Abstract
Patients with chronic ulcerative colitis (UC) are at high risk for developing colorectal cancer. In this study, archival formalin-fixed paraffin-embedded colonic tissue from patients with UC who developed carcinoma (CA) or high-grade dysplasia (HGD) was examined for changes in expression of the proinflammatory and mitogenic neurokinin-1 receptor (NK-1R). Laser capture microscopy was used to microdissect epithelia from areas of colons that showed histologic evidence of CA, HGD, and epithelia that were not dysplastic or cancerous but did contain evidence of prior inflammation (quiescent colitis). mRNA was extracted from the dissected tissue, and PCR array analysis was performed on extracted mRNA. Two antibodies were necessary to separately estimate the protein levels of the truncated (tr-NK-1R) and full-length (fl-NK-1R) receptors by immunohistochemistry. mRNA expression of tr-NK-1R increased 14-fold (P = 0.02) when comparing the HGD and CA groups. In contrast, the fl-NK-1R transcript showed no significant differences among groups. The protein levels of the total NK-1R increased by 40% (P = 0.02) in HGD and 80% (P = 0.0007) in CA compared with quiescent colitis. There were no significant changes in protein levels of the fl-NK-1R. We conclude that the increase in total NK-1R protein in HGD and CA is attributable to an increase in tr-NK-1R, suggesting there may be a functional role for tr-NK-1R in malignant transformation in colitis-associated cancer. The tr-NK-1R could prove useful as a diagnostic marker to identify patients at risk for neoplasia and may serve as a useful therapeutic target in the treatment of colitis-associated cancer.
Keywords: colon cancer, receptor splice variants, substance P, IBD
Chronic inflammation is associated with a higher rate of cancer development in various organs (1). More specifically, patients with ulcerative colitis (UC) are at high risk for developing colorectal cancer (2); both extent and duration of intestinal inflammation further increase risk (3, 4). These associations suggest that chronic intestinal inflammation is a causative factor in carcinogenesis in patients with UC, and that there is a causal link between chronic inflammation and increased risk of cancer. These observations raise the question of the signaling pathways by which long-standing inflammation might underlie the development of cancer.
Substance P (SP) is a proinflammatory neuropeptide described by von Euler and Gaddum (5) and later isolated and characterized by Chang and Leeman (6). SP is synthesized by a variety of cells, most notably neurons and inflammatory cells such as macrophages (7). SP's primary receptor, the neurokinin-1 receptor (NK-1R), can mediate a variety of physiologic and pathophysiologic responses, including cell proliferation, migration, and inflammation (8). The NK-1R has been identified in epithelial cells in rodent models of colonic inflammation (9, 10) and in patients with UC as well as Crohn disease (11–14). However, not all of these studies are in agreement regarding epithelial NK-1R expression in UC: some studies have shown an increase (12, 13), whereas others have not (11, 14). The NK-1R has been identified in many cancers, including skin, gastrointestinal, laryngeal, pancreatic, ovarian, brain, thyroid, and breast cancers (15–19). NK-1R has also been shown to mediate the antitumor activities of NK-1R antagonists in cell lines derived from melanoma, gastric, colon, and laryngeal cancers (15, 17, 20). There is also evidence from a rat model of colitis-associated cancer (CAC) suggesting that NK-1R may play a functional role in the disease; treatment with an NK-1R antagonist reduced cyclooxygenase-2 levels and reduced expression of the proliferation marker ki67 (21). It is important to note that, although many studies have shown the presence of NK-1R in both UC and various cancers, few have attempted to differentiate between NK-1R isoforms.
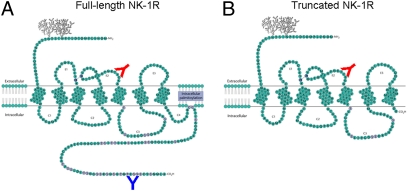
Kage et al. (22) showed that NK-1R, a G protein-coupled receptor (GPCR), has two isoforms that arise from the same TACR1 gene: a full-length form containing 407 amino acids (Fig. 1A) and a truncated form containing 311 amino acids that arises from a splice variant and lacks a cytoplasmic C-terminal tail (Fig. 1B) (23). The truncated isoform of NK-1R has received little attention in the study of cancer; however, recent evidence does suggest that it may play a role in breast cancer. tr-NK-1R is present in breast cancer tissue (24), and transfection of truncated but not full-length NK-1R into nontumorigenic breast epithelial cell lines creates a transformed phenotype with greatly increased growth capability (25). Signaling studies have shown that unlike fl-NK-1R, tr-NK-1R can neither initiate calcium influx via Gq, nor activate PKC or NF-κB; however, ERK activation still occurs but at a slower rate (26). In light of these findings, we were particularly interested in examining the expression of tr-NK-1R in the transition from colonic inflammation to CAC.
