Abstract
The present study investigated whether kisspeptin–G protein-coupled receptor 54 (GPR54) signaling plays a role in mediating mating-induced ovulation in the musk shrew (Suncus murinus), a reflex ovulator. For this purpose, we cloned suncus Kiss1 and Gpr54 cDNA from the hypothalamus and found that suncus kisspeptin (sKp) consists of 29 amino acid residues (sKp-29). Injection of exogenous sKp-29 mimicked the mating stimulus to induce follicular maturation and ovulation. Administration of several kisspeptins and GPR54 agonists also induced presumed ovulation in a dose-dependent manner, and Gpr54 mRNA was distributed in the hypothalamus, showing that kisspeptins induce ovulation through binding to GPR54. The sKp-29–induced ovulation was blocked completely by pretreatment with a gonadotropin-releasing hormone (GnRH) antagonist, suggesting that kisspeptin activates GnRH neurons to induce ovulation in the musk shrew. In addition, in situ hybridization revealed that Kiss1-expressing cells are located in the medial preoptic area (POA) and arcuate nucleus in the musk shrew hypothalamus. The number of Kiss1-expressing cells in the POA or arcuate nucleus was up-regulated or down-regulated by estradiol, suggesting that kisspeptin neurons in these regions were the targets of the estrogen feedback action. Finally, mating stimulus largely induced c-Fos expression in Kiss1-positive cells in the POA, indicating that the mating stimulus activates POA kisspeptin neurons to induce ovulation. Taken together, these results indicate that kisspeptin–GPR54 signaling plays a role in the induction of ovulation in the musk shrew, a reflex ovulator, as it does in spontaneous ovulators.
Keywords: brain, copulation, Kiss1r, metastin, ovary
Mammals have two major repertoires in their ovulatory mechanism: spontaneous ovulation and reflex ovulation. In the spontaneous ovulator, estrogen surge is a key regulator to induce a gonadotropin-releasing hormone (GnRH)/luteinizing hormone (LH) surge leading to ovulation (1). In this case, the brain's detection of an increase in circulating estrogen secreted from the mature follicles triggers ovulation. The reflex ovulator, on the other hand, has a characteristic that is unique in the ovulatory mechanism: The mating stimulus is a dominant factor inducing ovulation. The reflex ovulator lacks behavioral and ovarian estrous cycles. Interestingly, spontaneous ovulators may have an inherent mechanism mediating mating-induced ovulation, because rats are known to show reflex ovulation when maintained in a constant-light condition (2, 3). It has been suggested that coitus-induced ovulation even occurs in women (4). Thus, from the evolutionary aspect, every mammalian species may have an innate mechanism for reflex ovulation that is likely a more ancient form of reproduction than spontaneous ovulation.
The musk shrew (Suncus murinus), a reflex ovulator (5, 6) and a member of the order Insectivora, is considered the most primitive of placental mammals (7). In this animal, the mating stimulus induces formation of a follicular cavity and then ovulation (5). The musk shrew, therefore, is a unique animal model for investigating the neuroendocrine mechanism mediating ovulation, because the mating stimulus is an external trigger activating GnRH neurons to induce ovulation (8). The neuroendocrine pathway involved in the mating-induced ovulation in the musk shrew is not yet fully understood.
Kisspeptin, a Kiss1 gene product, has been identified as an endogenous ligand of G protein-coupled receptor 54 (GPR54) (9, 10). Accumulating evidence suggests that in many mammalian species the kisspeptin–GPR54 system plays a critical role in inducing gonadotropin secretion through stimulation of GnRH release (11–17). Kisspeptin neurons play a role in the generation of the surge mode of GnRH/LH release to mediate the estrogen-positive feedback effect and then ovulation (18–21) as well as the pulsatile GnRH/LH release responsible for follicular maturation (22, 23). Thus, kisspeptin may mediate mating-induced ovulation through GnRH/LH release in the musk shrew.
The present study aimed to identify suncus kisspeptin and then to investigate whether the mating stimulus induces ovulation through activation of kisspeptin neurons in the musk shrew brain. First, we identified Kiss1 and Gpr54 genes, and then we tested whether deduced suncus kisspeptin and various other kisspeptins mimicked mating to induce ovulation. The distribution of Kiss1 mRNA and the effect of estradiol on the Kiss1 expression were determined in the musk shrew brain. Finally, we examined whether the mating stimulus induces c-Fos expression in Kiss1-expressing cells to determine whether this stimulus subsequently activates kisspeptin neurons to induce ovulation in the musk shrew.
Results
Characterization of Suncus Kiss1 and Gpr54 cDNA and Their Expression in Various Tissues.
