Abstract
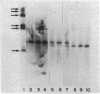
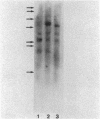
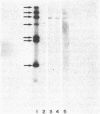
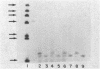
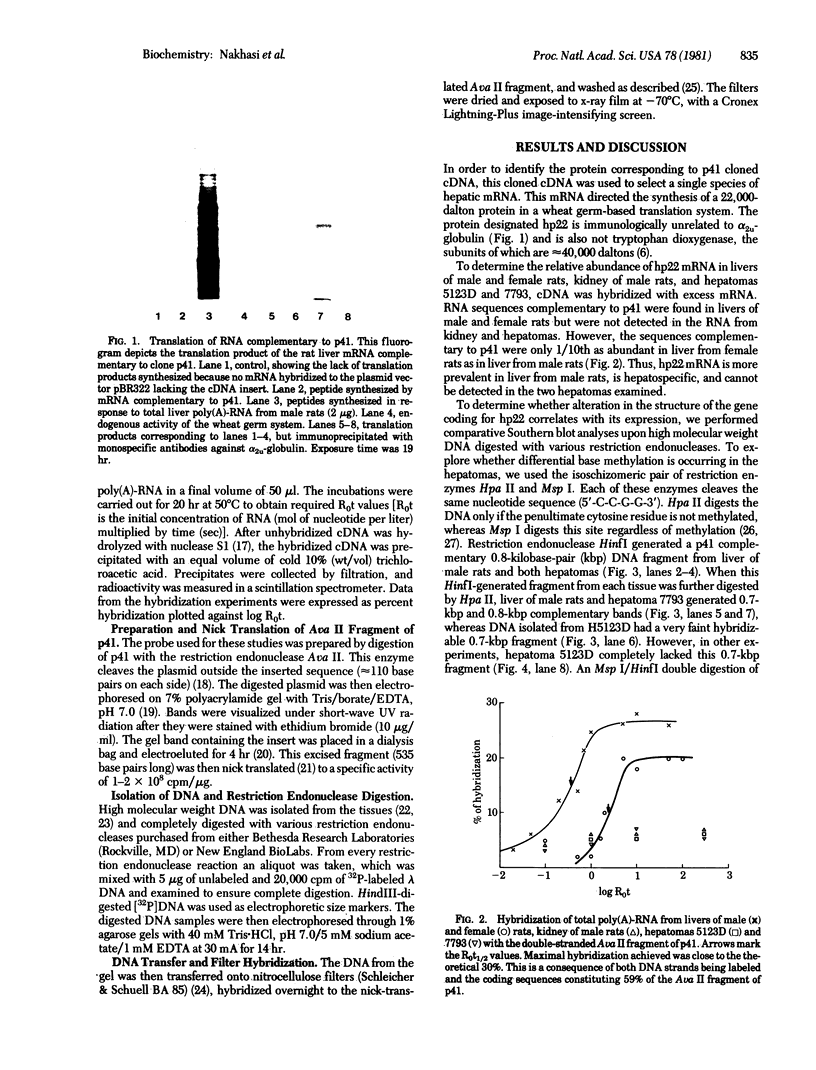
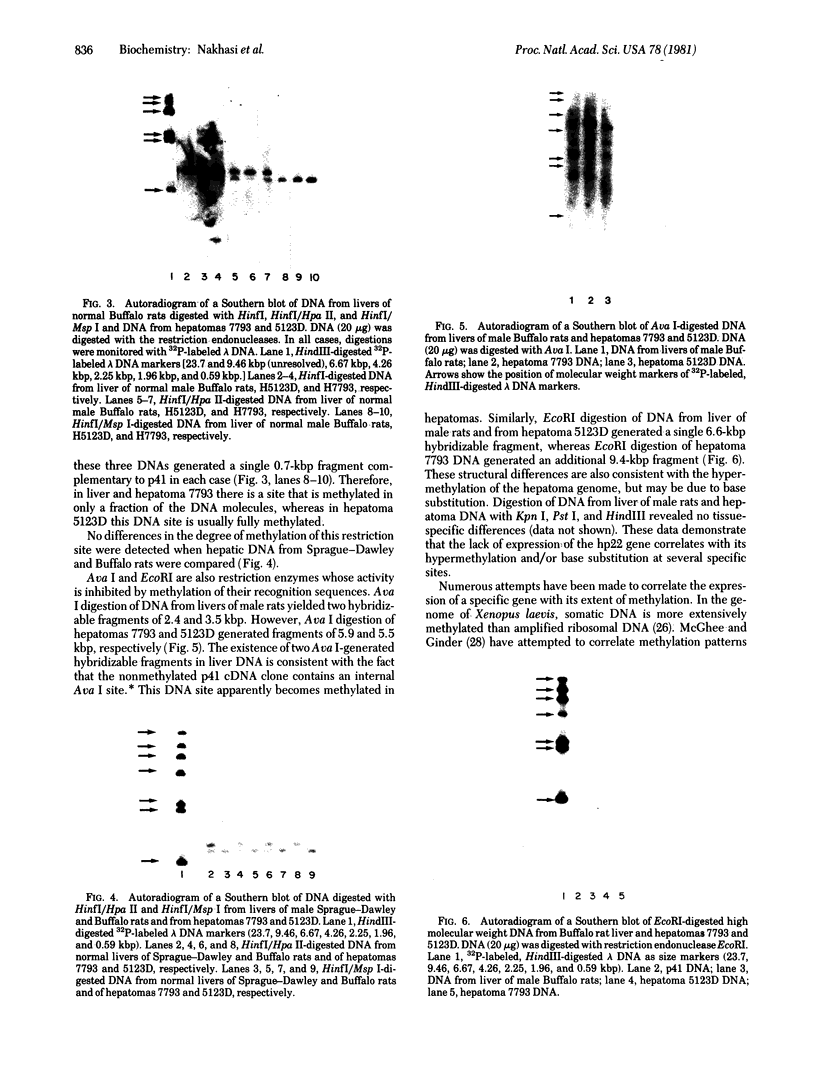
When liver cells undergo malignant transformation, certain genes cease being expressed. We have studied the structure of one such gene, whose protein product we have designated hepatic protein 22 (hp22), which is not expressed in the two Morris hepatomas studied. We have prepared a chimeric clone of pBR322 containing cDNA sequences complementary to mRNA coding for this protein. By using this cloned cDNA, we have examined changes in expression of this gene and changes in the restriction pattern of the DNA isolated from normal liver and these hepatomas. In both hepatomas, studies using the isoschizomeric pair of restriction enzymes Msp I and Hpa II have indicated hypermethylation of a cytosine residue within or proximal to the hp22 gene. Other differences in the restriction pattern between normal liver and hepatoma DNA were also detected with EcoRI and Ava I. Thus, in the nontranscribed form of this gene, the DNA has undergone covalent modification, distinguishing these two hepatomas from each other and from normal liver.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigelson P., Kurtz D. T. Hormonal modulation of specific messenger RNA species in normal and neoplastic rat liver. Adv Enzymol Relat Areas Mol Biol. 1978;47:275–312. doi: 10.1002/9780470122921.ch4. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Feigelson P. Multihormonal induction of hepatic alpha2u-globulin mRNA as measured by hybridization to complementary DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4791–4795. doi: 10.1073/pnas.74.