Abstract
OBJECTIVE
Sirtuin 1 (SIRT1) and its activator resveratrol are emerging as major regulators of metabolic processes. We investigate the site of resveratrol action on glucose metabolism and the contribution of SIRT1 to these effects. Because the arcuate nucleus in the mediobasal hypothalamus (MBH) plays a pivotal role in integrating peripheral metabolic responses to nutrients and hormones, we examined whether the actions of resveratrol are mediated at the MBH.
RESEARCH DESIGN AND METHODS
Sprague Dawley (SD) male rats received acute central (MBH) or systemic injections of vehicle, resveratrol, or SIRT1 inhibitor during basal pancreatic insulin clamp studies. To delineate the pathway(s) by which MBH resveratrol modulates hepatic glucose production, we silenced hypothalamic SIRT1 expression using a short hairpin RNA (shRNA) inhibited the hypothalamic ATP-sensitive potassium (KATP) channel with glibenclamide, or selectively transected the hepatic branch of the vagus nerve while infusing resveratrol centrally.
RESULTS
Our studies show that marked improvement in insulin sensitivity can be elicited by acute administration of resveratrol to the MBH or during acute systemic administration. Selective inhibition of hypothalamic SIRT1 using a cell-permeable SIRT1 inhibitor or SIRT1-shRNA negated the effect of central and peripheral resveratrol on glucose production. Blockade of the KATP channel and hepatic vagotomy significantly attenuated the effect of central resveratrol on hepatic glucose production. In addition, we found no evidence for hypothalamic AMPK activation after MBH resveratrol administration.
CONCLUSIONS
Taken together, these studies demonstrate that resveratrol improves glucose homeostasis mainly through a central SIRT1-dependent pathway and that the MBH is a major site of resveratrol action.
Diabetes and obesity are evolving as two of the major diseases of the 21st century, with a significant increase in morbidity and mortality worldwide. As a result, there is an increasing need to identify potential therapeutic targets for the management of these disorders. Sirtuin 1 (SIRT1) is a NAD+-dependent protein deacetylase and a member of the group of mammalian proteins collectively referred to as “sirtuins” (SIRT1–7). SIRT1 deacetylates several substrates, including transcription factors that are involved in multiple cellular processes, and is rapidly emerging as an important regulator of metabolism and aging (1). SIRT1 is nutritionally regulated, and its expression increases during caloric restriction and fasting (2,3). As such, it provides a link between nutrient availability and energy balance. Further, SIRT1 activation results in improved glucose tolerance, increased insulin secretion, and resistance to diet-induced obesity (4–7). Thus, SIRT1 acts as a master metabolic sensor with the ability to integrate environmental signals to the metabolic needs of the organism.
Resveratrol, a plant-derived polyphenol commonly found in grapes and red wine, is an effective SIRT1 activator. Oral administration of resveratrol alleviates hyperglycemia and diet-induced obesity and improves mitochondrial function (8–10). In addition, resveratrol enhances neuronal survival and reduces cerebral ischemia and neurodegenerative conditions in an SIRT1-dependent manner (11–13). Other SIRT1 activators that are structurally distinct from resveratrol have been developed, and studies have shown that they too demonstrate similar metabolic benefit (14–16). Thus, SIRT1-activating molecules such as resveratrol show significant therapeutic potential for the management of metabolic disorders.
The arcuate nucleus of the mediobasal hypothalamus (MBH) is a major center where signals involved in the determination of nutrient availability and energy balance converge. Our group and others have shown that hormones and nutrient substrates when acting centrally have a direct role in the acute regulation of insulin action (17–21). Recent studies have revealed that SIRT1 is highly expressed and regulated in the MBH and that chronic intracerebroventricular administration of resveratrol reduces diet-induced hyperglycemia (22,23). In addition, hypothalamic SIRT1 appears to have a role in mediating energy balance (24,25). However, the specific contribution of central SIRT1 to the effects of resveratrol on glucose metabolism has not been systematically investigated. In the studies presented, we have elucidated the specific effect of MBH resveratrol on glucose homeostasis and insulin action. We have also established that the effect of central resveratrol on hepatic glucose production is mediated primarily in an SIRT1-dependent manner. Further, we have demonstrated that acute administration of resveratrol centrally or systemically effectively and consistently modulates glucose homeostasis. In addition, we have shown that the hypothalamic KATP channel and vagus nerve innervation to the liver are required for central resveratrol action on hepatic glucose production.
RESEARCH DESIGN AND METHODS
Animal preparation.
Twelve-week-old SD male rats (Charles River Laboratories International, Inc., Wilmington, MA) were housed in single cages and exposed to a standard 12-h light:dark cycle. The rats had stereotaxic-guided surgery for placement of bilateral cannulae into the MBH using the following coordinates: 3.3 mm caudal to the bregma and 9.6 mm below the skull surface (19,26). One week before clamp studies, indwelling catheters were implanted into the carotid artery and jugular vein. The study protocol was approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Basal pancreatic insulin clamp.
Rats were restricted to 20 g of food on the night before the study. For the MBH studies, the animals received a continuous infusion of each compound at a rate of 0.006 μL/min. The agents used were vehicle (5% DMSO), resveratrol (200 μmol/L) (EMD Bioscience, Darmstadt, Germany), resveratrol coinfused with SIRT1 inhibitor (500 nmol/L), 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide (Axxora LLC, San Diego, CA), or glibenclamide (100 μmol/L) (Sigma-Aldrich, St. Louis, MO) depending on the study protocol. For the AMPK activation studies, AICAR 50 nmol/L (Toronto Research Chemicals, Ontario, Canada) was injected directly to the MBH. For the systemic studies, the animals received vehicle (5% DMSO) or resveratrol (10 µmol/kg). The compounds used were dissolved in DMSO, and the infusions were given continuously over 6 h. The basal pancreatic insulin clamp was carried out according to our standard protocol (27).
Selective hepatic branch vagotomy.
The animals had stereotaxic-guided placement of double cannulae into the MBH followed by hepatic branch vagotomy, which was performed as previously described (18,26). A control group of sham-operated animals had implantation of double cannulae that was subsequently followed by abdominal surgery to expose the contents of the gastrointestinal tract. No vagotomy was done. After recovery over 7–8 days, venous and arterial catheterizations were performed for clamp study.
Analytic procedures.
Plasma glucose was measured by the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter Instruments, Brea, CA). Plasma insulin was determined by radioimmunoassay. The radioactivity of [3-3H] in plasma to assess glucose kinetics was determined as previously described (26,28,29). For the hepatic glucose flux studies, phosphoenolpyruvate (PEP) and uridine diphosphoglucose (UDP-glucose) concentrations and specific activities in liver were determined by two sequential chromatographic separations. Hepatic 14C-PEP and 3H-UDP glucose and 14C-UDP glucose-specific activities were obtained by high-performance liquid chromatography. Calculations for G6Pase flux, PEP, and gluconeogenesis were performed as previously described (30–32). Glycogenolysis was calculated as the difference between glucose production and gluconeogenesis. Epinephrine levels were determined by ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO). Corticosterone levels were measured by ELISA (IBL America, Minneapolis, MN). Glucagon levels were determined by radioimmunoassay (RIA Kit; Linco Research, Inc., St. Charles, MO).
Cell culture and maintenance.
