Abstract
Pulsatile release of GnRH-1 is critical for reproductive function. However, the cellular mechanism of GnRH-1 neurosecretion is still elusive. In this study, we examined the neurosecretory process of GnRH-1 neurons using time-lapse image acquisition followed by immunocytochemistry with confocal microscopy. To monitor exocytotic processes, cultured GnRH-1 neurons derived from monkey embryos were labeled with the lipophilic dye, FM1-43, or its fixable form FM1-43Fx, in the presence or absence of depolarization signals, and changes in vesicles labeled with FM1-43 were analyzed. The results show FM1-43 was taken up into the cell and labeled puncta in the soma and neuroprocesses in the absence of depolarization signals, indicating that GnRH-1 neurons were spontaneously active. Depolarization of GnRH-1 neurons with high K+ or veratridine challenge increased the intensity and size of puncta in both soma and neuroprocesses, and the veratridine-induced changes in puncta were blocked by tetrodotoxin, indicating that changes in the puncta intensity and size reflect neurosecretory activity. Subsequent double immunocytochemistry for GnRH-1 and the synaptic vesicle marker, vesicle-associated membrane protein, demonstrated that the FM1-43Fx-labeled puncta were synaptic vesicles with the GnRH-1 peptide. Additional double immunocytochemistry for GnRH-1 and the marker of the neurosecretory active zone, Bassoon, indicated that the FM1-43Fx-labeled puncta were located at the sites of neurosecretory active zones in GnRH-1 neurons. These results suggest that GnRH-1 neurons have the capacity to release the peptide from the soma and dendrites. Collectively, we hypothesize that soma-dendritic release of the peptide may be a mechanism of synchronized activity among GnRH-1 neurons.
The mammalian form of GnRH-1 is a central molecule involved in the regulation of reproduction. This neurohormone is synthesized as the precursor form in the hypothalamus and preoptic area and subsequently cleaved to form the decapeptide. GnRH-1 is released from neuroterminals in the median eminence into the portal circulation and controls synthesis and release of pituitary gonadotropins (LH and FSH). The release of both GnRH-1 and LH is pulsatile (1–4), and changes in the pulsatile pattern govern gamete maturation, steroid hormone secretion, ovulation, and maintenance of luteal function (5, 6). An abnormality in the pulsatile pattern of LH secretion (and presumably an abnormality in pulsatile GnRH-1 secretion) is associated with reproductive disorders, such as polycystic ovarian syndrome, anorexia nervosa, and amenorrhea (7, 8).
Despite the importance of pulsatile GnRH-1 release, the cellular and molecular mechanisms of GnRH-1 neurosecretion are still elusive. This is because it is difficult to study GnRH-1 neurons in situ. GnRH-1 neurons are small in number, do not form nuclei in the preoptic area and hypothalamus (they scatter widely), and neuroterminals are located far from the cell body. Accordingly, we have made a cell culture system to examine cellular and molecular mechanisms of GnRH release and their maturation (9, 10).
Endocrine cells, such as pancreatic β-cells and pituitary cells, release peptides synchronously from their cell bodies (11–14). Similarly, during lactation and dehydration, oxytocin and vassopressin neurons, respectively, release peptides from dendrites and cell soma (15–17) for synchronized activity (18, 19). However, it is unclear whether GnRH-1 neurons release the decapeptide from cell soma and dendrites as a tool for their synchronized neurosecretion.
