Abstract
Cholangioscopy remains another modality in the investigation of biliary strictures. At cholangioscopy, the “tumour vessel” sign is considered a specific sign for malignancy. Through its ability to not only visualise mucosa, but to take targeted biopsies, it has a greater accuracy, sensitivity and specificity for malignant strictures than endoscopic retrograde cholangiopancreatography guided cytopathological acquisition. Cholangioscopy however, is time consuming and costly, requires greater technical expertise, and should be reserved for the investigation of undifferentiated strictures after standard investigations have failed.
Keywords: Biliary strictures, Cholangioscopy, Endoscopic retrograde cholangiopancreatography, Endoscopy, Cholangiocarcinoma
INTRODUCTION
Multiple modalities are now available for the investigation of obstructive jaundice: endoscopic retrograde cholangiopancreatography (ERCP), magnetic resonance cholangiopancreatography, endoscopic ultrasound (EUS), computed tomography (CT) and transabdominal ultrasound (TUS). TUS is accurate in identifying biliary obstruction, but is less accurate in identifying the aetiology[1-3]. Whilst there have been refinements in techniques in the other aforementioned imaging modalities, making a conclusive diagnosis in the setting of a biliary stricture or intraductal tumour may be difficult[4,5]. Biliary strictures may be caused by benign and malignant tumours, as well as inflammatory processes. Management strategies with optimal outcomes are clearly dependent on knowing the correct diagnosis. Whilst features suggestive of malignancy are assessed for on cholangiography and EUS, these criteria are known to be non specific[6] and histopathology remains the gold standard for diagnosis.
Conventional tissue sampling during ERCP is routinely performed in the investigation of biliary strictures; however it remains sub-optimal in making a diagnosis. Brush cytology remains the mainstay of obtaining a cytological sample from a biliary stricture at ERCP. It is simple to perform and reported to have few complications[7]. Brush cytology performed at the time of ERCP has a sensitivity of 30% to 57%[8-10] and is thought to be limited by the poor cellular yield.
Cholangioscopy involves direct visualisation of the biliary tree via a fibre optic or video scope. It may be performed percutaneously or “peroral”, where a scope is passed down the therapeutic channel of a duodenoscope, and has become an additional modality in the investigation and management of biliary disease. Initially, the percutaneous approach was favoured because these cholangioscopes were larger than the operating channel of a conventional therapeutic duodenoscope. Subsequently, cumbersome mother daughter systems were developed to visualise the biliary tree via the ampulla. Peroral cholangioscopy (POCS) was first described in the in 1976 by Kawai et al[11]. Initially the domain of specific tertiary hospitals, the clinical use of cholangioscopy is expanding. Our focus is to review the utility of cholangioscopy in biliary strictures: in particular details of cholangioscopes available, the evidence available in relation to the diagnostic realms of cholangioscopy, cholangioscopy assisted biopsy and the utility of chromocholangioscopy (CC) and POCS with narrow band imaging (NBI).
INSTRUMENTS
Peroral cholangioscopes
Peroral cholangioscopes are passed down the 4.2 mm working channel of a therapeutic duodenoscope to visualise the biliary tree. There are currently devices available from Olympus, Pentax, and Boston Scientific.
Olympus and Pentax provide reusable fibre optic and video cholangioscopes. There are four different sized diameter per-oral cholangioscopes available - 2.6, 2.8, 3.1 and 3.4 mm. The 2.6 and 2.8 mm diameter cholangioscopes have a 0.75 mm working channel that allows the passage of a 0.025 inch guide wire. The 2.8, 3.1 and 3.4 mm diameter cholangioscopes with a 1.2 mm working channel permit use of 1.9-3 French electrohydraulic lithotripsy fibers, 0.035 inch guide wire and biopsy forceps. These cholangioscopes come in 187, 190 and 200 cm lengths and have bi-directional (up-down) movement.
These scopes consist of a dial for two way tip deflection, air/water buttons and suction channels. The core of the cholangioscope is predominantly fibre optic cables through which the image is transmitted from the tip of the endoscope to the eyepiece, with a light guide for illumination, angulation wires for tip deflection, an air/ water nozzle and a working channel.
There are newer 3.4 and 5.3 mm cholangioscopes available using charge couple device (CCD) technology and a NBI system incorporated into a video cholangioscope (Olympus Inc., CHF-B260 and CHF-BP260). They are currently still undergoing FDA approval and have not yet been used in North America[12,13].