Nucleotide sequencing revealed that suncus Kiss1 cDNA is 696 bp, and the ORF consists of 402 bp encoding a 133-amino acid polypeptide (Fig. 1A). The ORF of Kiss1 cDNA is considered the kisspeptin precursor. Comparison of the overall amino acid sequence of the kisspeptin precursor revealed that the suncus kisspeptin precursor exhibits 43.6%, 43.6%, and 45.1% similarity to those of human, rat, and mouse, respectively. The position of the dibasic sequence of suncus kisspeptin precursor, which is predicted to be cleaved by a subtilisin-like proprotein convertase, is different from that in other species reported. The suncus kisspeptin precursor has two dibasic sequence positions, so we predicted two suncus kisspeptins, one with 40 amino acid residues (sKp-40) and one with 29 (sKp-29) (Fig. 1B). The core sequence of kisspeptin, Kp-10, was highly conserved compared with other mammalian species (80% against human and 90% against mouse or rat) (Fig. 1B). The third amino acid residue of Kp-10, tryptophan, is fully conserved in the mammals that have been reported previously [e.g., human (9), rodents (24, 25), ruminants (16), and pigs (17)], but is replaced by arginine in the musk shrew.
Fig. 1.
Identification of suncus Kiss1 gene. (A) Nucleotide and deduced amino acid sequences of the suncus Kiss1 gene. Predicted sequences of signal peptide, dibasic sequence regions, and Kp-10 are indicated by double underlining, underlining, and shading, respectively. The asterisk indicates a stop codon. (B) Comparison of amino acid sequences of predicted mature kisspeptin in suncus, human, rat, and mouse. Identical and similar amino acid residues are marked with asterisks and dots, respectively. The shaded box shows the Kp-10 region that is especially conserved through the mammalian species.
Suncus Gpr54 cDNA was 1,696 bp, including the ORF of 1,155 bp, encoding a 384-amino acid polypeptide (Fig. S1A). Comparison of the amino acid sequences of GPR54 reveals a 77.7%, 77.8%, 77.8%, and 76.2% similarity to those of human, pig, rat, and mouse, respectively. Comparison of the amino acid sequences of GPR54 transmembrane (TM) domains shows a 87.2%, 86.5%, 87.2%, and 87.2% similarity to those of human, pig, rat, and mouse, respectively. Phylogenic analysis performed by ClustalW program (http://clustalw.ddbj.nig.ac.jp/) showed that suncus GPR54 belongs to the group containing sheep and goats but is different from the group of other mammalian species (e.g., rat, mouse, pig, and human) (Fig. S1B). Kiss1 mRNA was found in the hypothalamus, lung, and testis, whereas Gpr54 mRNA was found in the hypothalamus, lung, ovary, uterus, and testis (Fig. 2).
Fig. 2.
Expression of suncus Kiss1 and Gpr54 genes in various tissues of the musk shrew. Representative photographs of RT-PCR products for Kiss1, Gpr54, and 18SrRNA are shown. RT-PCR products of Kiss1, Gpr54, and 18SrRNA were detected at 587 bp, 337 bp, and 117 bp, respectively.
Kisspeptin Mimics Mating Stimulus to Stimulate Ovulation via GnRH Release.
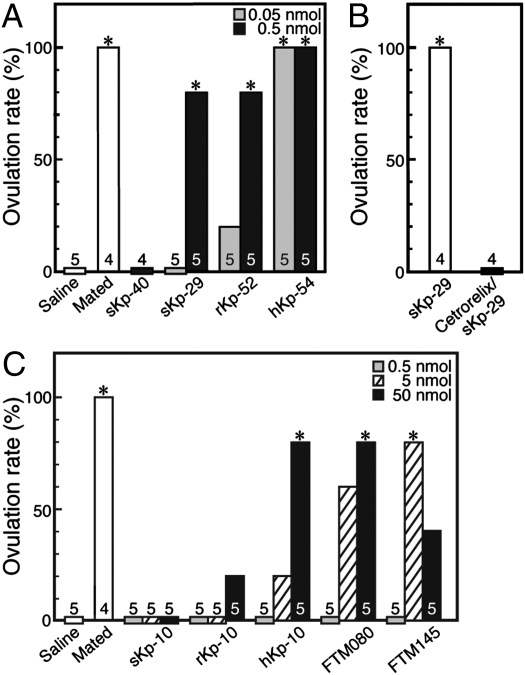
Of the two suncus kisspeptin candidates, sKp-29 mimicked the mating stimulus-induced presumed ovulation in female musk shrews (Fig. 3A). Another candidate, sKp-40, did not induce ovulation in any individuals examined (n = 4), even at the higher dose (0.5 nmol) (Fig. 3A). The injection of kisspeptins, such as rat Kp-52 (rKp-52) or human Kp-54 (hKp-54), also induced presumed ovulation at 0.5 nmol (P < 0.05, Fisher's exact test). hKp-54 was more potent than the other kisspeptins, sKp-29 and rKp-52. When the animals were pretreated with cetrorelix, a GnRH antagonist, sKp-29–induced ovulation was blocked completely (Fig. 3B).
