Abstract
Atrial fibrillation (AF) is the most common sustained arrhythmia and an important source for mortality and morbidity on a population level. Despite the clear association between AF and death, stroke, and other cardiovascular events, there is no evidence that rhythm control treatment improves outcome in AF patients. The poor outcome of rhythm control relates to the severity of the atrial substrate for AF not only due to the underlying atrial remodelling process but also due to the poor efficacy and adverse events of the currently available ion-channel antiarrhythmic drugs and ablation techniques. Data suggest, however, an association between sinus rhythm maintenance and improved survival. Hypothetically, sinus rhythm may also lead to a lower risk of stroke and heart failure. The presence of AF, thus, seems one of the modifiable factors associated with death and cardiovascular morbidity in AF patients. Patients with a short history of AF and the underlying heart disease have not been studied before. It is fair to assume that abolishment of AF in these patients is more successful and possibly also safer, which could translate into a prognostic benefit of early rhythm control therapy. Several trials are now investigating whether aggressive early rhythm control therapy can reduce cardiovascular morbidity and mortality and increase maintenance of sinus rhythm. In the present paper we describe the background of these studies and provide some information on their design.
Keywords: Atrial fibrillation, Rhythm control, Morbidity and mortality, Ablation
Introduction—Scope of the problem
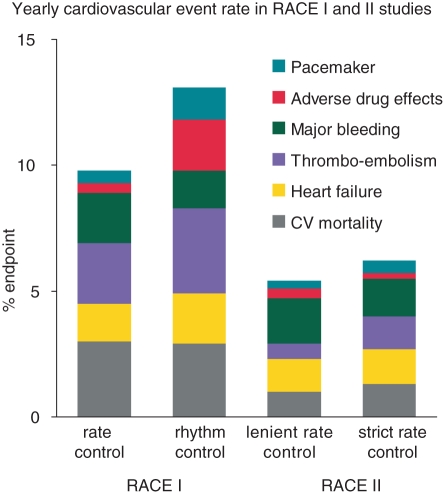
Atrial fibrillation (AF) is the most common sustained arrhythmia and an important source of mortality and morbidity on a population level. Atrial fibrillation is found in 1–2% of the general population. More than 6 million people in Europe are affected and this is expected to double during the next 30–50 years.1–3 The estimated lifetime risk of developing AF is one in four for the population having reached the age of 55.4 Atrial fibrillation is not a benign disease. It is associated with a doubled risk on death, a five-fold increased risk of stroke, increased risk of heart failure and hospitalization, a reduced exercise capacity and left ventricular function, and an impaired quality of life which may be worse in women than in men.5 Despite the clear association between AF and death, stroke, and other cardiovascular events, there is no evidence that rhythm control treatment improves outcome in AF patients. All published studies have shown that rate control is not inferior to rhythm control for the prevention of mortality and morbidity (Tables 1 and 2).6–12 This disappointing outcome may relate to the low long-term maintenance rate of sinus rhythm in the rhythm control groups of these studies, being 63% after 5 years in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial and 39% after 2.3 years of follow-up in the Rate Control versus Electrical Cardioversion (RACE) trial. Also the Atrial Fibrillation and Congestive Heart Failure trial (AF-CHF) observed no difference in cardiovascular mortality (primary outcome) between patients with a left ventricular ejection fraction (LVEF) ≤35%, symptoms of congestive heart failure and a history of AF randomized to rate or rhythm control, nor in the secondary outcomes including death from any cause and worsening of heart failure.11 In addition, a post-hoc time-dependent analysis did not show that sinus rhythm was associated with improved outcome.13 The negative outcome of rhythm control therapy may also be a consequence of ‘positive patient selection’. The enrolled patients were selected by not having severe AF-related symptoms and having survived a phase of AF-related complications. Furthermore, according to the guidelines used when the rate vs. rhythm control studies were performed, anticoagulant therapy was often withdrawn from patients in the rhythm control arms based on the assumption that sinus rhythm was present, resulting in a potentially avoidable excess risk of ischaemic stroke.5,14–16 On the other hand, it should be noted that during rate control therapy morbidity and mortality are still significant. Even with oral anticoagulation the residual stroke or systemic embolism rate in patients with AF remains relatively high, ranging between 1.1 and 2.4%, depending on the presence of risk factors.17–20 Although recent studies showed a trend towards reduction of events including stroke (Figure 1),7,20 further improvement of therapy to reduce AF-associated events clearly is warranted.