The Spyglass Direct Visualization System (Microvasive Endoscopy, Boston Scientific Co., Natick, Mass, United States) is composed of a reusable optical probe that traverses a disposable access and delivery catheter (Figure 1). The 3.4 mm diameter disposable catheter consists of the optical probe port, an irrigation port and 1.2 mm accessory channel. The 0.77 mm, 6000 pixel, reusable optic probe, is a collection of light fibers and optical fiber bundles. This system offers 4-way deflected steering, and a specific miniature biopsy forceps (Spybite®) which is commercially available with this system.
Figure 1.
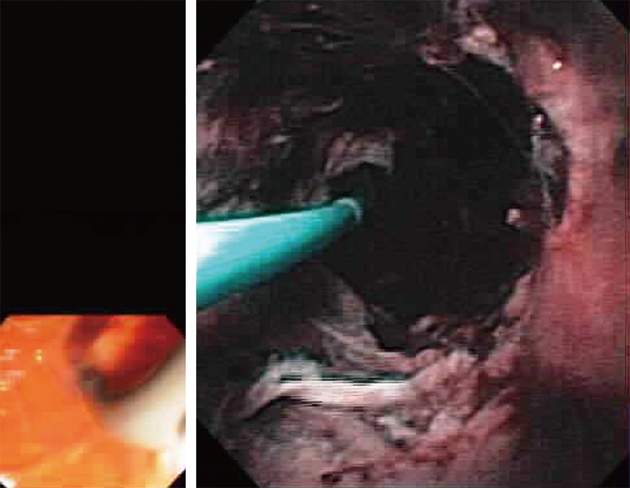
Video cholangioscopy of benign common hepatic stricture secondary to primary sclerosing cholangitis.
Percutaneous/intraoperative cholangioscopes
Percutaneous/intraoperative cholangioscopes used are usually shorter (35, 38, 45 and 70 cm) working length, wider diameter (4.8, 4.9 and 6.0 mm) and have a larger accessory channel (2.0, 2.2 and 2.6 mm). This wider channel facilitates instrumentation with more therapeutic devices - intraductal lithotripsy fibers, baskets, and forceps.
Paediatric endoscopes
Paediatric endoscopes have also been used to directly visualise the biliary tree intubated under fluoroscopic and endoscopic guidance, and the use of a biliary placed guidewire or balloon catheter[14,15]. These techniques overcome the disadvantages of using the “mother daughter” system which is relatively expensive in capital expenditure, time consuming, cumbersome, usually requires two endoscopists, is fragile and has a smaller working channel. This method has been reported with a variable success (45.5%-100%) of biliary intubation, but is currently not routinely performed[14,15].
A double balloon enteroscope overtube (TS 13140, total length 1450 mm, Fujinon Corp., Omiya, Japan) has been used in combination with an Olympus paediatric gastroscope (GIF-N230 or N260; Olympus Optical Co, Ltd, Tokyo, Japan) to perform cholangioscopy. The gastroscope was used to intubate the duodenum, the overtube advanced over the gastroscope and post-insufflation used as an anchor whilst the gastroscope intubated the biliary tree under endoscopic and fluoroscopic guidance. In this single case series of 12 patients, the device had a success of 83.3%[16]. Lithotripsy and biopsies were able to be performed through the working channel of the paediatric gastroscope.
Wilson Cook Inc. (Winston Salem, NC, United States) has released a cholangioscopy access balloon. These are 315 cm long 4 Fr catheters with either a 15 or 20 mm balloon that is inserted into the biliary tree through the duodenoscope. The balloon is then insufflated, securing its position, and the duodenoscope is exchanged for a paediatric gastroscope with tension on the catheter to prevent looping. After intubation of the biliary tree, the balloon may be deflated if biopsies are to be taken or therapeutic intervention needs to be performed. At this current time, there are no clinical trials evaluating the use of this device and it awaits FDA approval for clinical use.