As a step toward understanding the mechanism of the neurosecretory process in GnRH-1 neurons and possible neurosecretion from the cell bodies, in the present study, we examined cultured GnRH-1 neurons derived from rhesus monkey embryos using FM1-43 and a fixable form of FM1-43 (FM1-43Fx). FM1-43 is a lipophilic dye, which incorporates into the plasma membrane but does not cross the membrane. Upon neurotransmitter exocytosis followed by endocytosis, the dye is incorporated into the vesicular membrane (20), and thus the intensity of fluorescence reflects events of vesicular exocytosis/endocytosis (21). Because the GnRH-1 peptide is packaged in large dense core vesicles (LDCV) (22), visualization of large dense core vesicles with FM dyes would provide useful information regarding GnRH-1 neurosecretion. Because we initially found that exposure of GnRH-1 neurons to FM dyes yielded distinct puncta in the soma and neuroprocesses (dendrites), we further examined 1) whether application of a depolarization signal, previously shown to cause GnRH-1 release (23), increases the intensity and size of puncta, and if so, whether blockade of the depolarization prevents these changes; 2) whether FM dye-labeled puncta coexpress GnRH-1 peptide and vesicle-associated membrane protein (VAMP) (24); and 3) whether GnRH-1 containing vesicles colocalized with Bassoon, a marker for neurosecretory active zones (25–27).
Materials and Methods
Animals
A total of eight fetuses at embryonic day (E)35–E38 obtained from female rhesus monkeys (Macaca mulatta) with time-mated pregnancy was used in this study. Fetuses were delivered by Cesarean section under isoflurane anesthesia. The procedures for time-mated pregnancy and calculation of fetal age were described previously (9). The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the National Institutes of Health and United States Department of Agriculture.
Tissue preparation and culture conditions
Primary cultures of GnRH-1 neurons were prepared as described previously (9). Briefly, the nasal placode, from which GnRH-1 neurons originate, and the terminal nerve region, which includes the GnRH-1 migratory pathway, were dissected out, minced, and plated on 25-mm round glass coverslips with an engraved grid (No. 1, Bellco Glass, Inc., Vineland, NJ). In two fetuses, a few pieces of minced nasal placode were also plated on glass bottom dishes (MatTek, Ashland, MA). To compare the properties of non-GnRH neurons, in three fetuses, a small portion of the forebrain telencephalon was dissected out, minced, and plated. Coverslips were coated with dried rat-tail collagen. Cultures were maintained in 35-mm tissue culture dishes (Corning, Corning, NY) containing growth medium (Medium 199 plus l-glutamine; Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 0.6% glucose, and 50 μg/ml gentamycin and incubated at 37 C with 1.5% CO2 and 98.5% air. On the fourth day of culture, cells on glass coverslips were exposed to an antimitotic agent, 5-fluoro-5deoxyuridine (30–40 μm), for 2 d to better visualize GnRH-1 neurons. Medium was replaced every 1–3 d as needed. All experiments were carried out during the 3rd–4th wk in culture.
Imaging with FM1-43 or FM1-43Fx
We conducted two experiments with either FM1-43 or FM1-43Fx. In experiment 1, to capture the events associated with GnRH-1 neurosecretion using a confocal microscope, GnRH neurons were cultured on glass bottom dishes, and experiments were conducted under static conditions. Cells were exposed to 10 μm FM1-43 (Molecular Probes, Eugene, OR) for 7 min and washed five times with serum-free M199 media. Then, they were subjected to time-lapse live cell imaging with a confocal microscope (model TCS SP2 AOBS; Leica Microsystems, Wetzlar, Germany) at 10-sec intervals with a ×60 objective lens. Live cell images were captured for 5-min as a control, and then 56 mm K+ was applied to the cells for 5 min, while imaging was continuously recorded. Subsequently, the cells were washed with media five times, and an additional 5 min of imaging was captured. After the experiments, all cultures were fixed with 4% paraformaldehyde for later GnRH-1 immunostaining.