Fig. 3.
Effects of various kisspeptins and GPR54 agonists on the ovulation rate. (A) The ovulation rate in musk shrews that were mated or injected with saline or sKp-40 (0.5 nmol per animal) or with sKp-29, rKp-52, or hKp-54 (0.05 or 0.5 nmol per animal). *P < 0.05 (Fisher's exact test). (B) Effect of cetrorelix, a GnRH antagonist, on sKp-29–induced ovulation. *P < 0.05 (Fisher's exact test). (C) The ovulation rate in musk shrews that were mated or were injected with saline, sKp-10, rKp-10, hKp-10, or the GPR54 agonists FTM080 or FTM145 (0.5, 5, or 50 nmol per animal). *P < 0.05 (Fisher's exact test). Numbers over each column indicate the number of animals treated.
Suncus Kp-10 (sKp-10) or rat Kp-10 (rKp-10) injection did not induce ovulation effectively at any dose (0.5, 5, or 50 nmol) (Fig. 3C). Human Kp-10 (hKp-10) induced presumed ovulation at the highest dose (50 nmol). The GPR54 agonists FTM080 and FTM145 also induced presumed ovulation effectively (Fig. 3C).
sKp-29 Challenge Mimics the Mating Stimulus to Induce Follicular Development and Corpus Luteum Formation.
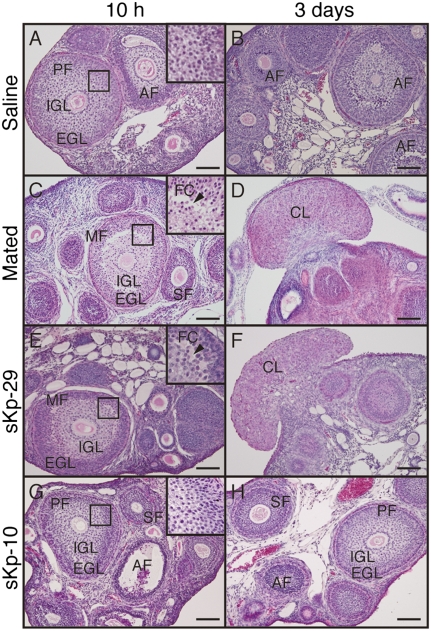
Saline-treated control animals had no mature follicles or corpus luteum (Fig. 4 A and B). sKp-29 challenge induced the slit-like follicular cavity and then corpora lutea formations 10 h and 3 d after treatment, respectively (Fig. 4 E and F). Similar formation of a follicular cavity and then corpora lutea was found at 10 h and 3 d, respectively, after the onset of mating (Fig. 4 C and D). sKp-10 did not cause any obvious morphological changes in ovaries (Fig. 4 G and H).
Fig. 4.
Effects of sKp-29 and sKp-10 on follicular development and corpus luteum formation. Photomicrographs of ovaries with H&E staining in animals that were mated (C and D) or injected with saline (A and B), sKp29 (E and F), or sKp-10 (G and H). Photographs were taken 10 h (A, C, E, and G) or 3 d (B, D, F, and H) after the onset of mating or injection. Mated and sKp-29–injected shrews showed slit-like follicular cavity (arrowheads) in ovarian follicles at 10 h and fungiform corpora lutea in the ovary at 3 d. Insets show the boxed area in each panel at higher magnification. AF, atretic follicle; CL, corpus luteum; EGL, extra granulosa layer; FC, follicular cavity; IGL, inner granulosa layer; MF, mature follicle; PF, premature follicle; SF, secondary follicle. (Scale bars: 100 μm.)
Distribution of Kiss1 mRNA and the Effect of Estradiol on Kiss1 Expression in the Suncus Hypothalamus.