HEK293 cells were maintained in low glucose Dulbecco's modified Eagle's medium supplemented with 10% FBS.
Construction of SIRT1-specific short hairpin RNA and viral preparation.
An SIRT1-specific short hairpin RNA (shRNA) with target sequence 5′AAGACTCAAGTTCACCTGAAA3′ (Gene Acc. Number: XM_228146) and control-shRNA with target sequence 5′AAGCGTCTTGACGGTAATCAA3′ were custom designed by Qiagen (Venlo, The Netherlands). The shRNA oligonucleotides were subcloned into an adeno-associated viral (AAV) vector under the control of a U6 promoter. The plasmid also had a green fluorescent protein (GFP) tag driven by a cytomegalovirus promoter. AAV vectors were packaged and purified initially by Applied Viromics (Fremont, CA) and subsequently by the Vector Core at the University of North Carolina. For in vitro validation, HEK293 cells were transduced with the viral constructs and allowed to incubate for 5 days. The cells were lysed with radioimmunoprecipitation assay buffer supplemented with protease inhibitor and protein phosphatase inhibitor. Western blot analysis was used to verify the expression of SIRT1. β-Tubulin was used as a loading control.
Infusion/perfusion and tissue processing.
Animals had stereotaxic surgery for placement of double intrahypothalamic (IH) cannulae. Immediately afterward, they received bilateral injections of the AAV constructs at a dose of ∼1 × 1012 pfu. After 2 weeks of recovery, the animals were perfused under ketamine anesthesia (100 mg/kg) via the ascending aorta with saline solution, followed by 250 mL of ice-cold 4% paraformaldehyde at pH 7.4. The brains were postfixed overnight in 4% paraformaldehyde and then stored in 0.1 mol/L PBS with 20% sucrose at 4°C for 48 h. Serial 40-µm coronal sections were cut on a cryostat throughout the hypothalamic brain region. Serial sections were collected and stored at −20°C until immunohistochemistry was performed.
Immunohistochemistry.
SIRT1 immunoreactivity was detected using a conventional avidin-biotin immunoperoxidase protocol (33). Briefly, sections were pretreated with 1% H2O2 for 30 min to quench endogenous peroxidase activity, followed by three rinses in 0.1 mol/L PBS and then incubation in 1% NaBH4 to reduce free aldehydes. Next the tissue sections were incubated with the SIRT1 antibody (Millipore, Billerica, MA) at a dilution of 1:1,000 at 4°C for 48 h. Then they were incubated with secondary antibody 1:500 dilution at room temperature for 2 h. Immune complexes were detected with the avidin–DH-biotinylated horseradish peroxidase-H-complex (Vectastain Elite ABC kit; Vector Laboratories, Inc., Burlingame, CA). The sections were mounted and dehydrated permanently in nonaqueous mounting media. The slides were photographed using a Zeiss Axioskop II light microscope equipped with a digital Zeiss Axiocam (Carl Zeiss, Oberkochen, Germany).
Western blot analysis.
Micropunches of specific hypothalamic nuclei were taken as previously described (34). Brain tissue was homogenized in 1× radioimmunoprecipitation assay lysis buffer supplemented with protease inhibitor and protein phosphatase inhibitor. Protein concentration was determined using the bicinchoninic acid) method (Thermo Fisher Scientific, Inc., Waltham, MA). In SDS-PAGE sample buffer, 25–50 µg of protein were heat denatured and resolved on 4–15% Criterion Precast gel (Bio-Rad Laboratories, Inc., Hercules, CA), and then transferred electrophoretically to nitrocellulose membrane. The membranes were then immunoblotted overnight at 4°C with primary antibodies for SIRT1, 1:3,000 (Millipore), p53(Acetyl K381), 1:2,000 (Abcam, Cambridge, U.K.), AMPK, 1:2,000 (Cell Signaling Technology, Inc., Danvers, MA), pAMPK, 1:1,000 (Cell Signaling), β-tubulin mouse monoclonal antibody, 1:10,000 (Covance, Princeton, NJ), or GFP, 1:4,000 (Stratagene, La Jolla, CA) as determined by the study. Subsequently, the membranes were incubated with fluorescent-labeled secondary antibodies, and the immunoblots were developed and quantified using the Odyssey Infrared Scanner (LI-COR Biomedical Sciences, Lincoln, NE).
RNA analysis and quantitative real-time PCR.
Gene expression of proopiomelanocortin (POMC) and agouti-related peptide (AgRP) was determined by RT-PCR as previously described (35). Total RNA was isolated from hypothalamic wedges using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized by reverse transcription using the Superscript III First Strand kit (Invitrogen). Quantitative real-time PCR was performed on a LightCycler 2 (Roche Applied Science, Basel, Switzerland) using the Fast Start DNA Master SYBR Green I kit (Roche Applied Science). 18S mRNA expression was used for normalization.
Statistical analysis.
For the experiments, all data are represented as the mean ± SEM. For comparisons involving two groups, we used the unpaired two-tailed Student t test. For comparisons involving two or more groups, ANOVA with post hoc analysis was used.
RESULTS
Expression and activation of SIRT1 in hypothalamic nuclei.
To determine the role of MBH SIRT1, we first analyzed the expression of the protein in multiple hypothalamic nuclei in SD male rats by Western blot analysis. We found that SIRT1 expression is markedly increased in the arcuate nucleus of the MBH compared with the surrounding nuclei (Fig. 1A). These findings are similar to those of recent reports in mice (22,25). We then investigated the effect of acute MBH administration of resveratrol on SIRT1 activity. SD male rats received acute infusions of vehicle (5% DMSO) or resveratrol (200 μmol/L). Acetylation of lysine 379 in p53 (Acp53) was used as a marker of SIRT1 activity (14,36). Resveratrol infusion resulted in the deacetylation of p53 primarily in the arcuate nucleus and the ventromedial hypothalamus. Neither resveratrol nor DMSO altered SIRT1 expression in the MBH (Fig. 1B). The studies indicate that acute administration of resveratrol is effective in activating hypothalamic SIRT1.
FIG. 1.
