Abstract
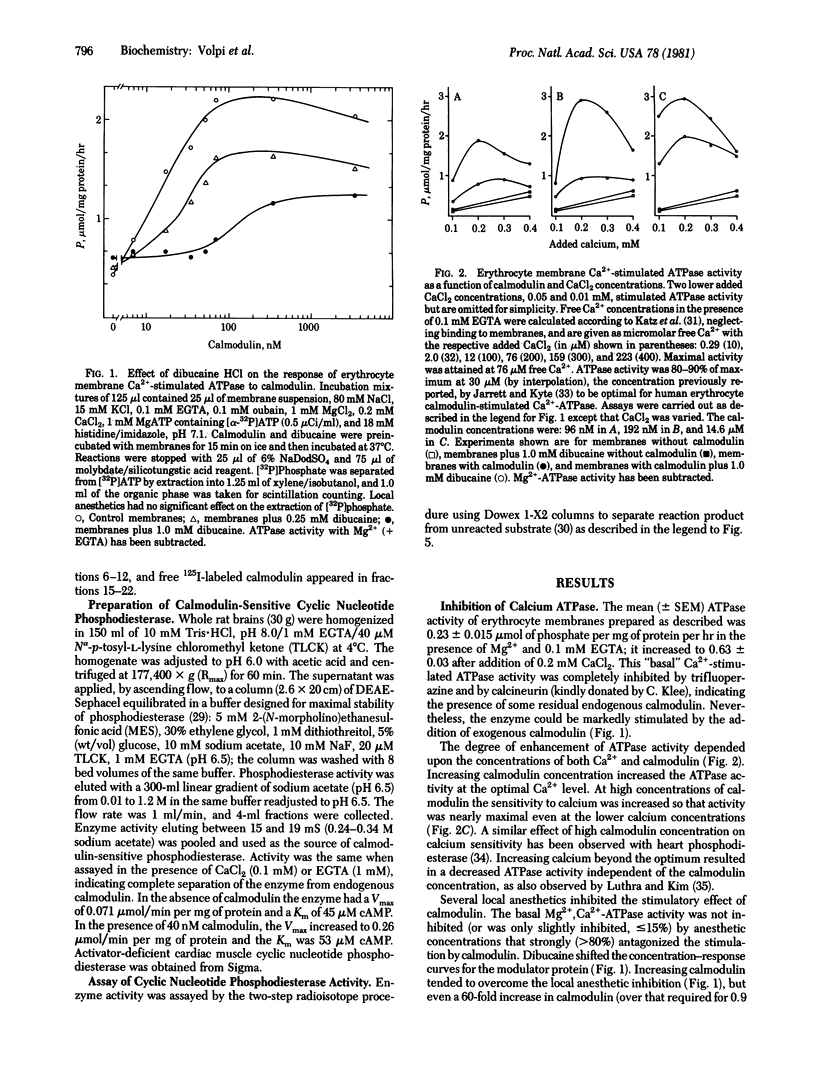
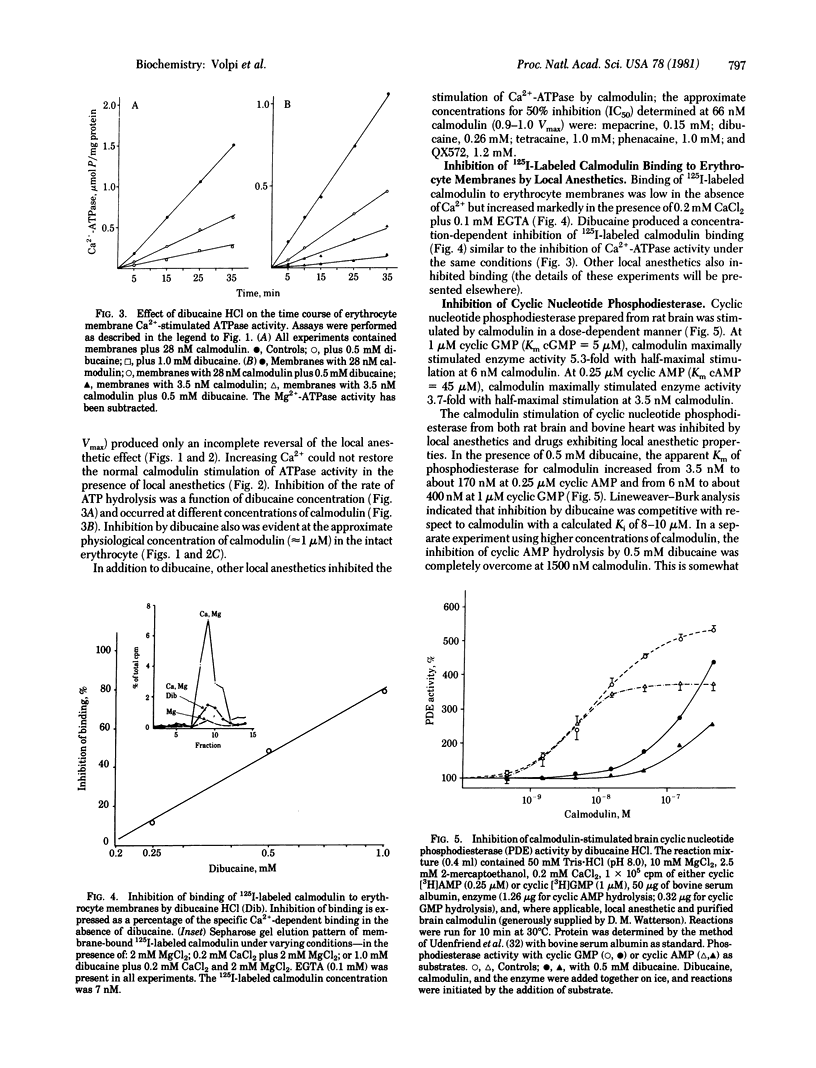
Local anesthetics such as dibucaine, QX572, tetracaine, and phenacaine, as well as other drugs with local anesthetic-like properties (e.g., mepacrine, propranolol, and SKF 525A) inhibit the specific calmodulin-dependent stimulation of erythrocyte Ca2+-ATPase (ATP phosphohydrolase, EC 3.6.1.3) and cyclic nucleotide phosphodiesterases (3',5'-cyclic-nucleotide 5'-nucleotidohydrolase, EC 3.1.4.17) from brain and heart. Basal activities of these enzymes in the absence of calmodulin are relatively unaffected by concentrations of local anesthetics that strongly inhibit the specific stimulation by calmodulin. Increasing calmodulin, but not Ca2+, overcomes the inhibitory action of the local anesthetics on brain phosphodiesterase. However, excess calmodulin does not fully restore activity of erythrocyte CA2+-stimulated ATPase. Although the mechanism(s) by which the local anesthetics act is unclear, they inhibit binding of 125I-labeled calmodulin to the erythrocyte membrane. Antagonism of calmodulin provides a molecular mechanism that may explain the inhibition of many Ca2+-dependent cellular processes by local anesthetics--e.g., Ca2+ transport, exocytosis, excitation-contraction coupling, non-muscle-cell motility, and aggregation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond G. H., Hudgins P. M. Inhibition of ATPase activity in human red cell membranes by tetracaine. Biochem Pharmacol. 1976 Feb 1;25(3):267–270. doi: 10.1016/0006-2952(76)90212-4. [DOI] [PubMed] [Google Scholar]
- Bressler R., Brendel K. Effect of local anesthetics on insulin secretion by pancreas pieces. Diabetes. 1971 Nov;20(11):721–728. doi: 10.2337/diab.20.11.721. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Anion permeability of the red blood cell. Naturwissenschaften. 1970 Apr;57(4):172–179. doi: 10.1007/BF00592968. [DOI] [PubMed] [Google Scholar]
- Eichhorn J. H., Peterkofsky B. Local anesthetic-induced inhibition of collagen secretion in cultured cells under conditions where microtubules are not depolymerized by these agents. J Cell Biol. 1979 Apr;81(1):26–42. doi: 10.1083/jcb.81.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B., Paimre M. Pharmacological action of local anesthetics on excitation-contraction coupling in striated and smooth muscle. Fed Proc. 1969 Sep-Oct;28(5):1643–1648. [PubMed] [Google Scholar]