Table 1.
Characteristics of rhythm control and rate control trials in patients with atrial fibrillation (adapted from Camm et al. with permission)1
| Patients reaching primary endpoint (n) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Trial | Patients (n) | Mean age (years) | Mean length of follow-up (years) | Inclusion criteria | Primary endpoint | Rate control | Rhythm control | P |
| PIAF8 | 252 | 61.0 | 1.0 | Persistent AF (7–360 days) | Symptomatic improvement | 76/125 (60.8%) | 70/127 (55.1%) | 0.32 |
| AFFIRM6 | 4060 | 69.7 | 3.5 | Paroxysmal AF or persistent AF, age 65 years or older, or risk of stroke or death | All-cause mortality | 310/2027 (25.9%) | 356/2033 (26.7%) | 0.08 |
| RACE7 | 522 | 68.0 | 2.3 | Persistent AF or flutter for <1 year and 1 to 2 cardioversions >2 years and oral anticoagulation | Composite: cardiovascular death, CHF, severe bleeding, PM implantation, thromboembolic events, severe adverse effects of antiarrhythmic drugs | 44/256 (17.2%) | 60/266 (22.6%) | 0.11 |
| STAF9 | 200 | 66.0 | 1.6 | Persistent AF (>4 weeks and <2years), left atrial size >45 mm, CHF NYHA II–IV, LVEF <45% | Composite: overall mortality, cerebrovascular complications, CPR, embolic events | 10/100 (10.0%) | 9/100 (9.0%) | 0.99 |
| HOT CAFÉ10 | 205 | 60.8 | 1.7 | First clinically overt persistent AF (≥7 and <2 years), 50–75-year old | Composite: death, thromboembolic events; intracranial/ major haemorrhage | 1/101 (1.0%) | 4/104 (3.9%) | >0.71 |
| AF-CHF11 | 1376 | 66 | 3.1 | LVEF≤35%, symptoms of CHF, history of AF (≥6 h or ECV <last 6 months) | Cardiovascular death | 175/1376 (25%) | 182/1376 (27%) | 0.59 |
AF, atrial fibrillation; AFFIRM, atrial fibrillation follow-up investigation of rhythm management; CHF, congestive heart failure; CPR, cardiopulmonary resuscitation; ECV, electrical cardioversion; HOT CAFE, how to treat chronic atrial fibrillation; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PIAF, pharmacological intervention in atrial fibrillation; PM, pacemaker; RACE, rate control versus electrical cardioversion for persistent atrial fibrillation; STAF, strategies of treatment of atrial fibrillation.
Table 2.
Comparison of adverse outcomes in rhythm control and rate control trials in patients with atrial fibrillation (adapted from Camm et al. with permission)1
| Trial | Deaths of all causes (in rate/rhythm) | Deaths from cardiovascular causes | Deaths from non-cardiovascular causes | Stroke | Thromboembolic events | Bleeding |
|---|---|---|---|---|---|---|
| PIAF8 | 4 | 1/1 | 1a | ND | ND | ND |
| AFFIRM6 | 666 (310/356) | 167/164 | 113/165 | 77/80 | ND | 107/96 |
| RACE7 | 36 | 18/18 | ND | ND | 14/21 | 12/9 |
| STAF9 | 12 (8/4) | 8/3 | 0/1 | 1/5 | ND | 8/11 |
| HOT CAFÉ10 | 4 (1/3) | 0/2 | 1/1 | 0/3 | ND | 5/8 |
| AF-CHF11 | 228/217 | 175/182 | 53/35 | 11/9 | ND | ND |
AF, atrial fibrillation; AFFIRM, atrial fibrillation follow-up investigation of rhythm management; HOT CAFE, how to treat chronic atrial fibrillation; ND, not determined; PIAF, pharmacological intervention in atrial fibrillation; RACE, rate control versus electrical cardioversion for persistent atrial fibrillation; and STAF, strategies of treatment of atrial fibrillation.
aTotal number of patients not reported.
Figure 1.