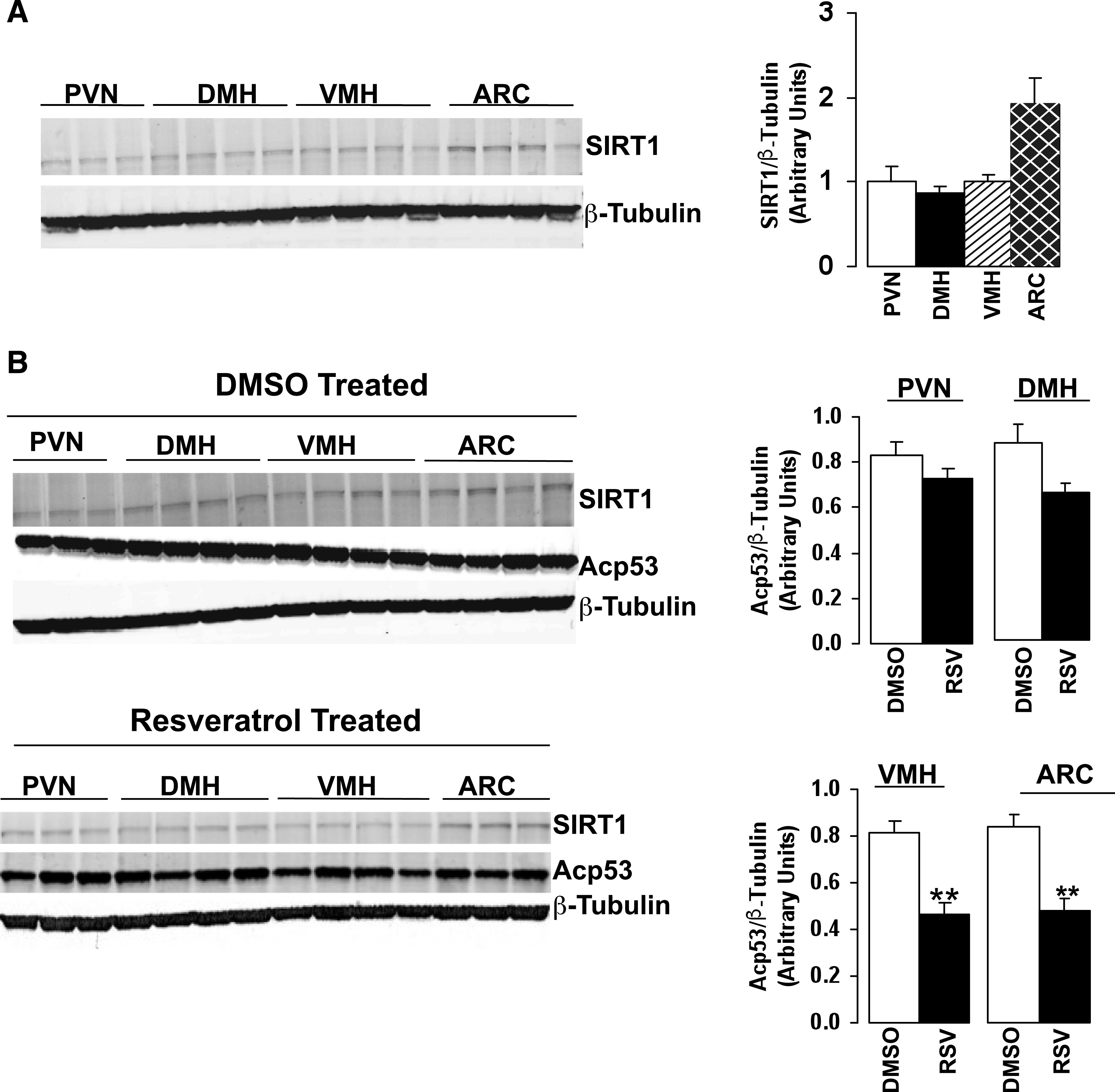
SIRT1 expression and acetylation of p53 in hypothalamic nuclei. A: Representative Western blot and quantification of SIRT1 expression in hypothalamic nuclei paraventricular nucleus, dorsomedial hypothalamus, ventromedial hypothalamus, and arcuate nucleus of male rats. B: SIRT1 expression and acetylation of p53 after MBH administration of vehicle (5% DMSO) or resveratrol. Experiment represents n = 3–4 per group. PVN, paraventricular nucleus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; ARC, arcuate nucleus. All values are mean ± SEM. **P < 0.005 compared with control.
Hypothalamic SIRT1 is essential for resveratrol’s effect on hepatic glucose production.
Given the importance of the MBH on the regulation of glucose homeostasis, we sought to determine its specific role on resveratrol action and the contribution of central SIRT1. Bilateral cannulae were implanted by stereotaxic surgery into the MBH of SD male rats. After adequate postsurgical recovery, vehicle (5% DMSO, CTRL) or resveratrol (RSV, 200 μmol/L) was infused into the MBH of conscious rats during basal pancreatic insulin clamp studies (Fig. 2A). The resveratrol dose selected was used to achieve maximal SIRT1 activation as previously described (36). Central resveratrol treatment significantly decreased the basal plasma glucose and basal insulin levels (Fig. 2B, Supplementary Table 1). In the resveratrol-treated animals, the glucose infusion rate (GIR) required to prevent hypoglycemia was approximately fourfold higher compared with the control group (RSV: 5.0 ± 0.5 vs. CTRL: 1.3 ± 0.6 mg/kg/min; P < 0.005), whereas the endogenous glucose production was significantly lower (RSV: 5.9 ± 0.7 vs. CTRL: 11.1 ± 0.8 mg/kg/min; P < 0.005). Overall, resveratrol suppressed glucose production by 55 ± 5 versus 9 ± 5% in the control group. Peripheral glucose uptake was not different between the two treatment groups, as might be expected in studies performed under basal insulin conditions (Fig. 2C–F). Thus, central resveratrol modulates glucose homeostasis by markedly repressing glucose output from the liver.
FIG. 2.
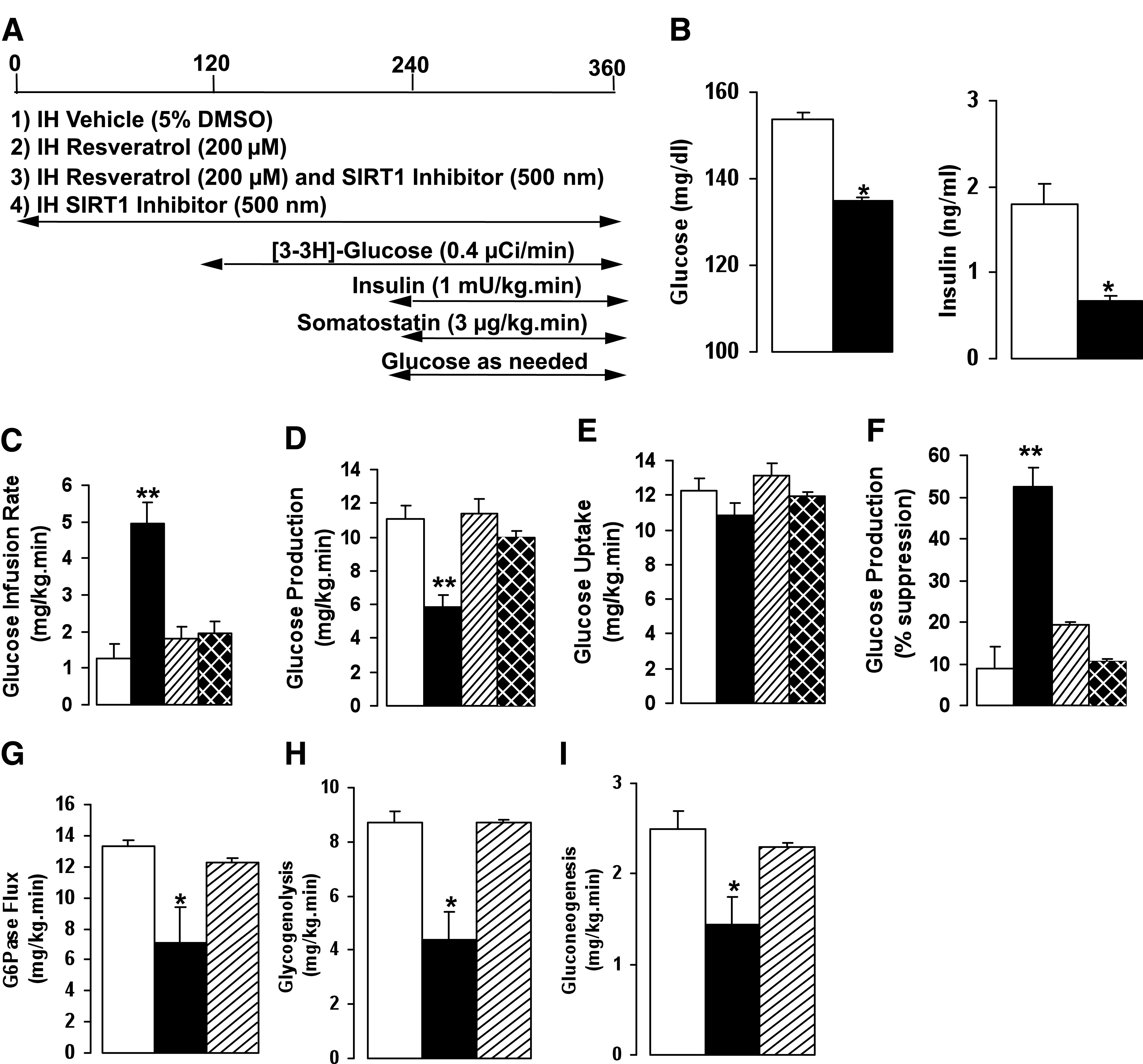
MBH infusion of resveratrol increases hepatic insulin sensitivity. A: Schematic representation of MBH infusions and insulin clamp protocol. B: Effect of MBH resveratrol on basal glucose and basal insulin levels. C–F: GIR, glucose production, and glucose uptake during basal pancreatic insulin clamp after treatment with vehicle, resveratrol, or SIRT1 inhibitor. G–I: Flux through G6Pase, glycogenolysis, and gluconeogenesis after resveratrol or SIRT1 inhibitor. All values are mean ± SEM. *P < 0.05, **P < 0.005 compared with control. For all studies: control, white bars; resveratrol, black bars; resveratrol and SIRT1 inhibitor, striped bars; SIRT1 inhibitor alone, hatched bars.