- Feinstein M. G., Fiekers J., Fraser C. An analysis of the mechanism of local anesthetic inhibition of platelet aggregation and secretion. J Pharmacol Exp Ther. 1976 Apr;197(1):215–228. [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Haslam R. J., Lynham J. A. Relationship between phosphorylation of blood platelet proteins and secretion of platelet granule constituents II. Effects of different inhibitors. Thromb Res. 1978 Apr;12(4):619–628. doi: 10.1016/0049-3848(78)90251-7. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett H. W., Kyte J. Human erythrocyte calmodulin. Further chemical characterization and the site of its interaction with the membrane. J Biol Chem. 1979 Sep 10;254(17):8237–8244. [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- Katz A. M., Repke D. I., Upshaw J. E., Polascik M. A. Characterization of dog cardiac microsomes. Use of zonal centrifugation to fractionate fragmented sarcoplasmic reticulum, (Na+ + K+)--activated ATPase and mitochondrial fragments. Biochim Biophys Acta. 1970 Jun 30;205(3):473–490. doi: 10.1016/0005-2728(70)90113-1. [DOI] [PubMed] [Google Scholar]
- Kazimierczak W., Peret M., Maśliński C. The action of local anaesthetics on histamine release. Biochem Pharmacol. 1976 Aug 1;25(15):1747–1750. doi: 10.1016/0006-2952(76)90409-3. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Cassidy P. S. Calcium-activated tension: the role of myosin light chain phosphorylation. Fed Proc. 1980 Apr;39(5):1558–1563. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Lacko L., Wittke B., Lacko I. Interaction of local anesthetics with the transport system of glucose in human erythrocytes. J Cell Physiol. 1977 Aug;92(2):257–263. doi: 10.1002/jcp.1040920214. [DOI] [PubMed] [Google Scholar]
- Larsen F. L., Vincenzi F. F. Calcium transport across the plasma membrane: stimulation by calmodulin. Science. 1979 Apr 20;204(4390):306–309. doi: 10.1126/science.155309. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Dedman J. R., Brinkley B. R., Means A. R. Localization of calmodulin in rat cerebellum by immunoelectron microscopy. J Cell Biol. 1980 May;85(2):473–480. doi: 10.1083/jcb.85.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Lloyd D. H., Stein T. M., Rogers J. A., 3rd Calcium displacement by local anesthetics. Dependence on pH and anesthetic charge. J Biol Chem. 1979 May 25;254(10):4119–4125. [PubMed] [Google Scholar]
- Luthra M. G., Kim H. D. Effects of calcium and soluble cytoplasmic activator protein (calmodulin) on various states of (Ca2+ + Mg2+)-ATPase activity in isolated membranes of human red cells. Biochim Biophys Acta. 1980 Aug 4;600(2):467–479. doi: 10.1016/0005-2736(80)90449-6. [DOI] [PubMed] [Google Scholar]