Yearly cardiovascular morbidity and mortality rate in the Rate Control Versus Electrical Cardioversion (RACE) I study (published in 2002) and the RAte Control Efficacy in permanent atrial fibrillation (RACE) II study (published in 2010).7,20
Interestingly, a subgroup analysis of the AFFIRM trial demonstrated an association between sinus rhythm maintenance and improved survival.14 This, together with the results of the recently published ATHENA trial, supports the idea that the presence of AF is one of the modifiable factors associated with death and cardiovascular morbidity in AF patients.21 Apart from the beneficial effect of dronedarone on a composite endpoint, predominantly driven by cardiovascular hospitalizations in the ATHENA trial, there are no other controlled data that show a benefit of rhythm control therapy beyond improved quality of life.22,23 Hence, current guidelines for the treatment of AF base the decision to add rhythm control therapy to the management of AF on individual factors interpreted by the physician and the patient. These factors include the severity of complaints and how these will affect the individual patients, and the severity of AF, i.e. how successful rhythm control is expected to be.1,24 Further elucidation of the mechanisms and signals involved in the process of sustaining AF might ultimately improve therapeutic strategies and outcome of rhythm control therapy both for maintenance of sinus rhythm and for prevention of morbidity and mortality.
Potential benefit of early rhythm control therapy
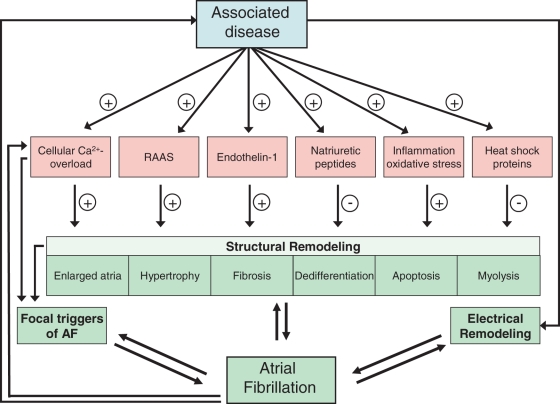
The causes underlying AF are multifactorial. Age, hypertension, congestive heart failure, valve disease, and diabetes are all well-known risk factors for the development of AF.25–28 Less well-known risk factors include, among others, endurance training, obesity, sleep apnoea syndrome, and chronic obstructive pulmonary disease.1,29 These risk factors together with an altered metabolism, autonomic changes, and genetic and environmental factors cause marked changes in the molecular function and structure of the atria, which is called structural remodelling. The induced molecular and structural changes in the atria include cellular calcium overload, activation of the renin−angiotensin−aldosterone system, inflammation, oxidative stress, enlarged atria, hypertrophy, fibrosis, dedifferentiation, apoptosis, myolysis, and amyloidosis (Figure 2).30,31 Structural remodelling ultimately creates a substrate for AF due to electrical dissociation between muscle bundles and local conduction heterogeneities facilitating the initiation and perpetuation of AF. Once AF develops, it causes marked changes in atrial electrophysiology (‘electrical remodelling’) and further deteriorates the structural remodelling process.32–35 The first manifestation of AF usually occurs after years of atrial remodelling.35–37 Thus, atrial remodelling in patients with AF is caused by both the associated diseases and AF itself and may contribute to AF-related complications. Ultimately, due to ongoing remodelling, patients progress to permanent AF.38,39
Figure 2.
Flow chart showing the series of events caused by stretch. Hypothetical scheme of stretch induced by hypertension, heart failure and possibly extreme endurance exercise leading to calcium overload, activation of the renin−angiotensin−aldosterone system and release of different factors, resulting in structural remodelling and finally in atrial fibrillation (Adapted with permission from De Jong et al.).30
The remodelling changes may still be reversible during early phases of the arrhythmia, probably even more if the duration of the underlying disease also is not too long,40,41 but may provoke relevant and permanent atrial damage during later stages of AF and associated diseases. This may explain the disappointing outcome of rhythm control therapy in prior studies, both for the prevention of recurrent AF and for cardiovascular morbidity and mortality. Most trials included patients in whom the extent of remodelling was severe and even irreversible due to a long history of both AF and the underlying heart disease. Since the underlying disease is also a major contributor to the remodelling process, in some patients a first episode of AF may already be untreatable, even with aggressive therapy, due to the presence of substantial structural changes.35,42 In patients with a shorter history of both AF and the underlying disease, the remodelling processes are assumingly less advanced, which may provide more opportunities for rhythm control strategies to be effective.30,34,43 By successfully eliminating AF the remodelling process may become less progressive, reducing the extent of fibrosis, inflammation, atrial hypertrophy, and other adaptation processes. Hypothetically, this may also lower the risk of complications associated with AF, like stroke and heart failure.
