction of CD8αα is directly linked to the degree of TCR signal strength, which leads to the selective survival of the most avid effector cells to become memory T cells. The constitutive expression of TL on the epithelial cells in the intestine continues selective pressure which mediates affinity maturation of the mucosal TEM population in response to repeated re-challenges.
The data here, using the Lm model are consistent with our previously published study using the LCMV model system, which also indicated a critical role for activation-induced CD8αα in the generation of CD8αβ memory T cells, Nevertheless, two subsequent studies challenged this conclusion and provided evidence that under certain conditions CD8αβ+ ΔE8I memory T cells could also be generated in a CD8αα-independent fashion30, 36. In those two studies, however, and similar to what we observed here using Lm immunizations via the i.v. route, there was a pronounced down-regulation of CD8αβ expression during the priming of the ΔE8I CD8αβ T cells, which was not observed in our previous study using the LCMV system or during oral immunizations using the Lm system as shown here. The fact that in the absence of CD8αβ downregulation in both of those cases, ΔE8I CD8αβ T cells failed to accumulate as memory cells, indicates that strong activation signals which result in down-regulation of CD8αβ co-receptor on the ΔE8I mutant cells may rescue CD8αβ memory precursor cells similarly to the biological activity of activation-induced CD8αα on normal wild-type CD8αβ effector cells.
Although, a sequence-based homologue for TL in humans has not been identified, TL and the non-classical MHC class I molecule, HLA-G, display striking similarities, including their restricted pattern of expression, the limited antigen presentation capacity, the relative strong affinity for CD8αα compared to CD8αβ and the ability to induce Fas-FasL cell death of activated CD8αβ T cells25, 37, 38. These parallels suggest that, similar to other non-classical MHC or HLA pairs, TL and HLA-G might represent functional homologues of each other, and they further suggest that an affinity-based selective memory differentiation program likely operates in humans as well. Consistent with this, we showed that activated but not naïve human CD8αβ T cells co-express CD8αα and CD8αα expression also coincided with the high-affinity CMVpp65-specific CD8αβ effector T cells. Another study has indicated that the endogenous protective immune response against HIV was characterized by a CD8αα-expressing CD8αβ subpopulation that exhibited strong antiviral activity39 and high-avidity HIV-specific CD8+ T cell clonotypes were largely preserved in patients who control viremia, but not in progressive chronic HIV infections. All together, these observations suggest that CD8αα induction on human CD8αβ T cells is also an activation-induced and affinity-based process that marks the avid effector cells.
Most infections, including Lm but also many viral infections such as HIV and SIV infections, are acquired across mucosal barriers, and several studies have demonstrated that CD8+ CTL responses play a crucial role in the initial containment and early control of pathogen replication40-44. Although such responses may be unable to provide sterilizing protection, they can control the pathogen load and delay or even prevent spreading and onset of disease, as well as reduce the potential for secondary transmission. Therefore, the finding that an endogenous TCR quality-based mechanism may select for the most avid effector cells to form immune memory cells that have the capacity to reside long-term at mucosal interfaces, has significant implications for the design of new and improved strategies to induce effective pre-existing protective immunity, not only systemically but also locally at the major and most vulnerable entry sites for pathogens.
Methods
Mice
C57BL/6 CD45.2 (Ly5.2), referred to as WT, and CD45.1 (B6.SJL, Ly5.1) congenic, B6.MRL-Faslpr/J and B6.Smn.C3-Faslgld/J mice were purchased from The Jackson Laboratory. OT-I TCR-transgenic mice were crossed onto the Ly5.1 congenic background. Additionally, ΔE8I IL-7Rα449F, and TL-Tg mice were crossed to OT-I mice. TL-Tg mice were generated by forced expression of the T18d gene under the Dd promoter and backcrossed to the C57BL/6 background for more than 12 generations. TL- mice were generated by deletion of the T3b gene in the C57BL/6 background. Mice were maintained by breeding under specific pathogen-free conditions in the animal facility of the La Jolla Institute for Allergy & Immunology. Unless otherwise noted, mice were maintained under specific pathogen-free conditions. Sentinel mice from the Rag1-/- colony were tested to be negative for Helicobacter spp. and Citrobacter rodentium. Animal care and experimentation were consistent with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the La Jolla Institute for Allergy and Immunology.