To further delineate the contribution of hypothalamic SIRT1 to resveratrol action, we used an SIRT1-specific cell permeable inhibitor (SI), 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide that has been shown to selectively decrease the catalytic activity of SIRT1 (37,38). Resveratrol (200 μmol/L) and the SIRT1 inhibitor (SI, 500 nmol/L) were coinfused into the MBH of conscious rats during pancreatic insulin clamp studies. The SIRT1 inhibitor significantly attenuated the effect of resveratrol on GIR compared with resveratrol treatment alone (RSV/SI: 1.8 ± 0.4 vs. RSV: 5.0 ± 0.5 mg/kg/min; P < 0.005). In addition, the effect of resveratrol on reducing hepatic glucose production was significantly negated (RSV/SI: 11.4 ± 0.9 vs. RSV: 5.9 ± 0.7 mg/kg/min; P < 0.005; Fig. 2C–F). Acute MBH infusion of the SIRT1 inhibitor alone showed no significant difference on hepatic glucose production compared with control (Fig. 2C–F). Thus, selective inhibition of central SIRT1 is sufficient to block the effect of central resveratrol on glucose production.
Flux through glucose-6-phosphatase (G6Pase) is the most distal step in hepatic glucose production, and this net flux is suppressed relative to any decrease in hepatic glucose production. Because the in vivo flux through G6Pase is through the net contribution of glucosyl units derived from gluconeogenesis and glycogenolysis, we have estimated their relative contribution to ascertain how resveratrol-induced activation of central SIRT1 affects hepatic glucose output. In animals treated with MBH resveratrol, the flux through G6Pase was significantly reduced, and this effect was markedly blunted with infusion of the SIRT1 inhibitor (Fig. 2G). Resveratrol significantly reduced the rate of glycogenolysis (RSV: 4.4 ± 1.1 vs. CTRL: 8.7 ± 0.4 mg/kg/min; P < 0.05), and gluconeogenesis also was decreased (RSV: 1.4 ± 0.3 vs. CTRL: 2.5 ± 0.2 mg/kg/min; P < 0.05). Coinfusion of the SIRT1 inhibitor reversed the effect of resveratrol on glycogenolysis and gluconeogenesis (Fig. 2H and I). Thus, acute activation of central SIRT1 by resveratrol suppresses glucose production through inhibition of glycogenolysis and to a lesser extent gluconeogenesis.
Recent reports have suggested that resveratrol’s actions may be mediated through not only SIRT1 but also other targets, such as AMPK (8,39,40). Resveratrol has been reported to activate AMPK in neuronal cell lines and in brain tissue in vivo (41). We assessed whether there was any change in AMPK activation (phosphorylation) in the hypothalamus after treatment with resveratrol. AICAR, a known AMPK activator, was used as a positive control. Overall, we found no evidence of AMPK activation in resveratrol-treated animals. However, enhanced AMPK activation was observed after AICAR treatment (Supplementary Fig. 1A). Thus, in our current study paradigm, we have no evidence of central AMPK activation after acute resveratrol administration.
A role for POMC and AgRP in mediating the effects of SIRT1 has been reported (24,25,42,43). We assessed the expression of these neuropeptides after acute resveratrol administration and found that the mRNA expression of POMC and AgRP was not significantly different compared with control (Supplementary Fig. 1B and C).
SIRT1 knockdown significantly reduced the effect of resveratrol on glucose production.
To provide confirmation that central SIRT1 is essential for resveratrol action, we used an shRNA to selectively decrease SIRT1 expression. In cell culture, SIRT1 silencing was verified to be at least 50% by Western blot analysis (Fig. 3A). Immunohistochemical analysis confirmed that hypothalamic injection of the SIRT1-shRNA in rats reduced SIRT1 expression in the MBH compared with control (Fig. 3B, Supplementary Fig. 2A–C). Lower-magnification photomicrographs further confirmed that SIRT1 silencing was restricted to the medial portion of the arcuate nucleus because regions rostral and caudal did not show any significant difference in SIRT1 expression between control and SIRT1-shRNA–treated groups (Supplementary Fig. 2D–I). In preparation for the clamp studies, control-shRNA or SIRT1-shRNA was injected into the MBH immediately after implantation of the hypothalamic cannulae. This was followed by vascular catheterization and clamp studies after 2 weeks (Fig. 3C and D). There were no significant differences between the baseline metabolic characteristics of the control-shRNA and the SIRT1-shRNA group (Supplementary Table 2). When animals treated with the control virus or SIRT1-shRNA was infused with vehicle (5% DMSO) during the clamp study, there was no significant change in hepatic glucose production or glucose uptake between the groups. Resveratrol treatment reduced hepatic glucose production in animals who received the control-shRNA. However, similar to the cell-permeable SIRT1 inhibitor, treatment with the SIRT1-shRNA significantly blocked the effect of resveratrol on hepatic glucose production, resulting in an approximately twofold reduction of the GIR (Fig. 3E–H). Treatment with the SIRT1-shRNA had no effect on AgRP expression, but a slight increase in POMC expression was observed (Supplementary Fig. 2J and K). Taken together, the data indicate that resveratrol mediates its effect on glucose production through an SIRT1-dependent pathway and that inhibition of central SIRT1 by using a cell-permeable inhibitor or shRNA silencing is sufficient to blunt the effect of resveratrol on hepatic glucose metabolism.