Fig. 1.
Schematic models of the full-length (A) and truncated (B) isoforms of the neurokinin-1 receptor. The truncated isoform lacks the majority of the intracellular C terminus as a result of alternative splicing. Predicted binding sites of two antibodies to NK-1R used in this study, one (red antibody) targeting an internal epitope on the second extracellular loop of the receptor and one (blue antibody) targeting a C-terminal epitope on the cytoplasmic tail of the receptor. Both antibodies are capable of binding the full-length receptor (A), whereas only the antibody targeting the second extracellular loop can bind the truncated receptor (B). [(A) Reproduced with permission from ref. 49 (Copyright 2010, Wiley); (B) modified in our laboratory.]
Here, we report on a group of patients who underwent a total colectomy for CAC. Because each colon contained tissues in various stages of the transition from quiescent disease to neoplasia, we were able to study not only carcinoma but also the precancerous stage of dysplasia (Fig. 2). The premalignant dysplastic stage is a useful marker for early neoplastic changes that could represent targets for early detection and treatment of CAC. The use of laser capture microdissection of archival formalin-fixed paraffin-embedded (FFPE) tissue allowed us to specifically isolate the affected epithelial cells to ensure that our results are specific to the diseased tissue. Our approach permitted a powerful means to examine changes in the full-length and truncated isoforms of NK-1R in patients with chronic intestinal inflammation, dysplasia, and CAC.
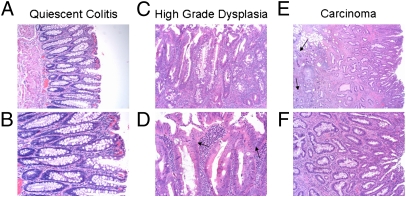
Fig. 2.
H&E stained sections from patients with CAC. Bright-field photomicrographs from a colon section displaying quiescent epithelium at (A) 100× magnification and (B) 200× magnification showing elongated and branched crypts typical of reparative processes after inflammation; HGD at (C) 100× and (D) 200× magnification; and CA at (E) 40× and (F) 100× magnification. In HGD, note the increased size and disorder of the nuclei (D, indicated by arrows) as well as a general disorder compared with quiescent epithelium. In CA, note as well the invasive pseudocrypts that extend beyond the mucosal membrane (E, indicated by arrows).
Results
mRNA Expression of Truncated TACR1 Is Increased Between High-Grade Dysplasia and Carcinoma Groups.
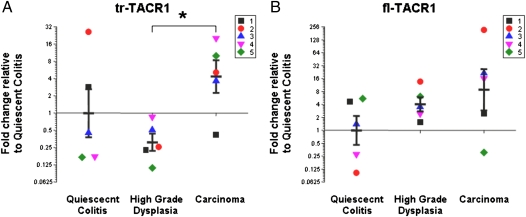
The mRNA expression of tr-TACR1 transcript is increased 14-fold (P = 0.02) when comparing the high-grade dysplasia (HGD) and CA groups (Fig. 3A). No significant differences were observed when comparing the two neoplastic groups to epithelium that was not dysplastic or cancerous but did contain evidence of prior inflammation (quiescent colitis, QC). In comparison, the fl-TACR1 transcript showed no significant differences among the groups (Fig. 3B; QC vs. CA, P = 0.30; QC vs. HGD, P = 0.23; HGD vs. CA, P = 0.49). The gene for the TACR1 ligand SP (TAC1) was also measured on the array, but no amplification was observed. Other negative results are included in Table S1.
Fig. 3.
(A) Increased mRNA expression of tr-TACR1 gene comparing high-grade dysplasia to carcinoma expressed as a fold-change compared with quiescent colitis (n = 5 for each group). (B) No significant change in the mRNA expression of fl-TACR1 gene (n = 5 for each group). Each graph contains individual observations (each patient is a distinct color/shape) and mean ± SEM. Results were calculated using the ΔΔCt method and expressed as a fold change of the quiescent colitis group as described in the RT2 Profiler PCR Array Data Analysis (http://www.sabiosciences.com/pcrarraydataanalysis.php). *P < 0.05 compared with high-grade dysplasia.