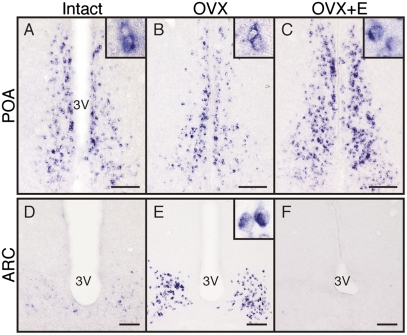
Kiss1 mRNA expression was found in the medial preoptic area (POA) and arcuate nucleus (ARC) in the suncus brain (Fig. 5 A–F). Kiss1 mRNA expression in the POA was slightly higher in intact and estradiol-treated ovariectomized (OVX+E) animals than in ovariectomized (OVX) animals (Fig. 5 A–C). Kiss1-expressing cells in the ARC were more abundant in OVX animals however, whereas Kiss1 signals in the ARC were quite weak in intact and OVX+E animals (Fig. 5 D–F).
Fig. 5.
Distribution of Kiss1 mRNA-expressing cells in the hypothalamus of intact or OVX shrews with or without estradiol treatment. Expression of Kiss1 mRNA in the POA of intact (A), OVX (B), and OVX+E (C) animals and in the ARC of intact (D), OVX (E), and OVX+E (F) animals. Insets show the sections at higher magnification. 3V, third ventricle. (Scale bars: 100 μm.)
Activation of Kisspeptin Neurons by Mating Stimulus.
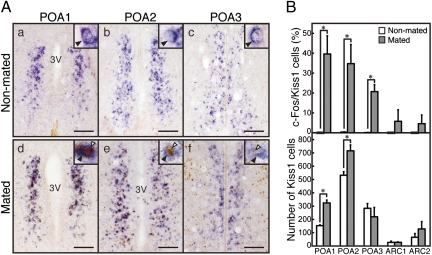
c-Fos immunoreactivity was found in few Kiss1-expressing cells in the POA of female suncus without mating (Fig. 6A), but c-Fos was highly expressed in Kiss1-expressing cells in the POA after mating. The percentage of POA Kiss1 cells coexpressing c-Fos was significantly higher in mated females (P < 0.05, student's t test) than in nonmated controls (Fig. 6B). The total number of Kiss1-expressing cells in the POA also was significantly higher in mated animals than in nonmated animals. In the ARC, Kiss1 and c-Fos expression was low even after mating; there was no significant difference between mated and nonmated females in the total number of Kiss1-expressing cells in the ARC or in the percentage of Kiss1 cells in the ARC coexpressing c-Fos (Fig. 6B).
Fig. 6.
Colocalization of Kiss1 mRNA (purple) and c-Fos immunoreactivities (brown) in the hypothalamus of nonmated and mated musk shrews. (A) Kiss1 mRNA (black arrowheads) and c-Fos immunoreactivities (white arrowheads) in the three parts of the POA of nonmated (a, b, and c) and mated (d, e, and f) animals. Insets show each section at higher magnification. 3V, third ventricle. (Scale bars: 100 μm.) (B) The percentage of Kiss1 mRNA-positive cells expressing c-Fos immunoreactivity and the number of Kiss1 mRNA-positive cells in the POA and ARC of nonmated and mated animals. Values are means ± SEM (n = 3). *P < 0.05 (student's t test).
Discussion
The musk shrew is a useful animal model for investigating the role of kisspeptin–GPR54 signaling in the induction of ovulation, because the shrew is a reflex ovulator and has maturing (but not mature) follicles that are ready to ovulate when the animal receives the mating stimulus. We first identified the suncus Kiss1 and Gpr54 genes and showed that sKp-29, deduced from cDNA sequence, can stimulate presumed ovulation through GnRH release in musk shrews. GPR54 agonists also were able to induce presumed ovulation. These results indicate that the musk shrew has a kisspeptin–GPR54 signaling system that stimulates GnRH release and then ovulation. We found that Kiss1-expressing cells are located in the POA and ARC, as reported in other mammalian species (15, 18, 26) that are spontaneous ovulators. Interestingly, the mating stimulus largely induced c-Fos expression in Kiss1-expessing cells in the POA but not in the ARC. Collectively, these findings imply that the mating stimulus activates POA kisspeptin neurons and then GnRH release to induce ovulation in the musk shrew.
The present study shows that estradiol positively regulates POA Kiss1 expression but negatively regulates the ARC Kiss1 expression in the musk shrew. The result is consistent with other mammalian species, such as rodents, in which kisspeptin expression is regulated positively by estrogen in the anteroventral periventricular nucleus and negatively in the ARC (18, 26). The ARC Kiss1 neurons might be a target of negative feedback of estrogen released from maturing follicles in the musk shrew, because Kiss1 expression remained at a low level in intact musk shrews, whereas ovariectomy dramatically increased Kiss1 expression.