Peroral vs percutaneous cholangioscopy
Percutaneous cholangioscopy (PTC) was often the favoured method of cholangioscopy. Wider, shorter and more maneuverable scopes enabled biopsies for diagnosis and therapeutic devices to be advanced through the working channel in a less cumbersome manner. The procedure itself requires only one endoscopist. The drawbacks of PTC are that it is performed through an operatively placed T-tube tract or a percutaneous transhepatic biliary drain (PTBD). To use the T-tube, 3 to 5 wk must be waited for the maturation of the tract, with inherent time delays. To perform PTCs via the PTBD, 2 to 4 wk must be allowed for a tract to mature after an internal/external 7 to 8 French pigtail catheter is initially deployed, with sequential dilatation commencing after several days to eventually deploy a 16 French diameter catheter across the tract. The final step before introduction of the cholangioscope is a pre-procedure dilation. A percutaneous plastic peel-away sheath (Wilson Cook Inc, Winston Salem, NC, United States) or metal sheath (Olympus Co., Tokyo, Japan) may be used for direct access if these time delays are not feasible. Drawbacks of this approach are the necessity for long hospitalization, cumbersome management of a drainage catheter and possible complications of bleeding/hemobilia, seeding metastasis along the sinus tract of percutaneous transhepatic drainage and risk of intraperitoneal metastasis[17].
POCS is usually performed with two experienced endoscopists and has a long procedure time. Alternatively a specially designed external cholangioscope fixation device or nurse/assistant may be used. ERCP is usually performed to delineate the biliary tree and pathology, and the cholangioscope is placed down the working channel of the duodenoscope and may be inserted freehand through the previously sphincteromised ampulla or it may be inserted over a long 450 cm guidewire that has been backloaded into the channel of the daughter scope. The cholangioscope is fragile and judicious use of the elevator is advised, with the latter method trying to overcome the need for vigorous use of the elevator. Maneuverability is technically more challenging due to the length of the POCS, and two way tip deflection and limitations also arise from traversal of the duodenoscope. Passage of the biopsy forceps can also be difficult due to the length and angles of the cholangioscope required to arrive at the target lesion.
Cholangioscopic findings in biliary strictures
Numerous studies have evaluated the use of cholangioscopy in undifferentiated strictures[18-22]. In a case series of 111 patients with biliary tumours/strictures, they aimed to correlate cholangioscopic findings with histopathology from cholangioscopic guided biopsies or surgically resected specimens[23]. They classified the cholangioscopic findings of cholangiocarcinoma into three categories: nodular, papillary and infiltrative types[23]. Nodular type cholangiocarcinoma is described to have nodular mucosa causing luminal narrowing, with the mucosa being irregular with intense neovascularization. This type of cholangiocarcinoma is short in length. Papillary type cholangiocarcinoma is described to have papillary mucosal projections, superficially spreading and neovascularization is usually not a feature. These papillary projections usually caused obstruction and were intermingled with pus and sludge. The authors describe a mucin secreting cholangiocarcinoma, that on cholangioscopy appear similar to papillary type cholangiocarcinoma with papillary or villous mucosal projections. The mucin secretion is thought to cause marked proximal ductal dilatation. Infiltrative type cholangiocarcinoma was not usually associated with a mass lesion. This malignancy has a smooth tapered narrowing, with the mucosa being white with subtle elevations on the margin of tumour vessels. Neovascularization was not described to be as predominant as with the nodular type cholangiocarcinoma. There have been no studies to prospectively evaluate if the cholangiographic type of cholangiocarcinoma prognosticates the clinical outcome.
Benign strictures were described in this case series to have a smooth surface mucosa and tapered luminal narrowing with no definite neovascularization[23]. Biliary papillomatosis consists of papillary projections into the lumen with intervening normal areas of mucosa, and is thought to predispose to papillary adenocarcinoma. The authors conclude that it is difficult to cholangiographically differentiate papillary adenoma from papillary adenocarcinoma; however they suggest that the degree of luminal obstruction may correlate to the risk of overt malignancy.
ASSESSMENT OF NEOVASCULARIZATION OR THE “TUMOUR VESSEL” SIGN
The “tumour vessel” is an abnormally proliferating and tortuous vascular structure on the mucosa adjacent to the stricture. Synonymous terms include “capillary sign”, neovascularization and vascular dilatation. A prospective study of patients with biliary strictures without a luminal mass in or around the stricture diagnosed at ERCP or percutaneous transhepatic cholangiography, evaluated the sensitivity and specificity of the “tumour vessel” sign for malignancy as seen at PTC[24]. In total, 63 patients were enrolled in the study. Malignancy was confirmed when malignant cells were seen in PTC directed biopsies and in the surgically resected specimen. A stricture was deemed benign if the resected specimen had no evidence of malignant cells or, after the 1 year follow up, there was no clinical or radiological evidence of disease progression. They found the sensitivity of this sign for malignancy to be 61% (25/41) and specificity was 100%. PTC directed biopsies had a sensitivity of 80% (33/41) and specificity of 100%. Concordance between two endoscopists for the “tumour vessel” sign was 100%. Six of eight strictures with a positive “tumour vessel” sign had false negative biopsies. The majority of these malignant strictures were infiltrative type cholangiocarcinomas, which is thought to spread beneath the epithelium, and the abundant fibrosis may lead to higher rates of falsely negative biopsies. For this particular reason, the authors suggested cholangioscopy be considered in patients with non diagnostic cytological specimens gathered at ERC in particular to assess for this specific sign.