Protein Levels of Truncated NK-1R Are Increased in High-Grade Dysplasia and Carcinoma.
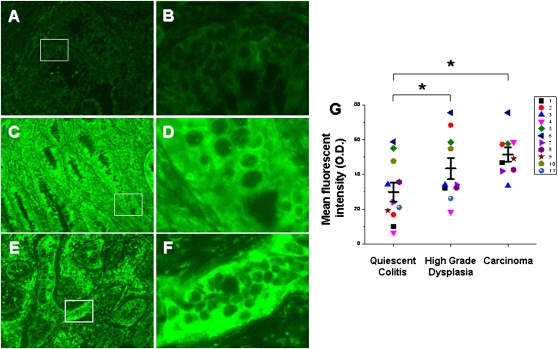
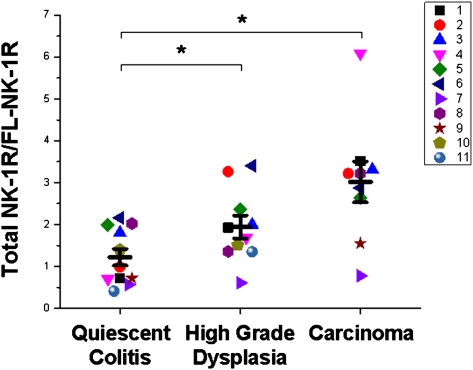
Two antibodies that bind NK-1R were used in this analysis; one binds the second extracellular loop and can therefore bind both receptor isoforms, and the other binds the cytoplasmic C-terminal tail and can bind only fl-NK-1R. The antibody that binds the second extracellular loop showed an increase in immunofluorescence among groups (P = 0.002). Multiple mean comparisons show that the protein levels of total NK-1R are increased by 40% (P = 0.02) in HGD and by 80% (P = 0.0007) in CA compared with QC (Fig. 4). The antibody that binds the C-terminal epitope showed no change in immunofluorescence among groups (Fig. 5), indicating no change in the protein levels of fl-NK-1R. We therefore conclude that the increase in total NK-1R protein in HGD and CA is attributable to an increase in tr-NK-1R. To further clarify these data, ratios were calculated from the total and full-length NK-1R immunofluorescent signals (Fig. 6; n = 11 QC, n = 10 HGD, n = 9 CA), which show a 60% increase from QC to HGD (P = 0.025) and a 150% increase from QC to CA (P = 0.001). This finding clearly demonstrates a significant increase in tr-NK-1R as epithelia transition to malignancy.
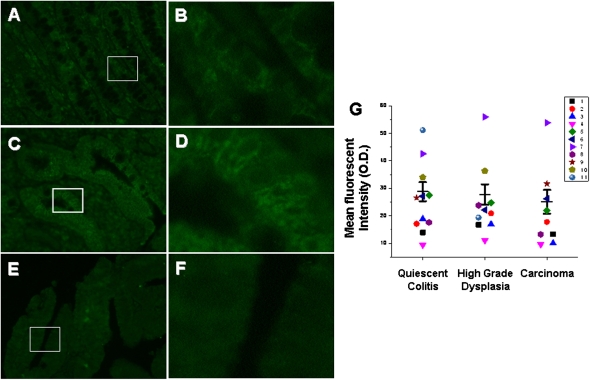
Fig. 4.
Increased amount of total NK-1R protein in epithelia from carcinoma and high-grade dysplasia compared with quiescent colitis. Representative images (A–F) of immunofluorescent staining using an antibody targeting an internal epitope of NK-1R. Quiescent epithelium (A, 40×; B, 200×), high-grade dysplasia (C, 40×; D, 200×), and carcinoma (E, 40×; F, 200×). Exposure time = 250 ms. (G) Quantification of mean fluorescent intensities with individual observations (a patient is a distinct color/shape) and means ± SE (n = 11 QC, n = 10 HGD, n = 9 CA). Each individual observation represents the average fluorescent intensity from five randomly selected fields. *P < 0.05 compared with quiescent colitis.
Fig. 5.