The low level of c-Fos expression in the ARC Kiss1 neurons after mating implies that ARC kisspeptin neurons are not involved in the mating-induced ovulation. Interestingly, unlike in other mammals, quite a large number of POA kisspeptin neurons remained in the OVX musk shrew. Rissman's group (27, 28) showed that the adrenal glands produce androgens that may be converted to estrogen in the POA to regulate sexual behavior in the female musk shrew. Likewise, Kiss1 expression in the POA after ovariectomy might be maintained by adrenal androgens in the musk shrew.
In the present study, we identified two deduced forms of candidate kisspeptin peptides, sKp-29 and sKp-40. sKp-29 showed a potent stimulatory effect on presumed ovulation, but sKp-40 had no effect on ovulation, indicating that sKp-29 is an endogenous kisspeptin in the musk shrew. Interestingly, suncus kisspeptin is much shorter than reported in other mammals, in which kisspeptin consists of 52–54 amino acids (9, 17, 24). Indeed, exogenous administration of sKp-29 mimicked the mating stimulus to induce presumed ovulation, and the sKp-29–induced ovulation was blocked completely by pretreatment with a GnRH antagonist. This result suggests that suncus kisspeptin stimulates GnRH release in the hypothalamus, as was reported in other spontaneous ovulators, and is potent enough to induce ovulation. The suncus kisspeptin sequence showed ∼80% similarity with 29-amino acid sequences at the C terminus in other mammalian species. Kp-10, the core sequence of kisspeptin, also is highly conserved in other mammalian species. The C-terminal amino acid residue of Kp-10 is tyrosine, which is conserved in the mammals previously reported except for primates, which have phenylalanine at the C terminus (6, 14, 26). Like GPR54 in other vertebrates (10, 29, 30), suncus GPR54 possesses features typical of the class I rhodopsin-like guanine nucleotide-binding protein coupled receptor (GPCR) family (31): an N/DPxxY motif in the TM7 and a D/ERY/W motif in junction between TM3 and intracellular loop 2.
Exogenous sKp-29, but not sKp-10, administration mimicked the mating-induced follicular development and ovulation in the musk shrew. The difference between sKp-29 and sKp-10 might be related to differences in degradation rate in the body. Indeed, previous studies, including ours, demonstrated that rKp-52 or hKp-54 shows much higher induction of LH release than Kp-10 (32, 33) and suggested that rKp-52 and hKp-54 are more stable than Kp-10. This notion is in line with the current results demonstrating that administration (5 nmol) of two stable GPR54 agonists consisting of five amino acids, FTM080 and FTM145, induced ovulation in musk shrews, but the same dose of sKp-10 or rKp-10 did not. In fact, in vitro analysis demonstrated that hKp-10 is degraded completely in murine serum within 1 h, whereas FTM080 and FTM145 have half-lives in murine serum of 6.6 h and 38 h, respectively (34). hKp-10–injected shrews showed a much higher ovulation rate than sKp-10– and rKp-10–injected shrews. The difference in sequences between hKp-10 and other Kp-10s, such as sKp-10 and rKp-10, was found in a C-terminal amino acid: phenylalanine in hKp-10 and tyrosine in the other Kp-10s (Fig. 1). Indeed, both hKp-54 and hKp-10 showed much higher bioactivity than any other kisspeptins, suggesting that the C-terminal phenylalanine may be involved in higher binding affinity or a lower degradation rate in the musk shrew.
Peripheral administration of sKp-29 induced ovulation via GnRH release, suggesting that there are two possible sites of interaction between kisspeptin and GnRH neurons in the musk shrew: GnRH cell bodies located in the POA and GnRH neuronal termini located in the median eminence. GnRH neuronal termini are more likely to be the sites at which exogenous kisspeptin induces GnRH surges, because peripheral administration of kisspeptins or GPR54 agonists induced ovulation. The sites at which kisspeptin stimulates GnRH release should be clarified in the future.
The musk shrew has two GnRH systems, GnRH1 and GnRH2. The research group led by Rissman (35) showed that GnRH2 fibers project to the hypothalamus in the musk shrew brain, suggesting that GnRH2 also may be involved in the ovulation, and an injection of GnRH2 induces ovulation, although the effect is one-tenth that of GnRH1. Moreover, these investigators showed that central administration of GnRH2, but not GnRH1, significantly restored mating behavior in underfed females, implying that GnRH2 also may be involved in energy balance and reproductive behavior (36). The role of GnRH in ovulation could be clarified by revealing the neural circuit that includes kisspeptin neurons.