- Nash-Adler P., Louis C. F., Fudyma G., Katz A. M. The modification of unidirectional calcium fluxes by dibucaine in sarcoplasmic reticulum vesicles from rabbit fast skeletal muscle. Mol Pharmacol. 1980 Jan;17(1):61–65. [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. The disruption of immunoglobulin caps by local anesthetics. Clin Immunol Immunopathol. 1976 Sep;6(2):264–269. doi: 10.1016/0090-1229(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978 Feb 2;271(5644):478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sobue K., Ichida S., Yoshida H., Yamazaki R., Kakiuchi S. Occurrence of a Ca2+- and modulator protein-activatable ATPase in the synaptic plasma membranes of brain. FEBS Lett. 1979 Mar 1;99(1):199–202. doi: 10.1016/0014-5793(79)80278-1. [DOI] [PubMed] [Google Scholar]
- Suko J., Winkler F., Scharinger B., Hellmann G. Aspects of the mechanism of action of local anesthetics on the sarcoplasmic reticulum of skeletal muscle. Biochim Biophys Acta. 1976 Sep 7;443(3):571–586. doi: 10.1016/0005-2736(76)90474-0. [DOI] [PubMed] [Google Scholar]
- Szász I., Sarkadi B., Gárdos G. Mechanism of Ca2+-dependent selective rapid K+-transport induced by propranolol in red cells. J Membr Biol. 1977 Jun 24;35(1):75–93. doi: 10.1007/BF01869941. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Thompson W. J., Epstein P. M., Strada S. J. Purification and characterization of high-affinity cyclic adenosine monophosphate phosphodiesterase from dog kidney. Biochemistry. 1979 Nov 13;18(23):5228–5237. doi: 10.1021/bi00590a030. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Feinstein M. B. Local anesthetics, chlorpromazine and propranolol inhibit stimulus-activation of phospholipase A2 in human platelets. Mol Pharmacol. 1979 Jul;16(1):171–180. [PubMed] [Google Scholar]
- Vargaftig B. B., Hai N. D. Selective inhibition by mepacrine of the release of "rabbit aorta contracting substance" evoked by the administration of bradykinin. J Pharm Pharmacol. 1972 Feb;24(2):159–161. doi: 10.1111/j.2042-7158.1972.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Voeikov V. L., Lefkowitz R. J. Effects of local anesthetics on guanyl nucleotide modulation of the catecholamine-sensitive adenylate cyclase system and on beta-adrenergic receptors. Biochim Biophys Acta. 1980 May 7;629(2):266–281. doi: 10.1016/0304-4165(80)90100-2. [DOI] [PubMed] [Google Scholar]
- Waite M., Sisson P. Effect of local anesthetics on phospholipases from mitochondria and lysosomes. A probe into the role of the calcium ion in phospholipid hydrolysis. Biochemistry. 1972 Aug 1;11(16):3098–3105. doi: 10.1021/bi00766a025. [DOI] [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Wong P. Y., Cheung W. Y. Calmodulin stimulates human platelet phospholipase A2. Biochem Biophys Res Commun. 1979 Sep 27;90(2):473–480. doi: 10.1016/0006-291x(79)91259-2. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]