No change in full-length NK-1R protein among groups. Representative images (A–F) of immunofluorescent staining using an antibody targeting the C-terminal epitope of NK-1R. Quiescent epithelium (A, 40×; B, 200×), high-grade dysplasia (C, 40×; D, 200×), and carcinoma (E, 40×; F, 200×). Exposure time = 500 ms. (G) Quantification of mean fluorescent intensities with individual observations (each patient is a distinct color/shape) and mean ± SE (n = 11 QC, n = 10 HGD, n = 9 CA). Each individual observation represents the average fluorescent intensity from five randomly selected fields.
Fig. 6.
Increased ratio of total NK-1R to full-length NK-1R in epithelia from carcinoma and high-grade dysplasia compared with quiescent colitis. For each observation, the mean fluorescent intensity for the total NK-1R was divided by the mean fluorescent intensity for the fl-NK-1R. Each individual patient is represented by a distinct color/shape pair, and means ± SEM are plotted. n = 11 QC, n = 10 HGD, n = 9 CA. *P < 0.05 compared with quiescent colitis.
Discussion
This study examined the expression of tr-NK-1R in CAC in humans. Using a unique group of UC patients in which quiescent colitis, high-grade dysplasia, and carcinoma were all present in the same colectomy specimen allowed us to demonstrate that there are significant increases in the truncated but not the full-length isoform of the NK-1R as colonic epithelia progresses from a nonneoplastic, quiescent stage to dysplasia and carcinoma. By using laser capture microdissection to specifically isolate epithelia, we showed an increase in truncated but not full-length NK-1R mRNA between high-grade dysplasia and carcinoma. Using immunohistochemistry, we show an increase in truncated but not full-length NK-1R protein between nonneoplastic, quiescent epithelium and both high-grade dysplasia and carcinoma. These results suggest that the increase in protein does not appear to be merely due to increased transcription of tr-TACR1 splice variant. At this time, the mechanisms that surround the tr-NK-1R protein increase remain unclear. The finding that tr-NK-1R is increased in CAC is significant because it may provide clues to plausible mechanisms that explain how chronic inflammation could initiate carcinogenesis. There is evidence that expression of tr-NK-1R can be increased in response to an inflammatory signal, can respond to that inflammatory signal, and promote oncogenesis. Increased expression of the receptor could therefore represent a cancer-promoting adaptation that creates or augments a growth stimulus from an inflammatory signal.
Although few studies have examined the downstream signaling pathways of the truncated receptor, an important question raised by this study is how the increased expression of tr-NK-1R might promote carcinogenesis in a setting of chronic inflammation. One possible answer comes from a rat model of CAC. Treatment with an NK-1R antagonist reduced cyclooxygenase-2 expression as well as expression of the proliferation marker ki67 (21), showing a potential functional role for NK-1R (although not necessarily tr-NK-1R) in CAC. In addition, Lai et al. (26) showed that upon ligand binding, tr-NK-1R, which lacks a C-terminal tail that binds and activates Gq, cannot initiate calcium influx or activate PKC and NF-κB; however, the truncated receptor can activate ERK in a delayed manner. The tr-NK-1R has also been reported to be resistant to desensitization (27, 28). Not only does the truncated receptor lack C-terminal residues that are important for desensitization, it is also incapable of activating PKC (26), a critical component of the feedback inhibition loop that causes desensitization of the full-length receptor (28). Because tr-NK-1R is desensitization resistant, its constitutive activation suggests that it is capable of providing a persistent growth stimulus to a cancer cell, perhaps in an ERK- and cyclooxygenase-2–dependent manner.