Because in experiment 1 we observed that changes in FM1-43-labeled puncta (defined as a distinct green spot with a diameter of 2–10 μm) occurred in association with neurosecretory events, in experiment 2 we examined 1) whether FM1-43Fx-labeled puncta were vesicles and 2) whether they localized at the active zone of exo/endocytosis using double immunocytochemistry after time-lapse live cell imaging. To monitor changes in the neurosecretory process, GnRH-1 neurons were successively exposed to 480-nm excitation at 10-sec intervals with a Lambda DG4 (Sutter Instrument, Novato, CA), while signals emitted at 500–575 nm were captured through a 73000v2 filter (Chroma Technology, Bellows Falls, VT) using a CCD camera (Photometrics, Tucson, AZ). The data were collected using Nikon Imaging software (NIS-Elements, Nikon, Melville, NY).
For time-lapse live cell imaging, cells cultured on glass coverslips were mounted in a Dvorak-Stotler recording chamber, GnRH-1 neurons were identified with a Nikon Ti-E inverted microscope (Nikon), and viewed through a ×20 epifluorescence objective lens with a 750 × 750 μm recording field. Experiments were conducted under continuous perifusion with serum-free M199 media [(pH 7.4) 95% O2 and 5% CO2] or experimental drugs (see below) in M199 media at a speed of 50 μl/min at room temperature under low light condition. FM1-43Fx changes in GnRH-1 neurons were continuously recorded at 10-sec intervals with the following protocol: the cells were 1) washed with media for 5 min, 2) loaded with 10 μm FM1-43Fx (Molecular Probes) alone for 15 min, 3) loaded with 10 μm FM1-43Fx containing 56 mm K+ or 5 μm veratridine for 5 min, 4) washed with media for 30 min, and 5) challenged with 56 mm K+ for 5 min, 5 μm veratridine for 5 min, or 5 μm tetrodotoxin (TTX) for 5 min followed by 5 μm veratridine plus 5 μm TTX for 5 min. Immediately after experiments, the cells were fixed with 4% paraformaldehyde for 20 min for later immunocytochemistry.
Immunocytochemistry
Fixed cells on coverslips were washed three times with PBS and incubated with blocking solution consisting of 1% BSA, 0.1% gelatin, and 0.25% Triton X-100 in PBS for 1 h at room temperature. Cells were then incubated with an antimouse monoclonal GnRH-1 antiserum [LRH13 supplied by Min K. Park (University of Tokyo, Tokyo, Japan) or HU4H supplied by Henryk F. Urbanski, Oregon National Primate Research Center, Beaverton, OR)] diluted to 1000× in blocking solution for 48 h at 4 C. In experiment 2, the cells were subsequently washed with PBS three times and incubated with polyclonal antirabbit antibody against VAMP (Synaptic System, Göttingen, Germany) diluted to 100× or polyclonal antirabbit antibody against Bassoon (Abcam, Cambridge, MA) diluted to 400× in blocking solution for 48 h at 4 C. After the cells were washed with PBS three times, they were exposed to antimouse Alexa Fluor 594 (Invitrogen, Carlsbad, CA) for GnRH-1 and antirabbit Alexa Fluor 350 (Invitrogen) with the Tyramide Signal Amplification kit (Invitrogen) for VAMP and Bassoon. Stained cells were mounted on glass slides using Vectashield mounting medium (Vector Lab, Burlingame, CA). To preserve FM1-43Fx fluorescence in the cells, all procedures were conducted in the dark.
Visualization of stained cells with confocal microscopy
Stained cells were visualized with an inverted confocal microscope Nikon A1R Eclipse Ti-E. Immunostained GnRH-1 neurons were located with a ×20 objective, and subsequently, z-series pictures in a specific region were obtained with a ×60 objective with oil. VAMP and Bassoon images were obtained with a laser excitation of 408 nm, and FM1-43Fx and GnRH-1 were obtained with a laser excitation of 488 and 561 nm, respectively. We defined colocalization of FM1-43Fx puncta with GnRH-1 and VAMP (or Bassoon) as follows. If three signals (FM1-43Fx, GnRH-1, and VAMP or Bassoon) overlap, the merged image appeared as a white spot. Among the white spots identified, we further confirmed that there was a red and a blue spot in each channel corresponding to a green punctum. However, because of the difference in the staining intensity with the three antibodies, sometimes the three individually observed signals appeared slightly off-white when merged.