FIG. 3.
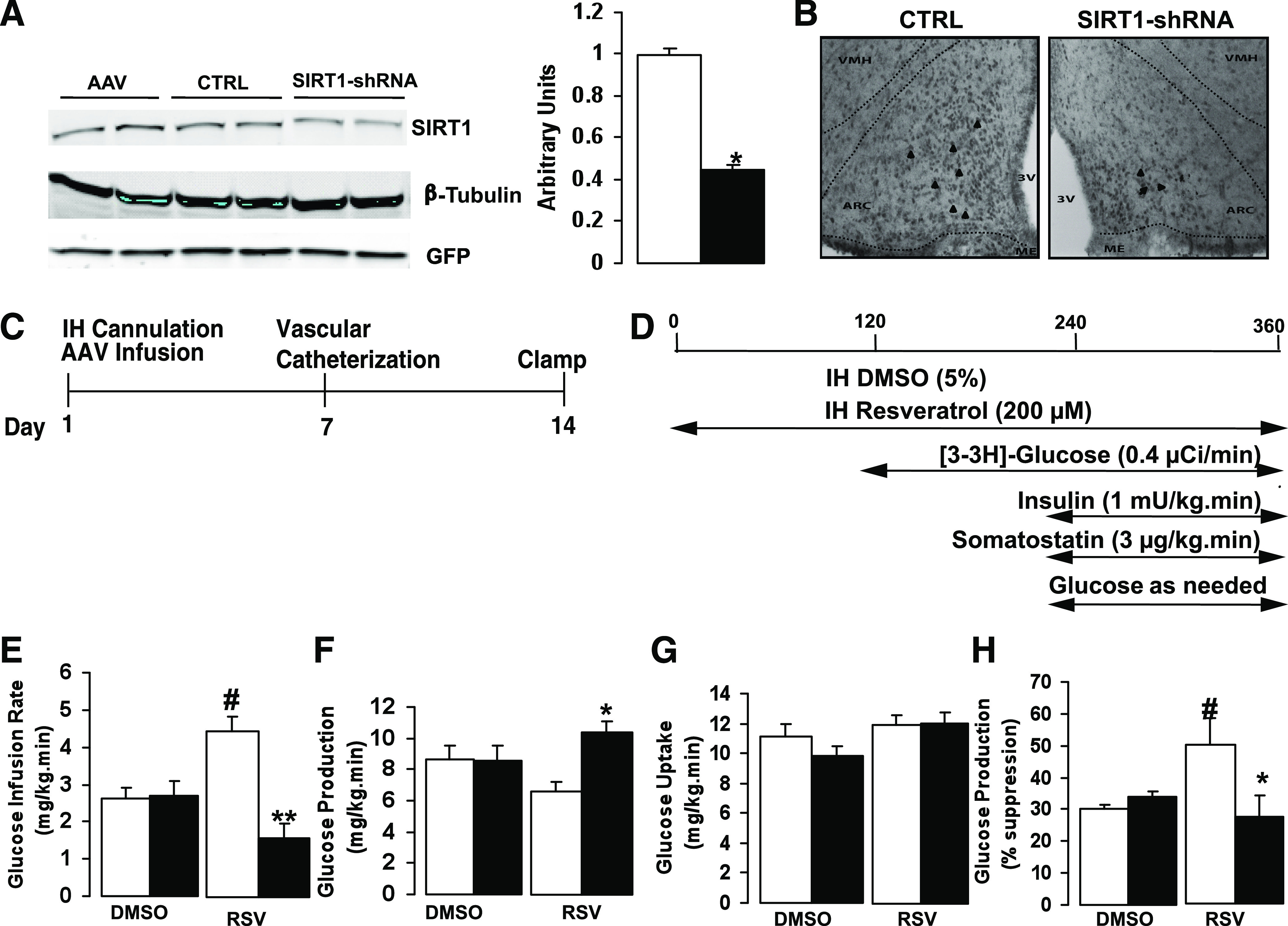
Effect of hypothalamic SIRT1 silencing on glucose production. A: Representative Western blot analysis of SIRT1 knockdown in HEK293 cells. SIRT1-shRNA decreases SIRT1 expression by ∼50% compared with control virus. B: Immunohistochemical staining of SIRT1 in the arcuate nucleus. C: Timeline of injections for viral constructs and animal preparation for the study. D: Schematic of MBH infusions and insulin clamp protocol. E–H: Effect of the SIRT1-shRNA on GIR, glucose production, and glucose uptake in animals treated with and without resveratrol. Experiments represent n = 6–8 per group. All values are mean ± SEM. *P < 0.05, **P < 0.005 SIRT1-shRNA compared with control-shRNA after resveratrol treatment; #P < 0.05 in control-shRNA group after resveratrol vs. DMSO treatment. For these studies: control-shRNA, white bars; SIRT1-shRNA, black bars. Arrowheads indicate SIRT1 immunoreactive cells. (A high-quality color representation of this figure is available in the online issue.)
Blockade of the hypothalamic KATP channel significantly reduced the effect of resveratrol.
Activation of the hypothalamic KATP channel has been shown to lower hepatic glucose production in response to hormonal stimuli, such as leptin, insulin, and nutritional substrates, whereas blockade of the KATP channel with sulfonylureas has the opposite effect (19,28,44). Thus, we asked whether resveratrol’s action on glucose production was dependent on KATP activation (Fig. 4A). SD male rats received MBH infusion of vehicle (5% DMSO), resveratrol (200 μmol/L), a coinfusion of resveratrol (200 μmol/L), and the KATP inhibitor glibenclamide (100 μmol/L) or glibenclamide alone (100 µmol/L) during basal pancreatic clamp study (Fig. 4B). We found that IH infusion of glibenclamide significantly reversed the effect of resveratrol on hepatic glucose production in a manner that was similar to what was observed with the SIRT1-specific inhibitor. However, glibenclamide alone had no appreciable effect compared with vehicle-treated controls (Fig. 4C–F). These findings suggest that the acute effects of resveratrol on hepatic glucose production are dependent on the activation of KATP channels in the MBH.
FIG. 4.