This study also raises the question of the source of the ligand for tr-NK-1R. Its primary ligand is SP, but other members of the tachykinin family can also activate NK-1R, including hemokinin-1 at equimolar affinity to SP (29, 30), and neurokinins A and B at lower affinity (31). There are several potential sources of SP that could activate tr-NK-1R in CAC. First, SP may be produced by the neoplasia to create an autocrine growth stimulus. In another inflammatory condition, oral lichen planus, chronic inflammation is capable of increasing the expression of SP in epithelial cells (32). In this study, however, mRNA expression of the TAC1 gene, which gives rise to β-preprotachykinin-A, the peptide that is cleaved to form SP and other neuropeptides, was undetectable. Based on these data, there does not seem to be an autocrine growth stimulus; however, because we were measuring mRNA from microdissected tissue from FFPE samples, we cannot rule out the possibility that we were below the limits of detection for that transcript. A second source of SP is the enteric nervous system. Enteric nerves project into the mucosa of the colon in close proximity to the epithelium. SP can be released by these nerves in response to an inflammatory stimulus (33), and studies have shown that the number of enteric nerves staining immunopositive for SP is increased in inflamed tissue from UC patients (34). A third source of SP is macrophages, which are increased in the mucosa of the colons of UC patients (35), and can produce SP when stimulated (7). Expression of both TAC1 and TAC4 (encoding hemokinin-1) genes is increased in the mucosa of UC patients compared with normal controls (36). The chronic inflammation associated with UC is therefore capable of not only providing the means to increase the expression of tr-NK-1R, but also the ligand to activate the receptor.
What little information there is on the specific expression of the truncated receptor suggests that it can be increased by inflammatory signaling. A study by Ramkissoon et al. (24) showed that the expression of tr-NK-1R is dependent upon the activation of the proinflammatory transcription factor NF-κB in breast cancer cell lines in vitro. NF-κB is activated by a number of inflammatory signals present in UC. Furthermore, studies from both human UC tissue (37) and animal models of colitis (38) show that the activation of NF-κB occurs in the disease, both in the epithelium and the mucosa, and is important to its pathogenesis. Based on these data, there exist signals that could increase the expression of tr-NK-1R in the milieu of chronic intestinal inflammation.
UC is characterized not only by chronic relapsing inflammation, but also by mucosal hypoxia caused by that inflammation (39), which represents another potential mechanism by which tr-NK-1R expression may be increased. It has been shown that patients with UC have increased expression of the hypoxia-sensitive transcription factors hypoxia-inducible factor-1α (HIF-1α) and HIF-2α (40), which remain elevated even in periods of remission, suggesting that hypoxia can persist during quiescent stages of the disease (41). In response to the hypoxia, inflamed mucosa have increased vascularity compared with normal tissue, potentially due to increased vascular endothelial growth factor, an HIF-1α downstream target (40). The mucosal hypoxia characteristic of UC is interesting in light of research showing hypoxia can activate NF-κB (42, 43), which is capable of specifically increasing expression of tr-NK-1R (24). In addition, hypoxia has been shown to induce alternative splicing events in other genes in vitro, and these splice variants are also associated with mutations to the tumor suppressor p53, a common finding in CAC (44). Evidence therefore suggests that both proinflammatory and hypoxic signaling may work together to increase the expression of tr-NK-1R in UC and CAC.
Alternative splicing of GPCRs has been observed in several cancers (reviewed in ref. 45), although these observations are not always coupled with evidence that splice variants contribute to malignancy. Recently, decreased transcriptome stability has been correlated with changes in expression of spliceosome components and poor patient survival in sporadic colon cancer (46). These findings suggest that increased splice variant expression may play an important role in carcinogenesis, which is relevant to our finding that tr-NK-1R increases in the transition from QC, nonneoplastic epithelia to dysplasia and carcinoma. In addition, factors that mediate the splicing of NK-1R may be targets for pharmacological intervention in CAC.
Though the colon specimens used in this study were from patients that had UC confirmed histologically by a pathologist, the samples were obtained anonymously. We therefore do not have access to information such as extent and duration of disease or details of the patient's history. This lack of information is a potential confounding factor that may have influenced the study; however, by including only patients that had areas of quiescent disease and nonneoplastic epithelium, and by using patient-matched samples, we believe we have controlled for this potential variability. In addition, the finding of no correlation between tr-TACR1 mRNA levels in QC lesions and later lesions was surprising. We believe that there are several explanations. First, we suggest the possibility that a larger study may have shown significance between the QC lesion and the CA lesion. Second, the QC lesion may contain a different set of alterations than the HGD or CA lesions, and the relationship between each individual gene may not be as straightforward as a pathologic progression of QC → HGD → CA might lead us to believe. However, most importantly, statistical significance is achieved between the HGD and CA groups, and this led us to examine changes in the truncated and full-length NK-1R protein levels.