In conclusion, in the musk shrew the mating stimulus activates kisspeptin neurons in the POA to induce ovulation through the release of GnRH. Thus, kisspeptin–GPR54 signaling plays a role in inducing ovulation in a reflex ovulator, as it does in spontaneous ovulators. The key role of kisspeptin–GPR54 signaling in reproduction is well conserved in reflex ovulators as well as in spontaneous ovulators.
Materials and Methods
Animals.
All animal experiments were conducted in accordance with the guidelines of the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University. Male and virgin female KAT line musk shrews (Suncus murinus) were used at 2–12 mo of age. KAT line musk shrews were captured in Katmandu, Nepal and bred at Nagoya University. Animals were housed in a temperature-controlled (25 ± 2 °C) room with 14 h light and 10 h darkness (lights on at 0600 h). All animals had free access to food (commercial trout pellets; Nippon Formula Feed Mfg Co., Ltd) and water. Some females were bilaterally ovariectomized 2 wk before the experiment to serve as the OVX group. Immediately after ovariectomy (i.e., 1 wk before the experiment), some of the OVX animals received s.c. Silastic tubing (i.d. 1.52 mm, o.d. 3.18 mm, 28 mm in length) filled with estradiol-17β (E) (Sigma Chemicals) dissolved in peanut oil at 200 μg/mL to serve as the OVX+E group.
Cloning Analysis.
Three OVX female musk shrews were used for Kiss1 and Gpr54 cDNAs cloning. Total RNA was extracted from the hypothalamic tissues of each OVX musk shrew using TRIzol (Invitrogen). The cDNA encoding suncus Kiss1 was obtained by 5′ RACE and 3′ RACE-PCR using the GeneRacer Kit (Invitrogen) with gene-specific primers according to the manufacturer's instructions. The gene-specific primers used in this study were designed based on the sequence deduced from the comparison of human, rat, and mouse mRNA sequences of Kiss1 and human, rat, mouse, sheep, goat, and pig mRNA sequences of Gpr54 (Table S1). All sequence data were obtained from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). The accession numbers for the mRNA sequences of Kiss1 are human (NM_002256), rat (NM_181692), and mouse (NM_178260); the accession numbers for the mRNA sequences of Gpr54 are human (AF343725), rat (AF115516), mouse (AF343726), sheep (HM135393), goat (GU142846), and pig (DQ459346).
RT-PCR Analysis.
Total RNA was extracted from the cerebral cortex, hypothalamus, pituitary, heart, lung, liver, spleen, kidney, ovary, and uterus of female musk shrews and from the testis of male musk shrews using TRIzol. RT-PCR analysis was done using a thermal cycler (PC-818; ASTEC). Primers for the amplification of partial cDNA sequences of Kiss1, Gpr54, and 18S ribosomal RNA (18SrRNA) are shown in Table S1.
Ovulation-Induction Test.
The amino acid sequence of suncus kisspeptin and its core sequence were deduced according to the cDNA sequence of the musk shrew. sKp-40 [suncus Kiss1 gene product (81-120)-amide], sKp-29 [suncus Kiss1 gene product (92-120)-amide], and sKp-10 [suncus Kiss1 gene product (111-120)-amide] were synthesized by Hayashi Kasei Co., Ltd. hKp-10 [human KISS1 gene product (112-121)-amide], rKp-10 [rat Kiss1 gene product (110-119)-amide], and rKp-52 [rat Kiss1 gene product (68-119)-amide] were synthesized by the Peptide Institute. hKp-54 was purchased from Phoenix Pharmaceuticals, Inc. FTM080 [4-fluorobenzoyl-Phe-Gly-Leu-Arg-Trp-NH2] and FTM145 (4-fluorobenzoyl-Phe-Gly-ψ[(E)-CH=CH]-Leu-Arg-Trp-NH2) were synthesized at Kyoto University as previously reported (34, 37, 38). Presumed ovulation was determined by microscopic examination of the fungiform corpus luteum formation in ovaries of individual animals. Female musk shrews (weight 44–63 g) were anesthetized briefly with diethyl ether and were injected s.c. with sKp-40, sKp-29, rKp-52, hKp-54, sKp-10, rKp-10, hKp-10, FTM080, or FTM145 (0.05–50 nmol/0.1 mL saline) or with saline (0.1 mL) alone at 1700 h (n = 5). Some of the animals injected with sKp-29 (0.5 nmol/0.1 mL saline) (n = 4) were pretreated with a GnRH antagonist (cetrorelix, 200 nmol/0.2 mL 5% mannitol s.c.; AnaSpec) or vehicle (5% mannitol) 30 min before the sKp-29 injection. Ovaries were collected 3 d after the injection or mating.