New modalities for safe and relatively effective rhythm control therapies: ablation, new antiarrhythmic drugs, and upstream therapy
The poor outcome of rhythm control relates to the severity of the atrial substrate for AF not only due to the underlying atrial remodelling process but also due to the poor efficacy and adverse events of the currently available ion-channel antiarrhythmic drugs and ablation techniques.44–55
While catheter ablation was not incorporated into the rate vs. rhythm control trials, today it is increasingly performed in patients with symptomatic AF. Pulmonary vein isolation is the cornerstone of all ablation procedures. In most centres this is performed with one long, encircling lesion around the right and another lesion around the left pulmonary veins. Several prospective randomized trials comparing ablation vs. antiarrhythmic drugs to maintain sinus rhythm consistently show that ablation therapy is significantly more effective in maintaining sinus rhythm compared with antiarrhythmic drugs with an overall risk reduction of AF recurrence by 65 to 70% at 1-year follow-up, keeping in mind that most of these trials have shortcomings in detecting recurrent AF.1,51–55 A recent meta-analysis showed a single-procedure success rate of ablation off antiarrhythmic drugs of 57%, a multiple procedure success rate off antiarrhythmic drugs of 71%, and a multiple procedure success rate on antiarrhythmic drugs of 77%. In comparison, the success rate for antiarrhythmic drug therapy was 52%.56 Whether these short-term success rates may be extrapolated to long-term success is yet unclear. Data on long-term outcome for catheter ablation for AF are scarce. Tzou et al.57 recently reported on 123 consecutive patients with paroxysmal or persistent AF who underwent pulmonary vein isolation and were free from AF and without antiarrhythmic drugs 1 year after ablation. Long-term ablation success, defined as freedom from AF off antiarrhythmic drugs after a single-ablation procedure, was 85% at 3 years and 71% at 5 years, with an ∼7% per year late recurrence rate after the first year. Predictors for late recurrences were the known risk factors for AF (progression),39 including higher age, hypertension, larger left atrial size, as well as more AF triggers being present during the electrophysiological procedure, and patients who present with persistent AF. The Bordeaux group showed comparable late success rates.58 Arrhythmia-free survival following the last catheter ablation procedure was, in 100 patients undergoing ablation in 2001–2002 in their experienced hands, 87, 81, and 63% at 1, 2, and 5 years, respectively. Valvular heart disease and non-ischaemic dilated cardiomyopathy independently predicted recurrences. Thus, although most recurrences transpire over the first 6–12 months, a slow but steady decline in arrhythmia-free survival is noted thereafter, even after three or more years of apparent arrhythmia control. Nevertheless, catheter ablation for AF appears to be more effective in maintaining sinus rhythm compared with antiarrhythmic drug therapy. It might be even more effective when considering the fact that most ablation studies included patients with a relatively long history of AF and the associated disease who had failed serial antiarrhythmic drug testing. Thus, success might be further improved by a better selection of patients and more efficacious and safe ablation techniques. The highest efficacy of catheter ablation is observed in younger patients with less severe atrial remodelling.57,59 The procedure is relatively safe with a 5% overall complication rate.56,60 Feared complications include tamponade, stroke, pulmonary vein stenosis, permanent diaphragmatic paralysis, and oesophageal fistula.60 Repeat procedures, however, are necessary in up to 40% of all patients. Only one small study investigated catheter ablation and antiarrhythmic drugs as first-line therapy in patients with a duration of AF of >8 months.51 Duration of the associated disease was not reported. At 1 year of follow-up 80% of patients who had undergone catheter ablation were free of AF. It is, however, unknown yet as to whether an early ablation therapy and a further optimization of ablation procedures could further improve success rate and reduce complications associated with the ablation procedure, and, in turn, may improve cardiovascular outcomes like mortality, cardiovascular hospitalizations, worsening of heart failure, and stroke.