The tr-NK-1R or its downstream signaling pathways could prove useful as diagnostic markers to assist in identification of patients with neoplasia. Also significant to this finding is the plan to treat inflammatory bowel disease patients with mesenchymal stem cells. Though these stem cells are immunosuppressive, they can also promote cancer. In the presence of early transformed cells, which may be detectable using tr-NK-1R as a marker, these stem cells could have deleterious effects. The tr-NK-1R could therefore serve to segregate patients into groups that should or should not receive stem cell treatment. Diminishing expression or activity of the truncated NK-1R may be a useful therapeutic strategy to prevent dysplasia from progressing to carcinoma, or for treating CAC directly. Overall, our findings are of such interesting biological relevance that they deserve to be validated in a clinically oriented study.
Methods
Sample Collection.
Cases were identified from patients with long-standing UC that underwent total colectomy at Boston University Medical Center by a single surgeon (J.M.B.). Cases were selected if the diagnosis was reconfirmed histologically by two independent gastrointestinal pathologists (M.J.O. and S.R.C.) and if each colon contained the following three stages of CAC in the same specimen: CA, HGD, and QC. Histologically, QC is the most abundant nonactively inflamed tissue in the UC colon and was therefore chosen as a reference group for the other two groups because it had been subjected to the same inflammatory milieu as the neoplastic groups but did not undergo neoplastic transformation. The advantage of obtaining all three groups from the same patient was that it controlled for multiple differences in genetic background, disease duration, severity, and history as well as environmental factors and therapeutic interventions. Additional cases were selected for immunohistochemistry (IHC) if there were blocks containing two of the three classifications. Representative images of these stages and their histologic descriptions are shown in Fig. 2.
Laser Capture Microscopy, RNA Extraction, Isolation, and Purification.
Successful RNA extraction from microdissected FFPE samples has been shown to be feasible (47) and capable of producing results similar to frozen fixed tissue, although RNA quantity and quality are reduced (48). Five patients were selected with archival FFPE blocks from each of the three stages (CA, HGD, and QC) cut to 7 μm and mounted on glass slides. One slide was microdissected for each patient/group. Slides were rehydrated, stained with Paradise Plus stain, and dehydrated. Multiple crypts were then microdissected (Fig. S1) by laser capture microscopy (Arcturus Pixcell 2e; Molecular Diagnostics, Inc.) using CapSure HS LCM Caps (LCM0214; Molecular Diagnostics, Inc.). RNA was extracted from the microdissected epithelial cells according to the manufacturer's protocol (Paradise Plus Kit; Molecular Diagnostics, Inc.). Briefly, microdissected samples were digested overnight with proteinase K and nucleic acids were bound to a column while other cellular material was washed through and DNA was digested on-column. Purified RNA was then reverse transcribed, preamplified, and run on a custom PCR array (Qiagen). No genomic DNA was detected using controls on the PCR array.
PCR Array.
Custom PCR arrays (Qiagen) containing primers for the full-length and truncated TACR1 transcripts were used. Results were normalized to one housekeeping control, tyrosine-3-monooxygenase/tryptophan-5-monoxygenase activation protein, zeta peptide that did not change between groups. Wells without amplification were assigned a cycle threshold (Ct) value of 40. Results were calculated using the ΔΔCt method and expressed as a fold change of the quiescent colitis group as described in the RT2 Profiler PCR Array Data Analysis (http://www.sabiosciences.com/pcrarraydataanalysis.php). Statistical comparisons between groups were performed using Bioconductor's R statistical program (HTqPCR Bioconductor package; http://www.bioconductor.org/). All data passed normality and equivariance tests. Results for a given gene were disregarded if multiple wells did not show amplification.
Immunohistochemistry.
Archived FFPE samples (n = 11 QC, n = 10 HGD, n = 9 CA) described above were cut to 5 μm, mounted on glass slides, deparaffinized, and rehydrated. To facilitate maximal antibody binding, antigen retrieval was performed using a BioGenex EZ-Retriever and CITRA-PLUS buffer (BioGenex) heated to 98 °C for 15 min. Slides were incubated with 10% goat serum in PBS and then probed with two primary antibodies for NK-1R (Fig. 1): one that binds an epitope on the second extracellular loop and one that binds an epitope on the C terminus (sc-14115 and sc-14116; Santa Cruz Biotechnology). Specificity of both antibodies was validated by probing a subset of samples with and without a blocking peptide (Fig. S2). Samples were then probed with a fluorescein-labeled secondary antibody (31509; Thermo Fisher Scientific), mounted (VECTASHIELD mounting medium; Vector Labs), and photographed (Nikon Deconvolution Wide-Field Epifluorescence Microscope; Nikon Instruments). Five representative fields from each slide were randomly selected, and their mean fluorescent intensities were quantified using ImageJ (National Institutes of Health). Average mean intensities were then analyzed by one-way repeated-measures ANOVA. All data passed normality and equivariance tests. Post hoc pairwise comparison of means was performed using the Holm–Sidak method (SigmaStat; Systat Software).