T cell isolation, cell sorting, CFSE labeling and adoptive transfer
CD8+ OT-I cells were purified by magnetic negative selection using the MACS CD8α+ T cell isolation kit according to the manufacturer's protocol (Miltenyi Biotec). CD44lo cells were sorted as naïve CD8 OT-I cells by FACSAria (Becton Dickinson). In some cases, sorted cells were CFSE-labeled. OT-I cells were resuspended at a concentration of 10 × 106 cells/ml in PBS and CFSE was added to a final concentration of 5 μM (Invitrogen). After 10 min of incubation at 37°C, labeling was quenched with ice-cold DMEM medium with 10% FCS. Cells were washed with PBS three times and intravenously injected into the recipient mice.
DC isolation and activation
Pooled spleens or MLNs were cut into small pieces and digested with collagenase D (Roche) at 23 °C for 30 min and EDTA (5 mM in final concentration) was added for the last 5 min. Digested tissues were further homogenized and then enriched for CD11c+ DCs by positive selection using a MACS cell separation (Miltenyi Biotec). For DC activation in vitro, freshly isolated spleen DCs were cultured with 1 mM CpG DNA (1826, IDT) for 1 day and analyzed for TL expression by using anti-TL (HD168).
In vitro T cell stimulation culture
For total splenocyte culture, 5 × 105 total splenocytes were cultured in 96-well plates in the presence of anti-CD3 and anti-CD28. For artificial APC–OT-I co-cultures, either the transfected adherent fibroblast cell line (MEC.B7) expressing the costimulatory molecule B7.1, or the OVA-expressing cell line (APC, MEC.B7.SigOVA) having the OVA-derived, H-2Kb-restricted peptide epitope OVA257-264 (SIINFEKL), along with B7.1, were used. Irradiated APCs were cultured at 1.5 × 105 cells per well in 24-well plates overnight to establish a single layer. Naïve OT-I cells (5 × 105) were added to the monolayer of APCs in 2 ml of medium. The TL expressing APCs (APC-TL) were generated by transfection of MEC.B7.SigOVA cells with a plasmid expressing T18d gene. TL expression was confirmed by staining with a TL-specific antibody (HD168). For DC/OT-I culture, purified DC were incubated with 1 nM SIINFEKL for 2 h and then washed. 2 × 105 CFSE-labeled naïve OT-I cells were cultured with 4 × 104 peptide-pulsed DCs, with or without 100 nM all-trans RA (Sigma), in 96-well plates for 3-4 d. RA receptor antagonist LE135 (TOCRIS bioscience) was used for some cultures.
Bone marrow-derived DC immunization
Bone marrow was flushed from the tibia and femurs of 8-10-week-old mice and red blood cells were lysed. Bone marrow cells were plated at 5 × 105 cells/ml in complete IMDM with 20 ng/ml rmGM-CSF (Kyowa-Hakko Kirin). Fresh media containing rmGM-CSF were added on day 3 and half of the media was gently replaced on day 6. 100 ng/ml LPS (Sigma) was added on day 6 for 1 d to induce DC maturation and then 1 mM SIINFEKL was added to the culture 2 h before collecting cells. After extensive washing, 5 × 105 DC were injected i.v. in mice, which had previously received naïve OT-I cells 1 d earlier.
qRT-PCR
RNA from the FACS-sorted DCs were extracted with TRIZol (Invitrogen) and complementary DNA (cDNA) were synthesized using the iScript cDNA Synthesis kit (Bio-Rad). Real time RT-PCR was performed with the Roche 480 real-time PCR System. Values were normalized by the amount of L32 in each sample. The primers for qPCR are as following: TL-forward 5′-TGTATGGCTGTGAGGTGGAG-3′, TL-reverse 5′-GCTCCCACTTGCTTCTGG T-3′; L32-forward 5′-GAAACTGGCGGAAACCCA-3′, L32-reverse 5′-GGATCTGGCCCTTGAACCTT-3′.