Because there was a possible interference between fluorescence spectra, we conducted two experiments. First, we used time-lapse imaging in labeled GnRH neurons to see if FM1-43 fluorescence was seen with 480- and 408-nm laser excitation. We found that FM1-43 signal was detected only when excited with 480 nm, but not 408 nm (data not shown). Second, we examined whether the Alexa Fluor 350 (VAMP and Bassoon) and Alexa Fluor 594 (GnRH-1) fluorescence interfered with fluorescence detected with the 480-nm laser. Cells were fixed without adding FM1-43Fx and immunostained using Alexa Fluor 594 and Alexa Fluor 350. Under the confocal microscope, a series of pictures was acquired using 408-, 480-, and 561-nm laser excitation. No fluorescence was detected in the 480-nm channel (data not shown). These observations suggest that there was no interference between the fluorescence spectra in this study.
Image analysis
Changes in FM1-43 puncta were assessed using the Nikon imaging software. A grid with a square region of interest was overlaid on a punctum of the soma or neuroprocess, and the fluorescence intensity was measured. Analysis of confocal images was conducted using NIH ImageJ64.
Statistical analysis
The effects of high K+ on the number and size of puncta in the soma and neuroprocesses were compared using one-way ANOVA repeated measures. Statistical significance was attained at P < 0.05.
Results
Spontaneous GnRH-1 exocytotic activity detected with FM1-43 and FM1-43Fx labeling
We obtained fluorescent time-lapse images at 10-sec intervals from individual GnRH-1 neurons with the Leica confocal microscope (experiment 1) or Nikon epifluorescence microscope (experiment 2). Exposure of GnRH-1 neurons to either FM1-43 for 7 min or FM1-43Fx for 15 min in the absence of depolarization stimuli resulted in labeling of puncta, small fluorescent spots, in various sizes on the cell bodies (Figs. 1A, before, and 2A, 1), especially soma membranes (Fig. 1C, before), and also the neuroprocesses (Fig. 2B, 1).
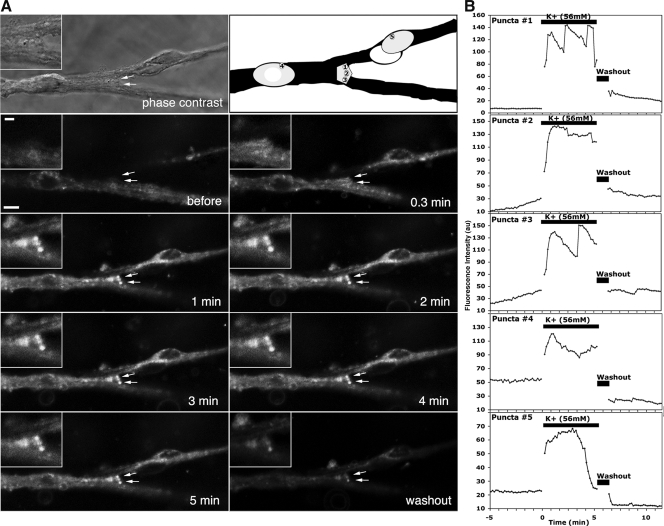
Fig. 1.