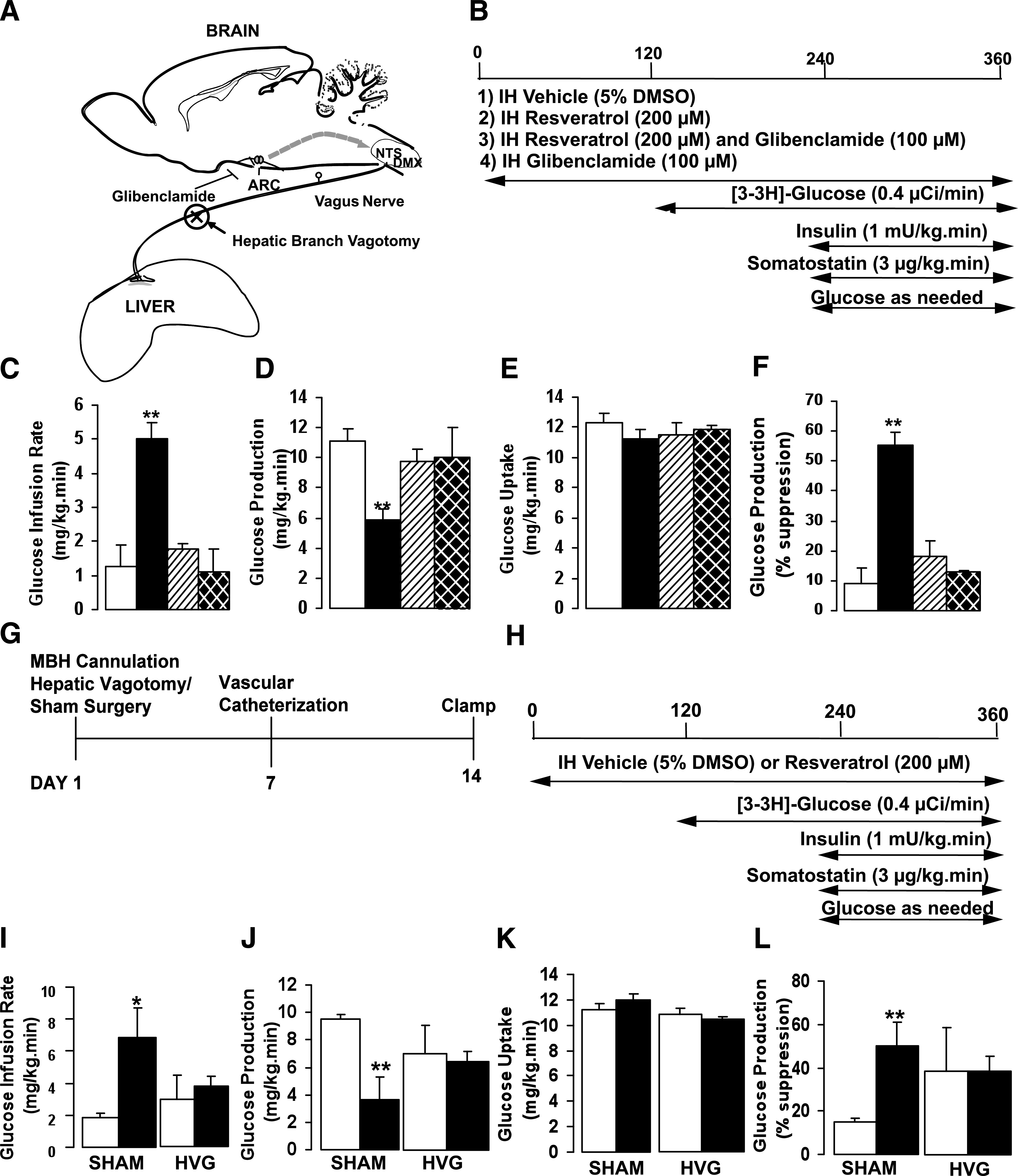
Hypothalamic KATP channel inhibition and hepatic branch vagotomy significantly reduce resveratrol action. A: Representative schematic of the sagittal section of the brain at the level of the arcuate nucleus. KATP channels in the arcuate nucleus have a role in regulating glucose production. Neural activity from the arcuate nucleus is transferred to the nucleus of solitary tract/dorsal motor vagal nucleus of the brain stem and subsequently relayed to the liver via the vagus nerve. B: Experimental protocol for resveratrol-glibenclamide studies. C–F: Central glibenclamide significantly attenuated the effect of resveratrol on hepatic glucose production. G and H: Timeline and basal insulin clamp protocol for hepatic vagotomy and sham studies. I–L: Effect of hepatic vagotomy or sham operation on GIR, glucose production, and glucose uptake after resveratrol treatment. For all studies n = 4–6 per group. All values are mean ± SEM. *P < 0.05, **P < 0.005 compared with control. ARC, arcuate nucleus; KATP, potassium sensitive ATP channel; NTS, nucleus of solitary tract; DMX, dorsal motor vagal nucleus; SHAM, sham-operated; HVG, hepatic vagotomy. For all studies: control, white bars; resveratrol, black bars; resveratrol and glibenclamide, striped bars; glibenclamide only, hatched bars.
Hepatic vagus nerve innervation is important for resveratrol action.
Previous work from our laboratory and others has shown that vagus nerve innervation is particularly important in modulating hepatic glucose production through central mechanisms (18,26,45). We investigated whether the acute effect of resveratrol on glucose production requires the vagus nerve. Before the clamp study, SD male rats received IH cannulae placement immediately followed by subdiaphragmatic hepatic branch vagotomy or sham surgery. The basal metabolic parameters between the sham and vagotomized groups were significant for a decrease in the corticosterone level in the vagotomy group (Supplementary Table 3). During the study, the vagotomized or sham-operated animals were treated with vehicle (5% DMSO) or resveratrol (200 μmol/L) (Fig. 4G and H). In the sham-operated animals, resveratrol significantly lowered hepatic glucose production compared with vehicle-treated control (RSV: 3.61 ± 0.85 vs. CTRL: 9.50 ± 0.33 mg/kg/min) with an overall percent suppression of ∼50% (Fig. 4I–L). However, in the vagotomized rats there was no significant difference in glucose production between the resveratrol-treated group and vehicle-treated controls (RSV: 6.71 ± 0.71 vs. CTRL: 7.3 ± 2.15 mg/kg/min). We then assessed the in vivo glucose flux and found that in the sham-operated animals resveratrol reduced the flux through G6Pase and markedly lowered gluconeogenesis and glycogenolysis compared with the vagotomized animals (Supplementary Fig. 3A–D). Thus, these findings indicate that vagus nerve innervation is required for mediating the effects of central resveratrol on hepatic glucose metabolism.
Resveratrol affects systemic insulin sensitivity by activating central SIRT1.
The previous studies have revealed the effect of hypothalamic resveratrol on glucose metabolism and the importance of central SIRT1. Recent reports have shown that chronic oral administration of resveratrol increases insulin sensitivity and reduces hyperglycemia (8–10). We investigated whether acute systemic infusion of resveratrol could recapitulate the metabolic findings of the chronic resveratrol studies and determined the involvement of central SIRT1 to the actions of systemic resveratrol. To this end, we infused vehicle (5% DMSO) or resveratrol (10 μmol/kg) systemically while vehicle (5% DMSO) or the SIRT1 inhibitor was infused IH into chronically catheterized SD male rats during pancreatic insulin-clamp studies (Fig. 5A). Systemic resveratrol significantly lowered plasma insulin levels, whereas basal glucose levels were unchanged (Fig. 5B). During the insulin clamp, the GIR required to maintain euglycemia was more than two times higher in the resveratrol-treated group compared with controls (RSV: 7.0 ± 0.7 vs. CTRL: 2.7 ± 0.6 mg/kg/min; P < 0.005). The need to infuse more glucose was mainly due to a reduction in hepatic glucose production in the resveratrol group compared with the vehicle-treated group (RSV: 5.3 ± 0.9 vs. CTRL: 9.6 ± 0.8 mg/kg/min; P < 0.05). Overall, the percent suppression of hepatic glucose production was 55 ± 5% with resveratrol treatment compared with 26 ± 6% with control. At the same time, no significant change was noted in peripheral glucose uptake between groups (Fig. 5C–F). These results indicate that acute systemic infusion of resveratrol is sufficient to increase insulin sensitivity. More important, after central inhibition of SIRT1, the effect of systemic resveratrol on glucose production was completely reversed.