THE CLINICAL UTILITY OF CHOLANGIOSCOPY
In a case series of 97 patients with biliary filling defects and strictures, Fukuda et al[20] evaluated the additional benefit of POC to ERCP and biopsies or brushings. Patients were excluded if there was an ampullary or pancreatic (based on CT or TUS) mass or in subjects with negative cytology who were not clinically followed up for a minimum of 12 mo. Criteria used to assess strictures for malignancy included: (1) the presence of tumour vessels; (2) easy oozing; and (3) irregular surface.
Patients were deemed to have malignancy if they had positive surgical specimens, evidence of malignant cytology by other means, or clinical progression at follow up. Of 76 strictures, 38 were malignant and 28 benign. ERCP and tissue sampling correctly identified 22 of the 38 malignant strictures giving sensitivity, specificity, accuracy, and a positive and negative predictive value of 57.9%, 100%, 78.1%, 100% and 68.6%, respectively. In comparison, the addition of POCS to ERCP and tissue sampling correctly identified all 38 malignant strictures and 33 of the 38 benign strictures. This improved the sensitivity to 100%; however the specificity was 87.2%. The five false positive strictures identified on POCS had abnormal tortuous dilated vessels present, which was previously thought to be specific for malignancy. The authors conclude that POCS without POCS directed biopsy is a useful adjunct to ERCP in the management of biliary strictures[20].
Shah et al[19] assessed the clinical utility of POCS and cholangioscopic assisted/directed biopsies in the investigation of indeterminate biliary strictures. Sixty-two patients were referred with a combination of non diagnostic cytology taken at ERCP and/or non specific imaging by CT, magnetic resonance imaging or positron emission tomography scanning. Eighteen patients had a final diagnosis of malignancy and 16 were diagnosed with cholangioscopy. One of the malignancies missed was proximal to a benign appearing biliary stricture secondary to primary sclerosing cholangitis and diagnosed intraoperatively in the caudate lobe. The other malignancy was a hilar cholangiocarcinoma diagnosed in the explanted liver, 7 mo after cholangioscopy with negative cholangioscopic assisted and directed biopsies. Based on cholangioscopy, two patients were incorrectly diagnosed with malignancy. Both had negative cholangioscopic assisted and directed biopsies and normal intraoperative biopsies. In the 16 patients correctly diagnosed with malignancy at cholangioscopy, biopsies were positive in 10 patients (63%). Overall the sensitivity for detecting malignancy by cholangioscopy with and without biopsy based on this cohort was 89%, specificity 96%, positive predictive value 89% and negative predictive value 96%. These studies suggest that cholangioscopy without biopsy has a relatively high sensitivity and specificity for malignant strictures as compared with ERCP and cytopathological acquisition. Whilst there are no head to head studies comparing ERCP and cholangioscopy, it is not unreasonable to consider cholangioscopy in the setting of a non diagnostic ERCP.
IMAGE ENHANCED VIDEO CHOLANGIOPANCREATOSCOPY
New techniques currently being evaluated in the investigation of biliary strictures include CC and NBI.
Chromocholangioscopy
Chromoendoscopy is commonly used in assessment, particularly of the borders of gastrointestinal malignancies. Methylene blue chromocholangioscopy has been evaluated in two studies in patients with biliary strictures[25,26]. Hoffman et al[26] performed per oral chromoendoscopy by injecting 15 mL of 0.1% methylene blue down the working channel of the cholangioscope. Excess dye was then removed with suction after 2 min. In 55 patients who underwent chromoendoscopic cholangioscopy for biliary strictures or filling defects, dye spray added on average 18 min to the procedure (range 10-45 min). Chromocholangioscopic images were correlated with cholangioscopic directed biopsies. In this pilot study, different staining patterns of methylene blue were seen in normal, inflammatory (diffuse uptake) and dysplastic mucosa (irregular uptake). In particular, the authors suggested the uptake of stain in inflammatory tissue helped to distinguish fibrotic from inflammatory strictures, especially in patients with primary sclerosing cholangitis. The authors report that most lesions were identifiable with white light cholangioscopy[26]. Whilst this study demonstrated clear difference in patterns, this has not been repeated, nor is chromocholangioscopy practice widespread.