Histochemical Analysis.
Follicular development was determined by histological examination of the slit-like follicular cavity formation in ovaries of individual animals. Female musk shrews (weight 42–58 g) received a single s.c. injection of sKp-29 or sKp-10 (0.5 nmol/0.1 mL saline) at 1000 h (n = 3). Ovaries were collected 10 h or 3 d after the injection or mating and then wee fixed with 10% neutral buffered formalin. The fixed ovaries were dehydrated through a graded ethanol series and embedded in Tissue Prep (Fisher Scientific). Sections (3-μm thickness) were made by a microtome and were mounted on a glass slide, deparaffinized, rehydrated, and then stained with H&E. Sections were examined under a light microscope.
In Situ Hybridization Analysis.
To detect Kiss1 mRNA, we performed in situ hybridization in the coronal cryostat sections (40-μm thickness) of hypothalamus taken from intact, OVX, and OVX+E animals as previously described (18). Digoxigenin (DIG)-labeled antisense and sense cRNA probes for suncus Kiss1 were synthesized by in vitro transcription from the cDNA clones (Table S1). Hybridization with DIG-labeled cRNA probes was carried out at 60 °C overnight.
In Situ Hybridization and Immunohistochemical Analysis.
Female shrews were assigned randomly to one of two groups: nonmated controls (n = 3) and mated animals (n = 3). The animals in the mated group then were mated with a male at 1100 h. The females were killed 1 h after the first ejaculation. They were perfused with the fixative, and brain tissue was obtained as described above. The nonmated females were perfused between 1300 and 1400 h. We performed dual staining for Kiss1 mRNA by in situ hybridization and immunohistochemistry for c-Fos. After Kiss1 mRNA was visualized by in situ hybridization without proteinase K treatment (18), the sections were washed with PBS and then incubated with a blocking buffer (3% normal goat serum, 1% BSA and 0.1% Triton X-100 in PBS) for 1 h. The sections were incubated with rabbit anti-cFos antibody (1:15,000; Ab-5; Calbiochem-Novabiochem) in a blocking buffer at 4 °C for 24 h. They were treated with biotin-conjugated goat anti-rabbit IgG (1:500; Vector Laboratories) in 0.1% Triton X-100 in PBS (PBST) for 1 h and then in avidin-biotinylated HRP complex (ABC Elite; Vector Laboratories) in PBST for 30 min. Finally, the sections were visualized with diaminobenzidine (DAB) solution (Dako). The number of Kiss1-expressing cells and c-Fos–immunoreactive cells was counted under a light microscope, and the total cell number in brain sections including the POA and ARC was obtained. The POA and ARC were identified using the brain mapping of Rissman and colleagues (39, 40). The POA was divided into three parts (POA1, POA2, and POA3) with four serial sections per part; 12 serial sections were examined. The ARC was divided into two parts (ARC 1 and ARC2) with seven serial sections per part; 14 serial sections were examined.
Statistics.
Statistical differences in the ovulation rates were determined by Fisher's exact test, and statistical differences in the number of Kiss1-expressing cells and the percentage of Kiss1-positive cells expressing c-Fos immunoreactivity were determined by unpaired student's t test.
Supplementary Material
Acknowledgments
We thank Dr. Sen-ichi Oda (Okayama University of Science) for providing the KAT line musk shrews and Shiori Minabe (Nagoya University) for technical support. This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences of Japan and by Grants-in Aid from the Japan Society for the Promotion of Science 23780292 (to N.I.), 23380163 (to H.T.), and 23580402 (to Y.U.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank, DNA Data Bank of Japan (DDBL), and European Molecular Biology Laboratory (EMBL) databases [accession nos. AB466296 (suncus Kiss1) and AB646738 (suncus Gpr54)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113035108/-/DCSupplemental.