Supplementary Material
Acknowledgments
We thank the Robert and Dana Smith Family Foundation and Departments of Surgery (Smithwick and Tyler Endowment Fund) and Pharmacology and Experimental Therapeutics (National Institutes of Health Training Grant 5T32GM008541-14 and the Neuropeptide Laboratory) at the Boston University School of Medicine for financial support of this work.
Footnotes
The authors declare no conflict of interest.
This work was presented in part at the annual meeting of the American Gastroenterological Association, May 1–5, 2010, New Orleans, LA and at Experimental Biology 2011, April 9–13, 2011, Washington, DC.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114275108/-/DCSupplemental.
References
- 1.O'Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411–1420. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- 2.Farraye FA, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 7.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 8.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- 9.Stucchi AF, et al. A neurokinin 1 receptor antagonist reduces an ongoing ileal pouch inflammation and the response to a subsequent inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1259–G1267. doi: 10.1152/ajpgi.00063.2003. [DOI] [PubMed] [Google Scholar]
- 10.Pothoulakis C, et al. Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275:G68–G75. doi: 10.1152/ajpgi.1998.275.1.G68. [DOI] [PubMed] [Google Scholar]
- 11.Goode T, et al. Neurokinin-1 receptor expression in inflammatory bowel disease: Molecular quantitation and localisation. Gut. 2000;47:387–396. doi: 10.1136/gut.47.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabrò A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalski CW, et al. Increase in substance P precursor mRNA in noninflamed small-bowel sections in patients with Crohn's disease. Am J Surg. 2007;193:476–481. doi: 10.1016/j.amjsurg.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 14.ter Beek WP, Biemond I, Muller ES, van den Berg M, Lamers CB. Substance P receptor expression in patients with inflammatory bowel disease. Determination by three different techniques, i.e., storage phosphor autoradiography, RT-PCR and immunohistochemistry. Neuropeptides. 2007;41:301–306. doi: 10.1016/j.npep.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M, et al. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab Invest. 2010;90:1259–1269. doi: 10.1038/labinvest.2010.92. [DOI] [PubMed] [Google Scholar]
- 16.Esteban F, et al. Expression of substance P and neurokinin-1-receptor in laryngeal cancer: Linking chronic inflammation to cancer promotion and progression. Histopathology. 2009;54:258–260. doi: 10.1111/j.1365-2559.2008.03193.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosso M, Robles-Frías MJ, Coveñas R, Salinas-Martín MV, Muñoz M. The NK-1 receptor is expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer cell lines. Tumour Biol. 2008;29:245–254. doi: 10.1159/000152942. [DOI] [PubMed] [Google Scholar]
- 18.Hennig IM, Laissue JA, Horisberger U, Reubi JC. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int J Cancer. 1995;61:786–792. doi: 10.1002/ijc.2910610608. [DOI] [PubMed] [Google Scholar]
- 19.Schulz S, Stumm R, Röcken C, Mawrin C, Schulz S. Immunolocalization of full-length NK1 tachykinin receptors in human tumors. J Histochem Cytochem. 2006;54:1015–1020. doi: 10.1369/jhc.6A6966.2006. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz M, et al. NK-1 receptor antagonists induce apoptosis and counteract substance P-related mitogenesis in human laryngeal cancer cell line HEp-2. Invest New Drugs. 2008;26:111–118. doi: 10.1007/s10637-007-9087-y. [DOI] [PubMed] [Google Scholar]
- 21.Pagán B, et al. Effect of a neurokinin-1 receptor antagonist in a rat model of colitis-associated colon cancer. Anticancer Res. 2010;30:3345–3353. [PMC free article] [PubMed] [Google Scholar]
- 22.Kage R, Leeman SE, Boyd ND. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 23.Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- 24.Ramkissoon SH, Patel PS, Taborga M, Rameshwar P. Nuclear factor-kappaB is central to the expression of truncated neurokinin-1 receptor in breast cancer: Implication for breast cancer cell quiescence within bone marrow stroma. Cancer Res. 2007;67:1653–1659. doi: 10.1158/0008-5472.CAN-06-3813. [DOI] [PubMed] [Google Scholar]