Safer and more effective antiarrhythmic drugs may also improve the success rate of a rhythm control strategy. Ion channel-blocking antiarrhythmic drugs may counteract the electrical remodelling, but leave other mechanisms like the structural remodelling process untouched. In addition, these drugs all carry a relatively high risk of adverse events including life-threatening arrhythmias like torsades de pointes. For that reason the recommended dosages often cannot be instituted, which might reduce success rates. A number of new class III and atrial selective drugs have been developed.61 However, most of these drugs were abandoned before approval due to the risk of proarrhythmia. Dronedarone, a novel benzofuran derivative structurally related to amiodarone, has recently been approved and may improve the outcome of rhythm control therapy. It has a beneficial safety profile both in patients without structural heart disease and in those with stable mild-to-moderate heart disease and seems to carry a very low risk for proarrhythmia.21,62 However, it is contraindicated in patients with impaired left ventricular function [New York Heart Association (NYHA) class III/IV] and haemodynamic instability because of the data of the ANDROMEDA study.63 This study investigated the effect of dronedarone on the risk of hospitalization for progressive heart failure in a placebo-controlled study in patients with NYHA class III or IV congestive heart failure, and a LVEF <35%. After 2 months this trial was terminated because of a higher mortality rate in the dronedarone treatment group due to progressive heart failure. Similar to sotalol, propafenone, and flecainide, dronedarone is less effective to maintain sinus rhythm compared with amiodarone,62,64 but its efficacy has not been tested in patients with early AF. Of significance are the beneficial results of dronedarone on improvement of outcome. Data from the recently published ATHENA trial on outcome in patients with AF showed a reduction of the primary composite outcome driven by cardiovascular hospitalizations (hazard ratio 0.74, 95% confidence interval 0.69–0.84, P < 0.0001).21 Additionally, a post-hoc analysis of ATHENA showed a reduction of stroke.65 Comparable beneficial outcome effects have been demonstrated for amiodarone,50 but this beneficial effect is counteracted by a high rate of non-cardiac adverse events.50,66 Adverse effects associated with dronedarone have also been reported but seem to be less harmful.21,62,64 Thyroid, ocular, or pulmonary side effects in these studies were not significantly different from placebo-treated patients. Similar to amiodarone, however, dronedarone is associated with an increase in serum creatinine, which are assumed to be the result of inhibition of tubular secretion, independent of renal function.67 This is particularly the case in patients who use other drugs increasing serum creatinine.62
Substrate-oriented antiarrhythmic drug therapy that modifies the structural atrial remodelling process may also improve the outcome of rhythm control. ‘Upstream therapy’ refers to the use of non-ion channel antiarrhythmic drugs that modify the atrial substrate to prevent the occurrence of new onset AF or recurrence of the arrhythmia. It includes treatment with renin−angiotensin−aldosterone system (RAAS) blockers [angiotensin-converting enzyme inhibitors (ACE-inhibitor), angiotensin receptor blockers, aldosterone receptor antagonists], statins, and omega-3 polyunsaturated fatty acids. The RAAS blockers may prevent or reduce atrial structural remodelling especially by decreasing fibrosis. In addition, these drugs improve haemodynamics by lowering of blood pressure and reduction of left ventricular and atrial wall stress, which also may have beneficial effects on the remodelling process. Statins, known for their lipid-lowering capacities, have a variety of pleiotropic properties including attenuation of inflammation through anti-atherogenic and antioxidant actions. Results of upstream therapy for the prevention of AF in animal experiments, hypothesis-generating small clinical studies, and retrospective analyses in selected patient categories have been encouraging. Larger prospective randomized trials, however, did fail to show any protective benefit against AF in patients with and without structural heart disease,40,68–70 while patients with known left ventricular dysfunction71 or with diabetes mellitus and left ventricular hypertrophy36 experience less new onset AF on ACE-inhibitor or sartans compared with placebo or beta-blockers. This suggests that inhibition of the renin−angiotensin system may be helpful to prevent AF in patients whose atria are exposed to marked volume or pressure overload by systolic or diastolic dysfunction. The randomized trials so far included patients in whom the extent of remodelling was severe and even irreversible due to a longer history of AF and underlying heart disease. In patients with a shorter history of AF and the underlying disease, remodelling processes are assumingly less advanced, providing greater opportunity for upstream therapies to be effective.
The need for staged therapy
Atrial fibrillation is responsible for a five-fold increase in the risk of ischaemic stroke. Therefore, oral anticoagulation therapy is the cornerstone for the treatment of AF patients with an increased risk of thromboembolic complications.72 Such treatment is needed independently from the therapeutical strategy decided, rate, or rhythm control. But even with oral anticoagulation the residual stroke or systemic embolism rate in patients with AF remains relatively high.17–20 The presence of AF seems one of the modifiable factors associated with death and cardiovascular morbidity in AF patients.
We can therefore hypothesize that if effective and safe methods for maintaining sinus rhythm with fewer adverse effects become available rhythm control therapy may become the first choice therapy in more patients. A promising strategy might be catheter ablation combined with safe antiarrhythmic drugs and substrate-oriented antiarrhythmic drugs with beneficial effects on outcome parameters. Catheter ablation is nowadays an effective therapy but only retrospective evidence supports the notion that catheter ablation may result in reduced mortality.73 Therefore, prospective randomized trials that include catheter ablation and new antiarrhythmic drugs for rhythm control are needed to reaffirm the concept that sinus rhythm maintenance may improve outcome. These trials preferably should be performed in patients with a short history of AF and the underlying disease, i.e. in patients with less severe remodelled atria.
