Abstract
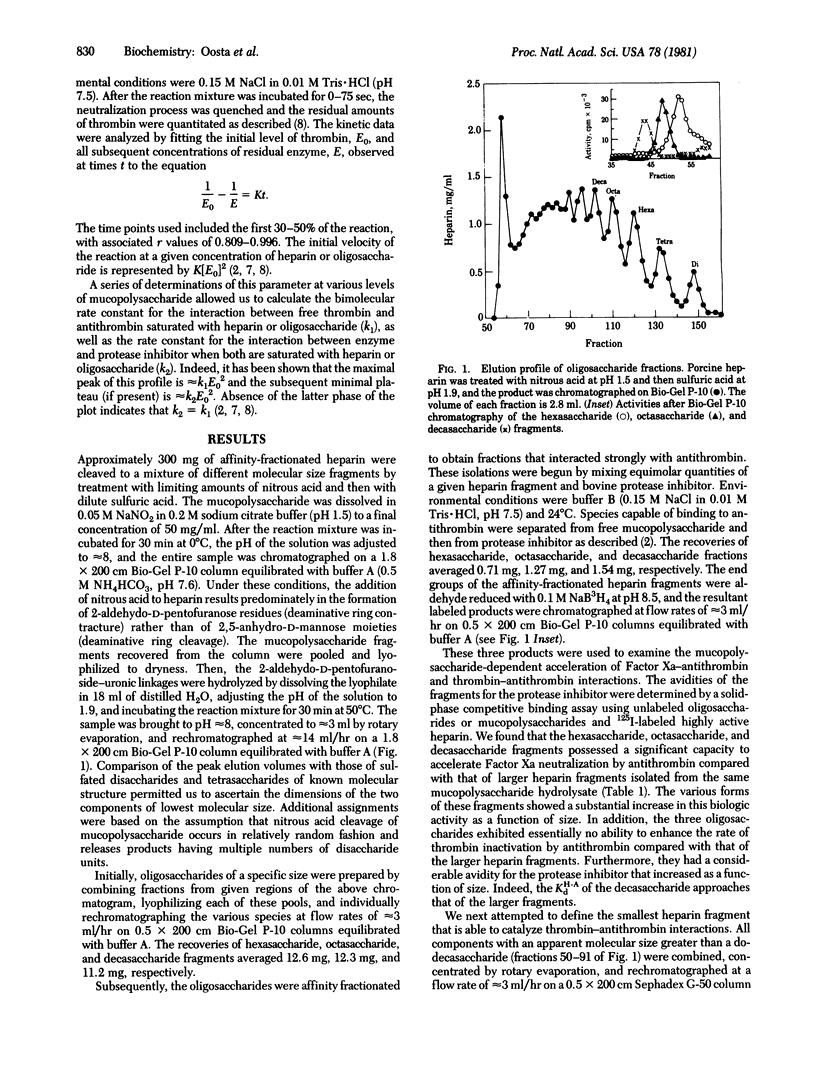
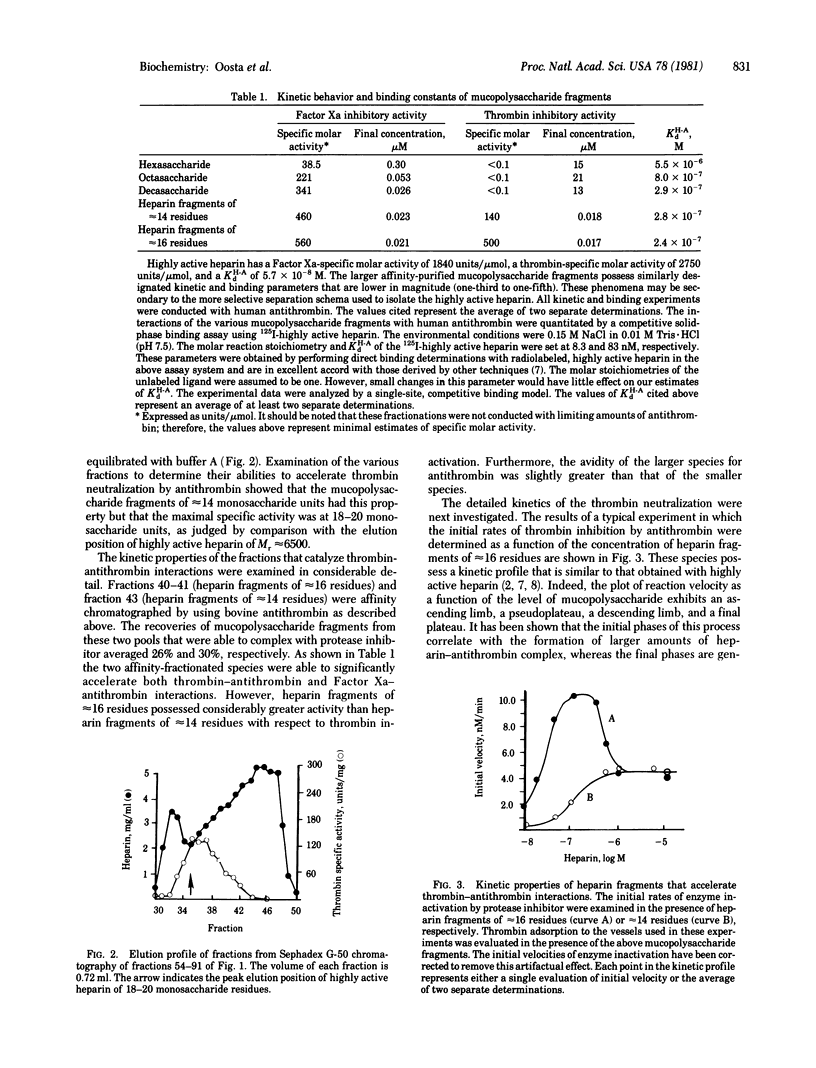
Affinity-fractionated porcine heparin was randomly scissioned by chemical techniques to give hexasaccharides, octasaccharides, decasaccharides, and mucopolysaccharide fragments of approximately 14 residues and approximately 16 residues that were able to complex with the protease inhibitor. Direct measurements of the kinetic behavior of the hexasaccharides, octasaccharides, and decasaccharides showed that these fractions greatly enhanced the rate of Factor Xa inactivation by antithrombin. Indeed, these species exhibited specific molar activities that ranged from 6.9% (hexaccharide) to 60.9% (decasaccharide) of that of the heparin fragment of approximately 16 residues. However, these oligosaccharides exhibited essentially no ability to accelerate thrombin-antithrombin interactions. The avidity of the hexasaccharides, octasaccharides, and decasaccharides for the protease inhibitor increased as a function of size with the respective dissociation constants ranging from 5.5 X 10(-6) M to 2.9 X 10(-7) M. These data suggest that the region of the heparin molecule needed for catalyzing Factor Xa-antithrombin interaction is intimately related to the antithrombin binding domain. The smallest complex carbohydrate fragment that accelerated the inactivation of thrombin by antithrombin had approximately 14 residues. This fraction had an avidity for the protease inhibitor of 2.8 X 10(-7) M and specific molar activities of 140 units per mumol (thrombin neutralization) and 460 units per mumol (factor Xa inactivation). The