- 25.Patel HJ, Ramkissoon SH, Patel PS, Rameshwar P. Transformation of breast cells by truncated neurokinin-1 receptor is secondary to activation by preprotachykinin-A peptides. Proc Natl Acad Sci USA. 2005;102:17436–17441. doi: 10.1073/pnas.0506351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JP, et al. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA. 2008;105:12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, et al. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci USA. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Déry O, Defea KA, Bunnett NW. Protein kinase C-mediated desensitization of the neurokinin 1 receptor. Am J Physiol Cell Physiol. 2001;280:C1097–C1106. doi: 10.1152/ajpcell.2001.280.5.C1097. [DOI] [PubMed] [Google Scholar]
- 29.Morteau O, Lu B, Gerard C, Gerard NP. Hemokinin 1 is a full agonist at the substance P receptor. Nat Immunol. 2001;2:1088. doi: 10.1038/ni1201-1088. [DOI] [PubMed] [Google Scholar]
- 30.Bellucci F, et al. Pharmacological profile of the novel mammalian tachykinin, hemokinin 1. Br J Pharmacol. 2002;135:266–274. doi: 10.1038/sj.bjp.0704443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 32.González Moles MA, et al. A role for the substance P/NK-1 receptor complex in cell proliferation and apoptosis in oral lichen planus. Oral Dis. 2009;15:162–169. doi: 10.1111/j.1601-0825.2008.01504.x. [DOI] [PubMed] [Google Scholar]
- 33.Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- 34.Keränen U, et al. Changes in substance P-immunoreactive innervation of human colon associated with ulcerative colitis. Dig Dis Sci. 1995;40:2250–2258. doi: 10.1007/BF02209015. [DOI] [PubMed] [Google Scholar]
- 35.Choy MY, Walker-Smith JA, Williams CB, MacDonald TT. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990;31:1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, et al. Distinct differences in tachykinin gene expression in ulcerative colitis, Crohn's disease and diverticular disease: A role for hemokinin-1? Neurogastroenterol Motil. 2011;23:475–483. doi: 10.1111/j.1365-2982.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- 37.Rogler G, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 38.Reed KL, et al. NF-kappaB activation precedes increases in mRNA encoding neurokinin-1 receptor, proinflammatory cytokines, and adhesion molecules in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2005;50:2366–2378. doi: 10.1007/s10620-005-3066-y. [DOI] [PubMed] [Google Scholar]
- 39.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giatromanolaki A, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okuda T, et al. Hypoxia-inducible factor-1 alpha and vascular endothelial growth factor expression in ischaemic colitis and ulcerative colitis. Aliment Pharmacol Ther. 2006;24(Suppl 4):182–188. [Google Scholar]
- 42.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 43.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-β, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turpin E, et al. Stress-induced aberrant splicing of TSG101: Association to high tumor grade and p53 status in breast cancers. Oncogene. 1999;18:7834–7837. doi: 10.1038/sj.onc.1203196. [DOI] [PubMed] [Google Scholar]
- 45.Körner M, Miller LJ. Alternative splicing of pre-mRNA in cancer: Focus on G protein-coupled peptide hormone receptors. Am J Pathol. 2009;175:461–472. doi: 10.2353/ajpath.2009.081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sveen A, et al. Transcriptome instability in colorectal cancer identified by exon microarray analyses: Associations with splicing factor expression levels and patient survival. Genome Med. 2011;3:32. doi: 10.1186/gm248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagedar NA, et al. Gene expression analysis of distinct populations of cells isolated from mouse and human inner ear FFPE tissue using laser capture microdissection—a technical report based on preliminary findings. Brain Res. 2006;1091:289–299. doi: 10.1016/j.brainres.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 48.Nonn L, Vaishnav A, Gallagher L, Gann PH. mRNA and micro-RNA expression analysis in laser-capture microdissected prostate biopsies: Valuable tool for risk assessment and prevention trials. Exp Mol Pathol. 2010;88:45–51. doi: 10.1016/j.yexmp.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bremer AA, Leeman SE. eLS. New York: Wiley; 2010. Substance P. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.