Bacterial infection and bacterial counts in organs
ActA- Lm-OVA for immunization were prepared from cultures in brain heart infusion broth. Lm-N4OVA and Lm-Q4OVA strains stably express chicken ovalbumin (AA134–387) containing either the native ligand SIINFEKL257–264 (N4) or the altered peptide ligand SIIQFEKL (Q4). Bacteria were washed and resuspended in Hanks' balanced salt solution (HBSS) prior to oral infection by gavage. Bacterial injection stocks were plated to confirm the CFU. To count the bacteria in organs, livers were sterilely dissected, homogenized and lysed with 0.1% Triton X-100. Serial dilutions were plated onto BHI plates and bacterial colonies were counted 24 h after incubation at 37 °C.
IEL preparation
In brief, small intestines were removed and separated from Peyer's patches. They were cut longitudinally and then into 0.5 cm pieces. The pieces were shaken for 40 min in Mg2+-free, Ca2+-free HBSS supplemented with 1 mM dithiothreitol and 5% FCS. Cells were collected from the washes and passed over a discontinuous 40/70% Percoll (Pharmacia Biotech) gradient at 900 g for 20 min. IEL were then isolated from the Percoll-gradient interface and washed free of Percoll.
Immunofluorescence staining and flow cytometry
For mouse samples, a standard surface staining protocol included pre-incubated with anti-CD16/CD32 Fc-receptor antibody (2.4G2) to block Fc-antibody binding. Then, cells were stained in cold PBS containing 0.5% FBS and 0.05% sodium azide with the relevant labeled antibodies and tetramers. The following antibodies were used: CD8α (clone 53.6.7), CD8β (clone 53-5.8), CD44 (clone IM7), CD45.1 (clone A20), CD45.2 (clone 104), CD103 (clone M290), CD127 (IL-7Rα, clone A7R34), and IFN-γ (clone XMG1. 2). All primary antibodies were directly conjugated to fluorophores (BD Biosciences or eBioscience). CD8αα was detected with PE-labeled TL-tetramers. OVA-specific CD8+ T cells were detected with PE-labeled Kb–OVA257-264 tetramers. For intracellular staining of IFN-γ, splenocytes or IEL were stimulated with OVA257-264 (5 μg/ml) for 5 h in the presence of brefeldin A at 37 °C. After surface staining, intracellular cytokine staining for IFN-γ was performed using a Cytofix/Cytoperm Kit (BD Biosciences), according to the manufacturer's directions. For detection of cell apoptosis and death, cells were stained with Annexin V-APC (BD Biosciences) using the manufacturer's protocol and cells were analyzed immediately after staining. For human samples, peripheral blood samples were obtained from healthy volunteers (26-78 years old). Informed written consent was obtained from all participants and the blood collection was approved by the ethics committee of the Innsbruck Medical University. Isolation of peripheral blood mononuclear cells (PBMC) was performed by density gradient centrifugation using Ficoll-Hypaque (Amersham Biosciences). Immunofluorescence surface staining of PBMCs was performed using the following conjugated antibodies: TCRαβ (FITC, clone T10B9.1A-31), CD3 (PerCP or APC-Cy7, clone SK7), CD4 (PerCP, clone SK3), CD8α (PerCP, clone SK1), CD8β (PE or APC, clone 2ST8.5H7), CD16 (FITC, clone B73.1), CD28 (APC or PE-Cy7, clone CD28.2), CD45RA (FITC, clone HI100) and CD45RO (FITC, clone UCHL1) (BD Biosciences), CMVpp65495-503 pentamer (PE or APC; ProImmune) and TL tetramer (PE). PBMC were pre-incubated with unlabeled anti-CD8α (clone SK1; 2.5 μg/mL; BD Biosciences) or anti-CD8β (polyclonal; 100 μg/ml; Abcam or clone 2ST8.5H7; 25 μg/mL; BD Biosciences) for 15 min at 23°C. After a washing step with PBS, TL tetramer (1:100) was incubated for 10 min at 23°C. Thereafter, PBMC were co-stained with the relevant antibodies for 30 min at 4-8°C. Plasma CMV IgG titers were analyzed by ELISA using Enzygnost® (Dade Behring). All the stained cells were analyzed on a FACSCalibur or FACSCanto II® flow cytometer (Becton Dickinson) and FlowJo software (Three Star) or FACSDiva® Software (BD Biosciences).