References
- 1.Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol. 1987;49:365–382. doi: 10.1146/annurev.ph.49.030187.002053. [DOI] [PubMed] [Google Scholar]
- 2.Hardy DF. The effect of constant light on the estrous cycle and behaviour of the female rat. Physiol Behav. 1970;5:421–425. doi: 10.1016/0031-9384(70)90246-5. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Grant K, Davidson JM, Greig F. Induced ovulation in albino rats exposed to constant light. J Endocrinol. 1973;57:7–22. doi: 10.1677/joe.0.0570007. [DOI] [PubMed] [Google Scholar]
- 4.Jöchle W. Current research in coitus-induced ovulation: A review. J Reprod Fertil Suppl. 1975;22:165–207. [PubMed] [Google Scholar]
- 5.Dryden GL. Reproduction in Suncus murinus. J Reprod Fertil Suppl. 1969;6:377–396. [Google Scholar]
- 6.Rissman EF, Silveira J, Bronson FH. Patterns of sexual receptivity in the female musk shrew (Suncus murinus) Horm Behav. 1988;22:186–193. doi: 10.1016/0018-506x(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 7.Simpson GG. The principles of classification and a classification of mammals. Bull Am Mus Nat Hist. 1945;85:1–350. [Google Scholar]
- 8.Dellovade TL, Ottinger MA, Rissman EF. Mating alters gonadotropin-releasing hormone cell number and content. Endocrinology. 1995;136:1648–1657. doi: 10.1210/endo.136.4.7895675. [DOI] [PubMed] [Google Scholar]
- 9.Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 10.Kotani M, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 11.Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 13.Irwig MS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 14.Shahab M, et al. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschini I, et al. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Ohkura S, et al. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides. 2009;30:49–56. doi: 10.1016/j.peptides.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Tomikawa J, et al. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod. 2010;82:313–319. doi: 10.1095/biolreprod.109.079863. [DOI] [PubMed] [Google Scholar]
- 18.Adachi S, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita M, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 21.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 22.Roseweir AK, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–5404. [PubMed] [Google Scholar]
- 25.Terao Y, et al. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 27.Rissman EF, Clendenon AL, Krohmer RW. Role of androgens in the regulation of sexual behavior in the female musk shrew. Neuroendocrinology. 1990;51:468–473. doi: 10.1159/000125376. [DOI] [PubMed] [Google Scholar]
- 28.Sharma UR, Rissman EF. Testosterone implants in specific neural sites activate female sexual behaviour. J Neuroendocrinol. 1994;6:423–432. doi: 10.1111/j.1365-2826.1994.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee DK, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 30.Moon JS, et al. Molecular cloning of the bullfrog kisspeptin receptor GPR54 with high sensitivity to Xenopus kisspeptin. Peptides. 2009;30:171–179. doi: 10.1016/j.peptides.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Oh DY, Kim K, Kwon HB, Seong JY. Cellular and molecular biology of orphan G protein-coupled receptors. Int Rev Cytol. 2006;252:163–218. doi: 10.1016/S0074-7696(06)52003-0. [DOI] [PubMed] [Google Scholar]
- 32.Pheng V, et al. Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J Reprod Dev. 2009;55:378–382. doi: 10.1262/jrd.20240. [DOI] [PubMed] [Google Scholar]
- 33.Thompson EL, et al. Kisspeptin-54 at high doses acutely induces testicular degeneration in adult male rats via central mechanisms. Br J Pharmacol. 2009;156:609–625. doi: 10.1111/j.1476-5381.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomita K, Oishi S, Ohno H, Peiper SC, Fujii N. Development of novel G-protein-coupled receptor 54 agonists with resistance to degradation by matrix metalloproteinase. J Med Chem. 2008;51:7645–7649. doi: 10.1021/jm800930w. [DOI] [PubMed] [Google Scholar]
- 35.Rissman EF, Alones VE, Craig-Veit CB, Millam JR. Distribution of chicken-II gonadotropin-releasing hormone in mammalian brain. J Comp Neurol. 1995;357:524–531. doi: 10.1002/cne.903570404. [DOI] [PubMed] [Google Scholar]
- 36.Kauffman AS, Rissman EF. A critical role for the evolutionarily conserved gonadotropin-releasing hormone II: Mediation of energy status and female sexual behavior. Endocrinology. 2004;145:3639–3646. doi: 10.1210/en.2004-0148. [DOI] [PubMed] [Google Scholar]
- 37.Tomita K, et al. Structure-activity relationship study on small peptidic GPR54 agonists. Bioorg Med Chem. 2006;14:7595–7603. doi: 10.1016/j.bmc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Tomita K, et al. SAR and QSAR studies on the N-terminally acylated pentapeptide agonists for GPR54. J Med Chem. 2007;50:3222–3228. doi: 10.1021/jm070064l. [DOI] [PubMed] [Google Scholar]
- 39.Dellovade TL, Blaustein JD, Rissman EF. Neural distribution of estrogen receptor immunoreactive cells in the female musk shrew. Brain Res. 1992;595:189–194. doi: 10.1016/0006-8993(92)91048-j. [DOI] [PubMed] [Google Scholar]
- 40.Dellovade TL, King JA, Millar RP, Rissman EF. Presence and differential distribution of distinct forms of immunoreactive gonadotropin-releasing hormone in the musk shrew brain. Neuroendocrinology. 1993;58:166–177. doi: 10.1159/000126529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.