Perspective: slowing down the progression of atrial fibrillation to prevent atrial fibrillation-related complications
Patients with a short history of AF and the underlying heart disease have not been studied before. It is fair to assume that the abolishment of AF in these patients is more successful and possibly also safer, which could translate into a prognostic benefit of early rhythm control therapy. Several trials are now investigating whether aggressive early rhythm control therapy can reduce cardiovascular morbidity and mortality and increase the maintenance of sinus rhythm. The Radiofrequency Ablation versus Antiarrhythmic drugs for Atrial Fibrillation Treatment (RAAFT) study (Clinical Trials.gov number NCT00393054)74 randomized 130 patients naïve to antiarrhythmic drugs to either atrial ablation or antiarrhythmic drugs as first-line treatment of symptomatic AF. Their primary endpoint is time to first recurrence of electrocardiographically documented symptomatic AF lasting >30 s. A second trial, the Catheter ABlation versus ANti-arrhythmic drug therapy for Atrial fibrillation trial (CABANA, Clinical Trials.gov number NCT00911508) currently randomizes patients to left atrial endocardial catheter ablation or current state-of-the-art therapy with either rate or rhythm control drugs. This study aims to include 3000 patients with risk factors for stroke. The hypothesis is that eliminating AF will be superior for reducing total mortality. The Routine versus Aggressive upstream rhythm Control for the prevention of Early atrial fibrillation in heart failure study (RACE 3, NCT00877643) includes patients with early AF (total AF history <2 years, total persistent AF duration <6 months, and ≤1 previous electrical cardioversion), and mild-to-moderate early heart failure (total heart failure history <1 year). Patients are randomized to aggressive upstream therapy with structured physical activity or routine rhythm control as described in the 2010 AF guidelines.1 The primary endpoint of the study is sinus rhythm after 1 year of follow-up, defined as sinus rhythm during ≥6/7th of assessable time of continuous 7 days Holter monitoring during the last week of the study.1,75 Finally, the Early treatment of Atrial fibrillation for Stroke prevention Trial (EAST, ISRCTN-Nr: 04708680) will soon start to include high-risk patients with shortlasting AF (known history of AF <1 year). This trial will randomize 3150 patients to early rhythm control therapy either by atrial catheter ablation or antiarrhythmic drugs (preferably dronedarone, and in case of a recurrence both modalities), or usual care as described in the 2010 AF guidelines.1 The primary outcome is cardiovascular mortality, stroke, transient ischaemic attack, and hospitalization due to worsening of heart failure or acute coronary syndrome. All authors will participate in the planned EAST trial. The above-mentioned studies will give us new insight into whether slowing down the progression of AF may prevent AF-related complications.
Funding
I.C.V.G. received research grants from Netherlands Heart Foundation, Interuniversity Cardiology Institute the Netherlands, Astra Zeneca, Biotronik, Medtronic, Sanofi-Aventis, Boehringer Ingelheim, St Jude Medical, Boston Scientific. L.S. received research grants from Polish Ministry of Science and Higher Education, European Union (EU), Biotronik. L.M. received research grants from Biosense Webster, Medtronic; Boston Scientific, St Jude Medical. S.T. received research grants from St Jude Medical. P.K. received research grants from 3M Medica/MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, sanofi-aventis, St Jude Medical, German Federal Ministry for Education and Research (BMBF), Fondation Leducq, German Research Foundation (DFG), European Union (EU). Funding to pay the Open Access publication charges was provided by AFNET.