Fluorescent time-lapse images (with a phase photomicrograph and its schema) of GnRH-1 cell somata (A), a neuroterminal (two arrows in A), and cell membrane of a soma (C) labeled with FM1-43 (from experiment 1). Changes in the FM1-43 intensity of GnRH-1 neurons and neuroterminals were obtained with a confocal microscope at 10-sec intervals. After a 5-min control period, high K+ was applied directly to the bath for 5 min in a static condition, cells were then washed three times with culture medium, and recording was continued for an additional 5 min. Images from right before K+ challenge, 0.3, 1, 2, 3, 4, 5 min after 56 mm K+, and 5 min after washout from two cultures are shown (A and C). Insets in the upper left are enlargements of puncta on the open neuroterminal that is also seen in the phase contrast picture (A). Quantitative changes in FM1-43 intensity of GnRH-1 neurons and neuroteminals: three puncta on a neuroterminal (marked 1–3 in the outline schema A) and two puncta on two somata (marked 4 and 5 in the outline schema A), and three puncta on the soma membrane (marked 1–3 in C, before) are shown in graph format (B and D). Note that changes in the intensity and size of puncta in the terminal and cell soma increased after depolarization by high K+ (B and D). Note that in all cases, the FM1-43 intensity increases and fluctuates during depolarization, although the patterns of intensity changes among the puncta differ (B and D). Neurons shown in this figure were GnRH-1 neurons, confirmed by post hoc immunocytochemistry (data not shown). Scale bar, 10 μm.
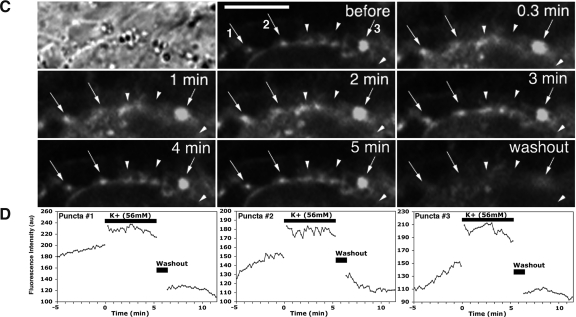
Fig. 2.
Epifluorescent time-lapse images with a phase photomicrograph of GnRH-1 cell bodies (A) and a neuroprocess (B) labeled with FM1-43Fx (from experiment 2). After a 5-min media infusion, FM dye was infused for 20 min, of which, during the last 5 min, high K+ was also infused, then culture medium was infused for 30 min, and finally high K+ was again infused for 5 min, while images at 10-sec intervals were continuously obtained. Immediately after the second high K+ challenge, cells were fixed with 4% paraformaldehyde for immunocytochemistry. In A and B, images at 15-min of infusion of FM1-43Fx alone (marked 1), 4.5 min in FM1-43Fx plus high K+ (marked 2), 2.5, 5, 15, and 30 min after exposure to FM1-43Fx plus high K+ (marked 3–6, respectively), and 2.5 min after second exposure to high K+ (marked 7) are shown. In the bottom panel, quantitative changes in the intensity of two puncta in the soma (marked a and b in A) and two puncta in the neuroprocess (marked c and d in B) during the entire experiment are shown. Open and hatched bars in the graphs indicate the exposure to FM1-43Fx and high K+, respectively. Note that exposure to high K+ increased the intensity of puncta. Scale bar, 10 μm.
Depolarization of GnRH-1 neurons with high K+ changes the intensity and size of puncta
Previously, we reported that depolarization with high K+ stimulated release of the decapeptide from cultured primate GnRH-1 neurons in a dose-dependent manner (23). If FM dye-labeled puncta reflect GnRH-1 neurosecretory activity, depolarization of GnRH-1 neurons with high K+ would cause changes in the intensity and/or size of puncta. Direct bath application (experiment 1) of 56 mm K+ for 5 min increased the intensity and size of FM1-43-labeled puncta in the somata, neuroterminals, and soma membranes (Fig. 1, A and C, 0.3–5 min). Subsequent washout of the cells reduced the intensity of puncta (Fig. 1, A and C, washout). Similarly, 56 mm K+ infusion to the chamber for 5 min (experiment 2) increased the intensity and size of puncta in the somata (Fig. 2A, 2, 3, 4, and 7) and neuroprocesses (Fig. 2B, 2, 3, 4, and 7) during the K+ exposure and 5 min after K+. In some cases, however, the K+-induced increase returned to the control level during the exposure.