Narrow band imaging
The NBI system developed by Olympus medical system is based on modifying/narrowing the bandwidth of spectral transmittance resulting in optical colour separation. The filter is placed in the optical illumination system and removes all light wavelengths except for two narrow wavelengths. The central wavelengths of each band are 415 nm and 540 nm. This is available on two video cholangioscopes (CHF-B260 and CHF-BP260). The shorter band is thought to give information about the capillary and pit patterns of the superficial mucosa, whilst the 540 nm wavelength gives more information about thicker capillaries in slightly deeper tissues. To date, published literature in relation to narrow band imaging cholangioscopy is limited to case reports and small caser series[12,27-30]. The largest case series is of 21 lesions in 12 patients who underwent POCS with narrow band imaging[12]. Their aim was to comparatively assess the clinical feasibility of using POCS with NBI, and the ability of POCS with NBI to identify biliary lesions in comparison to conventional white light POCS. In particular they assessed the ability to determine distal and if possible, proximal margins, and surface vasculature. In this small case series, only two POCS with conventional white light cholangioscopies were rated as “excellent” in comparison with 12 lesions with NBI. NBI identified four strictures not seen with standard POCS. The authors suggest observation of surface structure and mucosal vessels was as good as or better than, conventional observation and that video cholangioscopy with NBI may be helpful in differentiating benign from malignant strictures and bile duct tumours showing superficial spread[12]. A limitation of NBI cholangioscopy is that bile and blood both appear as dark red fluid, possibly limiting views. These preliminary findings need to be further evaluated with randomized controlled studies.
CONCLUSION
Cholangioscopy remains another modality in the investigation of biliary strictures. Through its ability to visualise mucosa and take targeted biopsies, it has a greater accuracy, sensitivity and specificity for malignant strictures than ERCP guided cytopathological acquisition[19,20]. Cholangioscopy however, is time consuming and costly, requires greater technical expertise, and should be reserved for the investigation of undifferentiated strictures after standard investigations have failed. There is still a paucity of literature with regards to image enhanced cholangioscopy and its role, which remain promising, in imaging technologies that require further investigation.
ACKNOWLEDGMENTS
The authors thank Professor Todd Baron, Mayo Clinic for Figure 1.
Footnotes
Peer reviewer: Yoshi Ueno, MD, Division of Gastroenterology, Tohoku University Hospital, Sendai 980-8574, Japan
S- Editor Tian L L- Editor Rutherford A E- Editor Zheng XM
References
- 1.Reinus WR, Shady K, Lind M, Scott R. Ultrasound evaluation of the common duct in symptomatic and asymptomatic patients. Am J Gastroenterol. 1992;87:489–492. [PubMed] [Google Scholar]
- 2.Thornton JR, Lobo AJ, Lintott DJ, Axon AT. Value of ultrasound and liver function tests in determining the need for endoscopic retrograde cholangiopancreatography in unexplained abdominal pain. Gut. 1992;33:1559–1561. doi: 10.1136/gut.33.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wachsberg RH, Kim KH, Sundaram K. Sonographic versus endoscopic retrograde cholangiographic measurements of the bile duct revisited: importance of the transverse diameter. AJR Am J Roentgenol. 1998;170:669–674. doi: 10.2214/ajr.170.3.9490950. [DOI] [PubMed] [Google Scholar]
- 4.Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–240. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 5.Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913–922. doi: 10.1634/theoncologist.11-8-913. [DOI] [PubMed] [Google Scholar]
- 6.Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870–876. doi: 10.1067/mge.2002.124206. [DOI] [PubMed] [Google Scholar]
- 7.Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, Bory R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–572. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 8.Venu RP, Geenen JE, Kini M, Hogan WJ, Payne M, Johnson GK, Schmalz MJ. Endoscopic retrograde brush cytology. A new technique. Gastroenterology. 1990;99:1475–1479. doi: 10.1016/0016-5085(90)91178-9. [DOI] [PubMed] [Google Scholar]