Conflict of interest: I.C.V.G. has received consulting fees/honoraria from Medtronic, MSD, Sanofi-Aventis, and Boehringer Ingelheim; travel support to attend meetings from the European Society of Cardiology (ESC) and the European Heart Rhythm Association (EHRA), a registered branch of ESC; and research grants from the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, Astra Zeneca,Biotronik, Medtronic, Sanofi-Aventis, Boehringer Ingelheim, St Jude Medical, and Boston Scientific. A.B. has received consulting fees/honoraria from Biotronik, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, MSD, Sanofi-Aventis, and St Jude Medical. E.A. has received consulting fees/honoraria from Sanofi Aventis, Meda Pharma, GlaxoSmithKline, Bayer, Boehringer Ingelheim, BMS-Pfizer, Medtronic, Biotronik, and St Jude medical. J.K. has received consulting fees/honoraria from Abbott, Biosense Webster, Biotronik, Boehringer Ingelheim, Boston Scientific, CardioFocus, GE Healthcare, Hansen Medical, Medtronic, Pfizer, Sanofi-Aventis, Siemens Healthcare, and St Jude Medical, and travel support to attend meetings from the European Society of Cardiology (ESC), the European Heart Rhythm Association (EHRA), and from the German Atrial Fibrillation Competence NETwork (AFNET). L.S. has received consulting fees/honoraria from Medtronic, Biotronik, Biosense Webster, St Jude Medical, and Sanofi-Aventis; travel support from the European Heart Rhythm Association and the European Society of Cardiology; and research grants from the Polish Ministry of Science and Higher Education, the European Union, and Biotronik. L.M. has received consulting fees/honoraria from Bard, Biosense Webster, Medtronic, Boston Scientific, St Jude Medical, Sanofi-Aventis, Biotronik, and Sorin Group, and research grants from Biosense Webster, Medtronic, Boston Scientific , and St Jude Medical. S.T. has received consulting fees/honoraria from Biosence Webster and Sanofi-Aventis, and research grants from St Jude Medical. P.K. has received consulting fees/honoraria from 3M Medica, MEDA Pharma, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer/BMS, Sanofi-Aventis, Servier, Siemens, and TAKEDA; travel support to attend meetings from the European Society of Cardiology (ESC), the European Heart Rhythm Association (EHRA), a registered branch of ESC, and from the German, Atrial Fibrillation Competence NETwork (AFNET); and research grants from 3M Medica/MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, Sanofi-Aventis, St Jude Medical, German Federal Ministry for Education and Research (BMBF), Fondation Leducq, German Research Foundation (DFG), and the European Union.
References
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Heeringa J, Van der Kuip DA, Hofman A, Kors JA, Van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 5.Rienstra M, Van Veldhuisen DJ, Hagens VE, Ranchor AV, Veeger NJ, Crijns HJ, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46:1298–306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 6.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation-Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–94. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–6. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 10.Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kaplon B, Kolodziej P, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 12.Testa L, Biondi-Zoccai GG, Dello Russo A, Bellocci F, Andreotti F, Crea F. Rate-control vs. rhythm-control in patients with atrial fibrillation: a meta-analysis. Eur Heart J. 2005;26:2000–6. doi: 10.1093/eurheartj/ehi306. [DOI] [PubMed] [Google Scholar]
- 13.Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc M, et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;55:1796–802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 15.Rienstra M, Van Veldhuisen DJ, Crijns HJ, Van Gelder IC. Enhanced cardiovascular morbidity and mortality during rhythm control treatment in persistent atrial fibrillation in hypertensives: data of the RACE study. Eur Heart J. 2007;28:741–51. doi: 10.1093/eurheartj/ehl436. [DOI] [PubMed] [Google Scholar]
- 16.Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van den Berg MP, Rienstra M, Ranchor AV, et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–11. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, et al. ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 18.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 19.Patel MR. Stroke Prevention Using the Oral Direct Factor Xa inhibitor Rivaroxaban Compared with Warfarin in Patients with Nonvalvular Atrial Fibrillation (ROCKET AF) Chicago: AHA; 2010. [Google Scholar]
- 20.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 21.Hohnloser SH, Crijns HJ, Van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 22.Hagens VE, Ranchor AV, Van SE, Bosker HA, Kamp O, Tijssen JG, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241–7. doi: 10.1016/j.jacc.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J Am Coll Cardiol. 2006;48:721–30. doi: 10.1016/j.jacc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, 3rd, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8:157–76. doi: 10.1016/j.hrthm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 25.Anne W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005;67:655–66. doi: 10.1016/j.cardiores.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Sanders P, Morton JB, Kistler PM, Vohra JK, Kalman JM, Sparks PB. Reversal of atrial mechanical dysfunction after cardioversion of atrial fibrillation: implications for the mechanisms of tachycardia-mediated atrial cardiomyopathy. Circulation. 2003;108:1976–84. doi: 10.1161/01.CIR.0000091408.45747.04. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–60. doi: 10.1161/01.hyp.25.6.1155. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 29.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of ‘not-so-lone atrial fibrillation. Europace. 2008;10:668–73. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 30.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–65. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 31.Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–7. doi: 10.1161/01.cir.0000034511.06350.df. [DOI] [PubMed] [Google Scholar]