largest heparin fragment examined contained approximately 16 residues. This fraction had an avidity for antithrombin of 2.4 X 10(-7) M and specific molar activities of 500 units per mumol (thrombin neutralization) and 560 units per mumol (Factor Xa inactivation). Detailed kinetic analyses showed that these two species are able to directly activate antithrombin to the same extent with respect to thrombin inhibition. However, the larger mucopolysaccharide fragment is also capable of approximating free enzyme with protease inhibitor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Choay J., Lormeau J. C., Petitou M., Sinay P., Casu B., Oreste P., Torri G., Gatti G. Anti-Xa active heparin oligosaccharides. Thromb Res. 1980 May 1;18(3-4):573–578. doi: 10.1016/0049-3848(80)90356-4. [DOI] [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenman B., Nyström C., Björk I. The size and shape of human and bovine antithrombin III. Eur J Biochem. 1977 Aug 15;78(1):195–203. doi: 10.1111/j.1432-1033.1977.tb11730.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1218–1222. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Grundberg H., Riesenfeld J., Lindahl U. The molecular size of the antithrombin-binding sequence in heparin. FEBS Lett. 1980 Aug 11;117(1):203–206. doi: 10.1016/0014-5793(80)80945-8. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Lindahl U., Tengblad A., Laurent T. C., Jackson C. M. On the molecular-weight-dependence of the anticoagulant activity of heparin. Biochem J. 1979 Jul 1;181(1):241–243. doi: 10.1042/bj1810241. [DOI] [PMC free article] [PubMed] [Google Scholar]