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. J., Burgoyne L. A. Interpretation of the properties of chromatin extracts from mammalian nuclei. Nucleic Acids Res. 1976 Apr;3(4):1101–1110. doi: 10.1093/nar/3.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Quasba P. K. Quantitation of milk proteins and their mRNAs in rat mammary gland at various stages of gestation and lactation. J Biol Chem. 1979 Jul 10;254(13):6016–6025. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pennica D., Lynch K. R., Cohen P. S., Ennis H. L. Decay of vesicular stomatitis virus mRNAs in vivo. Virology. 1979 Apr 30;94(2):484–487. doi: 10.1016/0042-6822(79)90480-x. [DOI] [PubMed] [Google Scholar]
- Ramanarayanan-Murthy L., Colman P. D., Morris H. P., Feigelson P. Pretranslational control of tryptophan oxygenase levels in Morris hepatoma and host liver. Cancer Res. 1976 Oct;36(10):3594–3599. [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Sargent T. D., Sell S., Bonner J. alpha-Fetoprotein and albumin genes of rats: no evidence for amplification-deletion or rearrangement in rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):695–699. doi: 10.1073/pnas.76.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E., Kurtz D. T., Morris H. P., Feigelson P. Comparison of in vivo translation rates and messenger RNA levels of alpha2U-globulin in rat liver and Morris hepatoma 5123D. Cancer Res. 1976 Oct;36(10):3588–3593. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Church G. M. The cleavage site of the restriction endonuclease Ava II. Nucleic Acids Res. 1978 Jul;5(7):2313–2319. doi: 10.1093/nar/5.7.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]