- 32.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 33.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–63. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 34.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 35.Cosio FG, Aliot E, Botto GL, Heidbuchel H, Geller CJ, Kirchhof P, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–7. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- 36.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–9. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 37.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 38.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 39.De Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–17. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 41.Smit MD, Van Gelder IC. Valsartan and recurrent atrial fibrillation. N Engl J Med. 2009;361:532. doi: 10.1056/NEJMc091057. [DOI] [PubMed] [Google Scholar]
- 42.Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–6. doi: 10.1016/s0008-6363(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 43.Tieleman RG, Van Gelder IC, Bosker HA, Kingma T, Wilde AA, Kirchhof CJ, et al. Does flecainide regain its antiarrhythmic activity after electrical cardioversion of persistent atrial fibrillation? Heart Rhythm. 2005;2:223–30. doi: 10.1016/j.hrthm.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Crijns HJ, Van Gelder IC, Van Gilst WH, Hillege H, Gosselink AM, Lie KI. Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter. Am J Cardiol. 1991;68:335–41. doi: 10.1016/0002-9149(91)90828-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Gelder IC, Crijns HJ, Tieleman RG, Brugemann J, De Kam PJ, Gosselink AT, et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med. 1996;156:2585–92. doi: 10.1001/archinte.156.22.2585. [DOI] [PubMed] [Google Scholar]
- 46.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 47.Cooper HA, Bloomfield DA, Bush DE, Katcher MS, Rawlins M, Sacco JD, et al. Relation between achieved heart rate and outcomes in patients with atrial fibrillation (from the Atrial Fibrillation Follow-up Investigation of Rhythm Management [AFFIRM] Study) Am J Cardiol. 2004;93:1247–53. doi: 10.1016/j.amjcard.2004.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–72. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 49.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Mahe I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166:719–28. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed S, Rienstra M, Crijns HJ, Links TP, Wiesfeld AC, Hillege HL, et al. Continuous vs. episodic prophylactic treatment with amiodarone for the prevention of atrial fibrillation: a randomized trial. JAMA. 2008;300:1784–92. doi: 10.1001/jama.300.15.1784. [DOI] [PubMed] [Google Scholar]
- 51.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 52.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 53.Noheria A, Kumar A, Wylie JV, Jr, Josephson ME. Catheter ablation vs antiarrhythmic drug therapy for atrial fibrillation: a systematic review. Arch Intern Med. 2008;168:581–6. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 54.Nair GM, Nery PB, Diwakaramenon S, Healey JS, Connolly SJ, Morillo CA. A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:138–44. doi: 10.1111/j.1540-8167.2008.01285.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 56.Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 57.Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:237–42. doi: 10.1161/CIRCEP.109.923771. [DOI] [PubMed] [Google Scholar]
- 58.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 59.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–41. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 60.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 61.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–65. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 62.Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–99. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- 63.Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 64.Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597–605. doi: 10.1111/j.1540-8167.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- 65.Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL, et al. Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174–80. doi: 10.1161/CIRCULATIONAHA.109.875252. [DOI] [PubMed] [Google Scholar]
- 66.Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–41. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 67.Tschuppert Y, Buclin T, Rothuizen LE, Decosterd LA, Galleyrand J, Gaud C, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol. 2007;64:785–91. doi: 10.1111/j.1365-2125.2007.02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, et al. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30:2327–36. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 69.Goette A. Angiotensin II-Antagonist in Paroxysmal Atrial Fibrillation Trial. Stockholm: ESC; 2010. ANTIPAF-Trial. [Google Scholar]
- 70.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–72. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 71.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999;100:376–80. doi: 10.1161/01.cir.100.4.376. [DOI] [PubMed] [Google Scholar]
- 72.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 73.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42:185–97. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 74.Khaykin Y, Wang X, Natale A, Wazni OM, Skanes AC, Humphries KH, et al. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J Cardiovasc Electrophysiol. 2009;20:7–12. doi: 10.1111/j.1540-8167.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 75.Van Gelder IC, Smit MD, Alings M, Crijns HJGM. Upstream therapy in patients with early atrial fibrillation. The relevance of the Routine versus Aggressive upstream rhythm Control for prevention of Early atrial fibrillation in heart failure (RACE 3) study. Neth Heart J. 2010;18:522–3. doi: 10.1007/s12471-010-0827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]