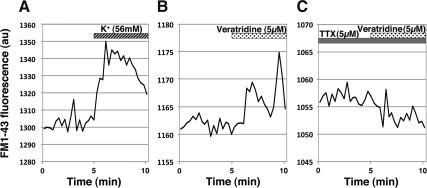
Fig. 3.
Examples of changes in epifluorescence levels of a punctum with 56 mm K+ challenge (A), 5 μm veratridine challenge alone (B), or 5 μm veratridine challenge in the presence of 5 μm TTX (C). Note that both veratridine and high K+ increased the fluorescence levels due to an increase in the intensity and size, whereas the veratridine-induced fluorescent increase was blocked by TTX. Note that the veratridine-induced fluorescence increase was not as large as the K+-induced fluorescence increase.
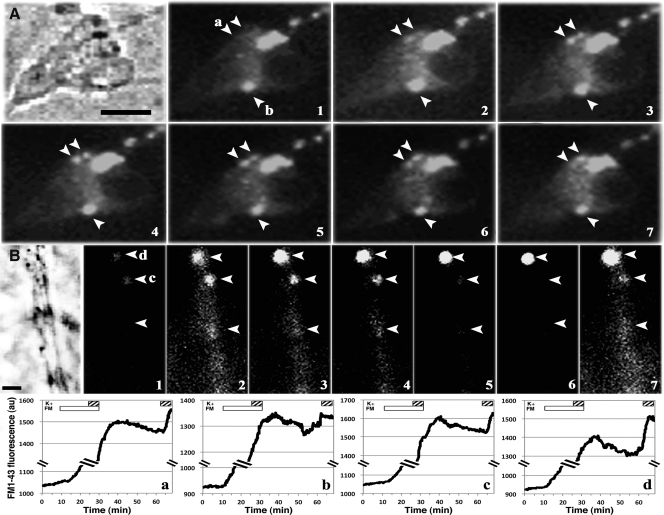
Fig. 4.
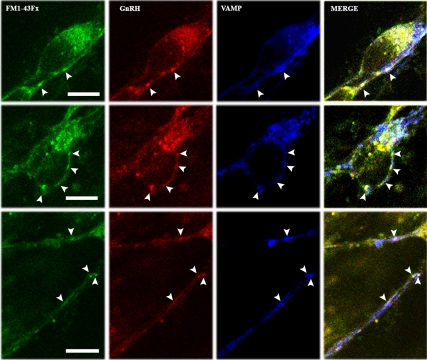
Puncta labeled with FM1-43Fx are synaptic vesicles. Examples from two GnRH-1 somata (top and middle panels) and two neuroprocesses (bottom panel) are shown. Cells were fixed immediately after the second 5-min infusion of 56 mm K+ (see Materials and Methods) and subjected to double immnocytochemical staining with GnRH-1 and VAMP antibodies. A single plane confocal microscope picture, which corresponds to the middle of the cells obtained from the z-series, indicates that puncta (arrowheads) labeled with FM dye (green) were also immunopositive with GnRH-1 (red) and VAMP (blue). White spots in the merged pictures of the three signals indicate colocalization of FM1-43Fx, GnRH-1, and VAMP. Scale bar, 10 μm.
Fig. 7.
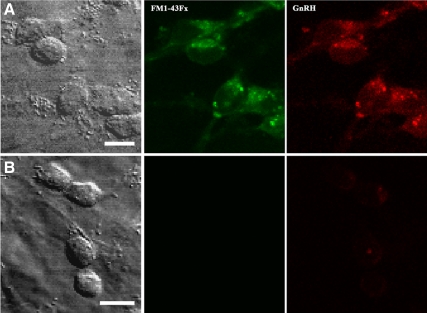
Comparison between GnRH-1 neurons (A) and non-GnRH neurons (B) in the same culture preparation from the GnRH-1 migratory pathway. Confocal images are shown. Although GnRH-1 neurons were GnRH-1 immunopositive (red) and clearly labeled with FM1-43Fx (green), non-GnRH neurons were GnRH-1 immunonegative and not labeled with FM1-43Fx. Scale bar, 10 μm.