In vivo cytotoxicity assays
In vivo cytolytic activity was determined using B6 splenocytes differentially labeled with CFSE. The cells highly labeled (CFSEhi) were used as target cells and pulsed with OVA257-264 (0.5 μg/ml; 90 min at 37°C, 5% CO2), whereas CFSElo labeled cells were pulsed with a negative control peptide, TRP-2180-188 (0.5 μg/ml). Peptide-pulsed target cells were extensively washed to remove free peptide and then co-injected intravenously in a 1:1 ratio to recipient mice. Sixteen hours later, spleens were removed and the ratio of CFSElo/CFSEhi cells was determined by flow cytometry.
Supplementary Material
Acknowledgments
We wish to thank M. Bevan for providing the Lm-OVA (N4 and Q4) reagents, M. Cheroutre for her contribution and L. Qiao, X.Z. Wang, and the members of the Cheroutre and Kronenberg laboratories for helpful discussions and technical assistance. D. Littman (New York University School of Medicine, New York, NY) for providing the ΔE8I mice. This work was supported by NIH RO1 grants: AI064584 and AI050265 (H.C.), AG10152 (M.K., H.C.) and by pilot projects from the Vanderbilt University Digestive Disease Research Center and the Vanderbilt-Meharry Center for AIDS Research (L.V.K), NIH training grant CA009385 (D.O,-V). The human project was supported by the Austrian Science Fund (Project S9308-B05 to B.G.-L.). D.H.-B. is supported by a European FLARE fellowship funded by the Austrian Federal Ministry of Science and Research.
Footnotes
This is manuscript 1063 from the La Jolla Institute for Allergy and Immunology.
Description of the individual contribution made by each author: Y.H. and Y.P. provided input into the conceptual development and execution of the studies, as well as preparation of the manuscript. Y.W.Z., A.L., R.A. and I.B. provided technical assistance and input into data analyses. D.O.-V. and L.V.K. contributed to generation of TL-deficient mice. M.A.T. contributed to the generation and backcrossing of TL-Tg mice. D.H.B. and B.G.-L. performed human sample experiments. N.A. provided IL-7Rα449F mice. S.P.S. helped with in vitro culture experiments. M.K. participated in discussions of the data and the preparation of the manuscript. H.C. conceived the ideas, generated the TL transgenic mice with assistance of M.A.T., wrote the manuscript and supervised the experiments.
Competing Interests statement. The authors declare no competing financial interests.