FIG. 5.
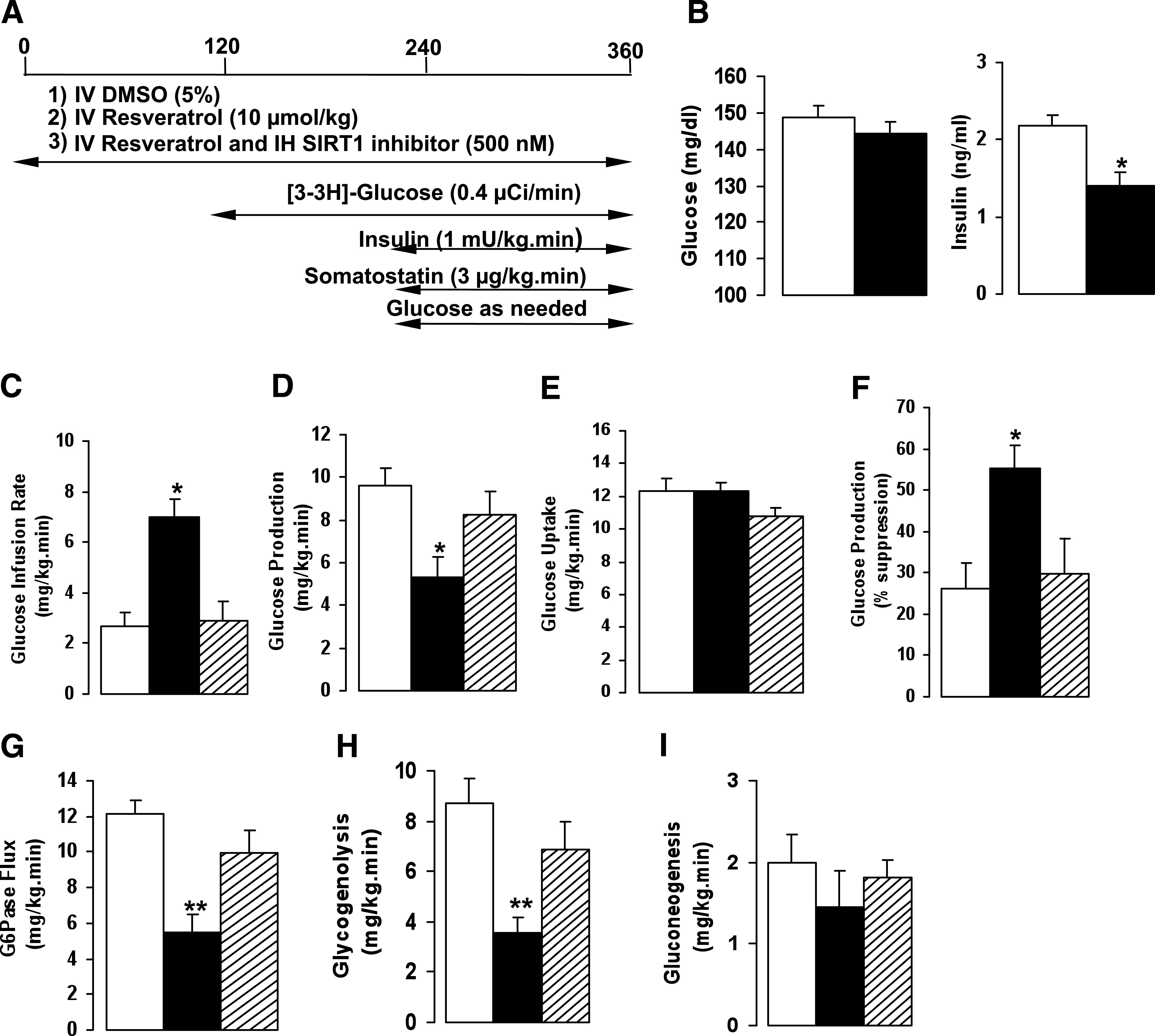
Central SIRT1 is required to regulate the effects of systemic resveratrol. A: Experimental protocol for basal insulin clamp. B: Effect of systemic resveratrol on plasma basal insulin and basal glucose levels. C–F: GIR, glucose production, or glucose uptake after systemic infusion of vehicle or resveratrol with or without IH SIRT1 inhibitor. G–I: Flux through G6Pase, glycogenolysis, and gluconeogenesis after treatment with vehicle, resveratrol, or IH SIRT1 inhibitor. All values are mean ± SEM. *P < 0.05, **P < 0.005 compared with control. For all studies: control, white bar; resveratrol, black bars; resveratrol and SIRT1 inhibitor, striped bars.
To determine whether systemic resveratrol regulates hepatic glucose output similar to that observed with central resveratrol, we determined the flux through G6Pase and the relative contributions of gluconeogenesis and glycogenolysis. As in central administration, systemic resveratrol causes a significant decrease in the G6Pase flux that was negated by central infusion of the SIRT1 inhibitor (Fig. 5G). As seen before with the hypothalamic studies, systemic resveratrol markedly reduced glycogenolysis and to a lesser extent gluconeogenesis. With the SIRT1 inhibitor in the hypothalamus, the reduction in glycogenolysis and gluconeogenesis seen with systemic resveratrol was abolished (Fig. 5H and I). The findings strongly suggest that the effect of systemic resveratrol on modulating glucose homeostasis is highly dependent on the activation of central SIRT1.
DISCUSSION
The deacetylase SIRT1 is emerging as a therapeutic target for the management of metabolic disorders. Several reports have demonstrated the role of SIRT1 and its activator resveratrol in the regulation of glucose metabolism (4,8,9,23). The studies presented reveal how acute administration of resveratrol directly to the MBH affects hepatic glucose production and insulin action and that MBH-specific inhibition of SIRT1 is sufficient to ablate these effects. Second, they also show that the systemic action of resveratrol is mediated via a central SIRT1-dependent pathway. Further, they reveal that resveratrol is dependent on the activation of the hypothalamic KATP channel for its effects on hepatic glucose production and that transection of the hepatic branch of the vagus nerve significantly attenuated the effect of central resveratrol on glucose production. Together, these findings reveal that resveratrol-induced activation of SIRT1 is important in mediating the regulation of glucose homeostasis and that these effects are primarily mediated through vagus nerve input to the liver.
Although it has been largely accepted that SIRT1 is a principal target of resveratrol, several reports have raised the question of whether the effects of resveratrol are due to its activation of SIRT1 (46). The studies presented in this report clearly demonstrate that pharmacologic or molecular inhibition of SIRT1 is sufficient to abolish the effect of resveratrol on glucose production and insulin action. These results are significant because they explicitly show that SIRT1 is required for the effect of resveratrol in modulating glucose homeostasis. A link between resveratrol action and the activation of AMPK in peripheral tissues has also been purported (8,9,40,47). Hypothalamic AMPK is a major nutrient sensor, and its activation is critical for maintaining glucose homeostasis and energy balance (48). Our studies found no evidence of AMPK activation after MBH resveratrol infusion. These results are similar to those reported in a recent study indicating that intracerebroventricular resveratrol treatment over several weeks did not alter AMPK activity in mice (23). Thus, it seems that central AMPK does not have a significant role on the effect of MBH resveratrol on glucose homeostasis.