- 9.Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–677. doi: 10.1136/gut.40.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart CJ, Mills PR, Carter R, O'Donohue J, Fullarton G, Imrie CW, Murray WR. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54:449–455. doi: 10.1136/jcp.54.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai K, Nakajima M, Akasaka Y, Shimamotu K, Murakami K. [A new endoscopic method: the peroral choledocho-pancreatoscopy (author's transl)] Leber Magen Darm. 1976;6:121–124. [PubMed] [Google Scholar]
- 12.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos) Gastrointest Endosc. 2007;66:730–736. doi: 10.1016/j.gie.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 13.Lew RJ, Kochman ML. Video cholangioscopy with a new choledochoscope: a case report. Gastrointest Endosc. 2003;57:804–807. doi: 10.1067/mge.2003.211. [DOI] [PubMed] [Google Scholar]
- 14.Larghi A, Waxman I. Endoscopic direct cholangioscopy by using an ultra-slim upper endoscope: a feasibility study. Gastrointest Endosc. 2006;63:853–857. doi: 10.1016/j.gie.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Moon JH, Ko BM, Choi HJ, Hong SJ, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Intraductal balloon-guided direct peroral cholangioscopy with an ultraslim upper endoscope (with videos) Gastrointest Endosc. 2009;70:297–302. doi: 10.1016/j.gie.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Choi HJ, Moon JH, Ko BM, Hong SJ, Koo HC, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Overtube-balloon-assisted direct peroral cholangioscopy by using an ultra-slim upper endoscope (with videos) Gastrointest Endosc. 2009;69:935–940. doi: 10.1016/j.gie.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Itoi T, Neuhaus H, Chen YK. Diagnostic value of image-enhanced video cholangiopancreatoscopy. Gastrointest Endosc Clin N Am. 2009;19:557–566. doi: 10.1016/j.giec.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Seo DW, Kim MH, Lee SK, Myung SJ, Kang GH, Ha HK, Suh DJ, Min YI. Usefulness of cholangioscopy in patients with focal stricture of the intrahepatic duct unrelated to intrahepatic stones. Gastrointest Endosc. 1999;49:204–209. doi: 10.1016/s0016-5107(99)70487-6. [DOI] [PubMed] [Google Scholar]
- 19.Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219–225. doi: 10.1016/s1542-3565(05)00979-1. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374–382. doi: 10.1016/j.gie.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67–73. doi: 10.1016/s0016-5107(99)70347-0. [DOI] [PubMed] [Google Scholar]
- 23.Seo DW, Lee SK, Yoo KS, Kang GH, Kim MH, Suh DJ, Min YI. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630–634. doi: 10.1067/mge.2000.108667. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–638. doi: 10.1067/mge.2000.108969. [DOI] [PubMed] [Google Scholar]
- 25.Maetani I, Ogawa S, Sato M, Igarashi Y, Sakai Y, Shibuya K. Lack of methylene blue staining in superficial epithelia as a possible marker for superficial lateral spread of bile duct cancer. Diagn Ther Endosc. 1996;3:29–34. doi: 10.1155/DTE.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman A, Kiesslich R, Bittinger F, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy in patients with biliary strictures: feasibility and outcome analysis. Endoscopy. 2008;40:563–571. doi: 10.1055/s-2007-995688. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa T, Horaguchi J, Noda Y, Kobayashi G, Ito K, Obana T, Takasawa O, Koshita S, Kanno Y, Fujita N. A case of distal bile duct cancer with extensive intraepithelial spread diagnosed preoperatively by peroral cholangioscopy combined with narrow band imaging. Nippon Shokakibyo Gakkai Zasshi. 2010;107:112–119. [PubMed] [Google Scholar]
- 28.Lu XL, Itoi T, Kubota K. Cholangioscopy by using narrow-band imaging and transpapillary radiotherapy for mucin-producing bile duct tumor. Clin Gastroenterol Hepatol. 2009;7:e34–e35. doi: 10.1016/j.cgh.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T. Evaluation of peroral videocholangioscopy using narrow-band imaging for diagnosis of intraductal papillary neoplasm of the bile duct. Dig Endosc. 2009;21 Suppl 1:S103–S107. doi: 10.1111/j.1443-1661.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 30.Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos) Gastrointest Endosc. 2008;67:574–576. doi: 10.1016/j.gie.2007.07.031. [DOI] [PubMed] [Google Scholar]