References
- 1.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheroutre H, Madakamutil L. Mucosal effector memory T cells: the other side of the coin. Cell Mol Life Sci. 2005;62:2853–2866. doi: 10.1007/s00018-005-5232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 4.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 7.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pamer EG. Immune responses to Listeria monocytogenes. Nature reviews Immunology. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 9.Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 10.Bahjat KS, et al. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect Immun. 2006;74:6387–6397. doi: 10.1128/IAI.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starks H, et al. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. Journal of immunology. 2004;173:420–427. doi: 10.4049/jimmunol.173.1.420. [DOI] [PubMed] [Google Scholar]
- 12.Madakamutil LT, et al. CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 13.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 14.Leishman AJ, et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 15.Hershberg R, et al. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci U S A. 1990;87:9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M, van Kaer L, Itohara S, Tonegawa S. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J Exp Med. 1991;174:213–218. doi: 10.1084/jem.174.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attinger A, et al. Molecular basis for the high affinity interaction between the thymic leukemia antigen and the CD8alphaalpha molecule. J Immunol. 2005;174:3501–3507. doi: 10.4049/jimmunol.174.6.3501. [DOI] [PubMed] [Google Scholar]
- 18.Olivares-Villagomez D, et al. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2008;105:17931–17936. doi: 10.1073/pnas.0808242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MA, Bevan MJ. Cutting edge: a single MHC class Ia is sufficient for CD8 memory T cell differentiation. J Immunol. 2005;175:2066–2069. doi: 10.4049/jimmunol.175.4.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eghtesady P, et al. Expression of mouse Tla region class I genes in tissues enriched for gamma delta cells. Immunogenetics. 1992;36:377–388. doi: 10.1007/BF00218045. [DOI] [PubMed] [Google Scholar]
- 21.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, et al. The crystal structure of a TL/CD8alphaalpha complex at 2.1 A resolution: implications for modulation of T cell activation and memory. Immunity. 2003;18:205–215. doi: 10.1016/s1074-7613(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 23.Contini P, et al. Apoptosis of antigen-specific T lymphocytes upon the engagement of CD8 by soluble HLA class I molecules is Fas ligand/Fas mediated: evidence for the involvement of p56lck, calcium calmodulin kinase II, and Calcium-independent protein kinase C signaling pathways and for NF-kappaB and NF-AT nuclear translocation. J Immunol. 2005;175:7244–7254. doi: 10.4049/jimmunol.175.11.7244. [DOI] [PubMed] [Google Scholar]
- 24.Contini P, et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33:125–134. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 25.Fournel S, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 26.Puppo F, et al. Soluble human MHC class I molecules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8(+) Fas (CD95)(+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 27.Arcaro A, et al. CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med. 2001;194:1485–1495. doi: 10.1084/jem.194.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ellmeier W, Sunshine MJ, Losos K, Hatam F, Littman DR. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- 30.Chandele A, Kaech SM. Cutting edge: memory CD8 T cell maturation occurs independently of CD8alphaalpha. J Immunol. 2005;175:5619–5623. doi: 10.4049/jimmunol.175.9.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 33.Osborne LC, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trautmann L, et al. Selection of T cell clones expressing high-affinity public TCRs within Human cytomegalovirus-specific CD8 T cell responses. J Immunol. 2005;175:6123–6132. doi: 10.4049/jimmunol.175.9.6123. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 36.Zhong W, Reinherz EL. CD8 alpha alpha homodimer expression and role in CD8 T cell memory generation during influenza virus A infection in mice. Eur J Immunol. 2005;35:3103–3110. doi: 10.1002/eji.200535162. [DOI] [PubMed] [Google Scholar]
- 37.Le Bouteiller P, Solier C. Is antigen presentation the primary function of HLA-G? Microbes Infect. 2001;3:323–332. doi: 10.1016/s1286-4579(01)01386-7. [DOI] [PubMed] [Google Scholar]
- 38.Sargent IL. Does ‘soluble’ HLA-G really exist? Another twist to the tale. Mol Hum Reprod. 2005;11:695–698. doi: 10.1093/molehr/gah196. [DOI] [PubMed] [Google Scholar]
- 39.Boulassel MR, Mercier F, Gilmore N, Routy JP. Immunophenotypic patterns of CD8+ T cell subsets expressing CD8alphaalpha and IL-7Ralpha in viremic, aviremic and slow progressor HIV-1-infected subjects. Clin Immunol. 2007;124:149–157. doi: 10.1016/j.clim.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol. 2007;178:7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 41.Belyakov IM, et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daucher M, et al. Virological outcome after structured interruption of antiretroviral therapy for human immunodeficiency virus infection is associated with the functional profile of virus-specific CD8+ T cells. J Virol. 2008;82:4102–4114. doi: 10.1128/JVI.02212-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 44.Vogel TU, et al. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J Virol. 2003;77:13348–13360. doi: 10.1128/JVI.77.24.13348-13360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.