Central or systemic administration of resveratrol lowered glucose production by decreasing hepatic glycogenolysis and gluconeogenesis. This effect was ablated with SIRT1 inhibition. It is interesting to note the marked suppression of glycogenolysis compared with gluconeogenesis after acute resveratrol treatment. This finding suggests that resveratrol has a potent inhibitory effect on hepatic glucose fluxes to reduce glucose output. A recent study has shown that selective activation of SIRT1 reduces gluconeogenesis in an insulin-resistant rodent model, thereby preventing hyperglycemia (14). However, there are other studies indicating that activation of SIRT1 enhances gluconeogenesis, whereas decreased expression of liver-specific SIRT1 reduces gluconeogenesis and therefore reduces glucose production (49,50). It is not immediately clear how to reconcile these different outcomes, and further work may be needed to determine whether there is a differential mechanism involved. Most of the available evidence, including that presented in this article, suggests that activation of SIRT1 has a significant effect on improving insulin sensitivity and glucose handling.
The effect of MBH resveratrol on glucose production raised pertinent questions regarding which hypothalamic neural pathway was being activated and how the message was being conveyed to the liver. Previous studies have shown that the hypothalamic KATP channel is adept at registering metabolic signals from multiple sources, including hormonal and nutrient substrates, and eventually relaying them to the liver. Our studies show that blockade of the KATP channel in the MBH is sufficient to disrupt the action of resveratrol on glucose production. Thus, activation of the KATP channel is necessary for resveratrol action. In the same way, selective hepatic vagotomy significantly reduced the effect of central resveratrol on glucose production, suggesting that vagal innervation is required for the regulation of glucose homeostasis by resveratrol. These findings emphasize the importance of the neural pathway(s) that link resveratrol action in the brain to the regulation of glucose production in the liver. Most important, these studies are the first to identify a neural mediated pathway to explain how MBH resveratrol affects hepatic glucose homeostasis.
In summary, our findings highlight the importance of the arcuate nucleus as a major site of resveratrol action in the regulation of glucose homeostasis. Further, they also provide support for the concept that central SIRT1 is required for resveratrol’s action. Taken together, these studies have emphasized that acute MBH administration of resveratrol has a significant effect on reducing hepatic glucose production and increasing insulin sensitivity. A thorough understanding of the central effect of resveratrol on glucose production and insulin action may provide a link to further understand how this pharmacologic agent favorably conveys many of the beneficial metabolic effects of caloric restriction and reduced energy states. Most important, these studies show that SIRT1 activators have therapeutic potential for use in the management of metabolic disorders.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (DK-45024 to R.G.-J., K08-AG-033098 to C.M.K., and R01-AG-18381, T32-AG-23475, and P01-AG-021654 to N.B.) and the Core Laboratories of the Albert Einstein Diabetes Research and Training Center (P60-DK 20541).
No potential conflicts of interest relevant to this article were reported.
C.M.K. and R.G.-J. designed experiments, researched data, and wrote and edited the manuscript. T.K.T.L. researched data and reviewed the manuscript. I.A.-C. researched data and reviewed the manuscript. L.H. researched data and reviewed the manuscript. G.S. researched data and reviewed the manuscript. N.B. researched data and reviewed and edited the manuscript. L.R. designed experiments and reviewed the manuscript.
The authors thank Bing Liu (Albert Einstein College of Medicine) and Xiaosong Li (Albert Einstein College of Medicine) for surgical expertise; Hong Zhang (Albert Einstein College of Medicine), Yuhua Wang (Albert Einstein College of Medicine), and Ya Su (Albert Einstein College of Medicine) for assistance with clamp studies; and Clive Baveghems (Albert Einstein College of Medicine) and Stanislaw Gaweda (Albert Einstein College of Medicine) for expert technical assistance with protein analysis and glucose fluxes.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0987/-/DC1.
L.R. is currently affiliated with Merck Research Laboratories, Rahway, New Jersey.
REFERENCES
- 1.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 2007;404:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390–392 [DOI] [PubMed] [Google Scholar]
- 3.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 2004;306:2105–2108 [DOI] [PubMed] [Google Scholar]
- 4.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 2008;105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007;6:759–767 [DOI] [PubMed] [Google Scholar]
- 7.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2005;2:105–117 [DOI] [PubMed] [Google Scholar]
- 8.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 10.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 2006;290:E1339–E1346 [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 2007;26:3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int 2009;54:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 2009;159:993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007;450:712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008;8:347–358 [DOI] [PubMed] [Google Scholar]
- 16.Yoshizaki T, Schenk S, Imamura T, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 2009;298:E419–E428 [DOI] [PMC free article] [PubMed]
- 17.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–275 [DOI] [PubMed] [Google Scholar]
- 18.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 [DOI] [PubMed] [Google Scholar]
- 19.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 20.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2005;2:411–420 [DOI] [PubMed] [Google Scholar]
- 21.Könner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 [DOI] [PubMed] [Google Scholar]
- 22.Ramadori G, Lee CE, Bookout AL, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 2008;28:9989–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009;150:5326–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE 2009;4:e8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki T, Kim HJ, Kobayashi M, et al. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology 2010;151:2556–2566 [DOI] [PubMed] [Google Scholar]
- 26.Lam TK, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 2005;11:320–327 [DOI] [PubMed] [Google Scholar]
- 27.Ono H, Pocai A, Wang Y, et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 2008;118:2959–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 [DOI] [PubMed] [Google Scholar]
- 29.Muse ED, Lam TK, Scherer PE, Rossetti L. Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest 2007;117:1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barzilai N, Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem 1993;268:25019–25025 [PubMed] [Google Scholar]
- 31.Rossetti L, Giaccari A, Barzilai N, Howard K, Sebel G, Hu M. Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats. Implications for the pathophysiology of fasting hyperglycemia in diabetes. J Clin Invest 1993;92:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez-Juárez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem 2004;279:49704–49715 [DOI] [PubMed] [Google Scholar]
- 33.Arrieta I, Díaz-Ibáñez LB, Morales T, et al. Progesterone receptor gene and protein expression in the anterior preoptic area and hypothalamus of defeminized rats. J Neurobiol 2003;56:338–346 [DOI] [PubMed] [Google Scholar]
- 34.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 2003;9:756–761 [DOI] [PubMed] [Google Scholar]
- 35.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006;9:227–233 [DOI] [PubMed] [Google Scholar]
- 36.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196 [DOI] [PubMed] [Google Scholar]
- 37.Napper AD, Hixon J, McDonagh T, et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem 2005;48:8045–8054 [DOI] [PubMed] [Google Scholar]
- 38.Solomon JM, Pasupuleti R, Xu L, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol 2006;26:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zang M, Xu S, Maitland-Toolan KA, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006;55:2180–2191 [DOI] [PubMed] [Google Scholar]
- 40.Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010;59:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A 2007;104:7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 2010;12:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich MO, Antunes C, Geliang G, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci 2010;30:11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997;390:521–525 [DOI] [PubMed] [Google Scholar]
- 45.Matsuhisa M, Yamasaki Y, Shiba Y, et al. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism 2000;49:11–16 [DOI] [PubMed] [Google Scholar]
- 46.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 2010;285:8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 49.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A 2007;104:12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erion DM, Yonemitsu S, Nie Y, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A 2009;106:11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]