Quantitative analyses of the puncta intensity in the somata and neuroteminals in both experiment 1 (Fig. 1, B and D) and experiment 2 (Figs. 2, bottom panel, and 3A) indicated that high K+ exposure resulted in an increase in fluorescent intensity within 1 min, and the intensity fluctuated during the 5-min period of the K+ exposure (Figs. 1, B and D, and 3A). Interestingly, the patterns of intensity changes during the depolarization were different among the puncta, regardless of their location in cell structures (Fig. 1, B and D). Exposure of cultures to high K+ significantly increased the size of puncta in somata from 3.4 ± 0.3 (mean ± sem) to 6.6 ± 0.3 μm2 in experiment 1 and 3.7 ± 0.4 to 7.5 ± 0.3 μm2 in experiment 2 (P < 0.001 for both) (Table 1) as well as in neuroprocesses from 4.9 ± 0.4 to 6.1 ± 0.3 μm2 in experiment 1 and 4.4 ± 0.7 to 8.4 ± 1.2 μm2 in experiment 2 (P < 0.01 for both) (Table 1). The number of puncta in the soma also increased transiently after high K+ (4.9 ± 0.6 to 5.9 ± 0.5 in experiment 1, 0.7 ± 0.2 to 1.5 ± 0.3 in experiment 2; P < 0.05 for both) (Table 1), whereas the number of puncta in the neuroprocess did not change (Table 1). The number of puncta per cell in experiment 2 was smaller than experiment 1, due to a lower resolution of the epifluorescence microscope at a ×20 objective.
Table 1.
Changes in the number and size of puncta during the high K+ exposure in GnRH-1 neuronal cell bodies and neuroprocesses in experiments 1 and 2
| No. of cell bodies or neuroprocesses | Puncta no./cell or neuroprocess |
Puncta size (μm2) |
|||
|---|---|---|---|---|---|
| Before K+ | After K+ | Before K+ | After K+ | ||
| Somata (experiment 1) | 15 | 4.9 ± 0.6 | 5.9 ± 0.5a | 3.4 ± 0.3 | 6.6 ± 0.3c |
| Neuroprocesses (experiment 1) | 16 | 4.6 ± 0.7 | 5.6 ± 0.5 | 4.9 ± 0.4 | 6.1 ± 0.3b |
| Somata (experiment 2) | 9 | 0.7 ± 0.2 | 1.5 ± 0.3a | 3.7 ± 0.4 | 7.5 ± 0.3c |
| Neuroprocesses (experiment 2) | 5 | 1.8 ± 0.2 | 1.8 ± 0.2 | 4.4 ± 0.7 | 8.4 ± 1.2b |
P < 0.05 vs. before high K+.
P < 0.01 vs. before high K+.
P < 0.001 vs. before high K+.
Changes in puncta size with depolarization are a sign of neurosecretion
In our earlier study, we found that the exposure of GnRH-1 neurons to the Na+ channel activator, veratridine, stimulated GnRH-1 release and that this veratridine-induced GnRH-1 release was blocked by the Na+ channel blocker TTX (23). To determine whether changes in the puncta intensity and size by a depolarization signal reflect a neurosecretory event, we examined the effects of veratridine on the FM1-43Fx-labeled puncta in the presence or absence of TTX. Quantitative analysis indicated that although veratridine increased the intensity and size of puncta in the absence of TTX (Fig. 3B), in the presence of TTX, the veratridine-induced increase in puncta did not occur (Fig. 3C).
FM1-43Fx puncta in somata and neuroprocesses colabel GnRH-1 and VAMP
Studies with photoconversion of FM1-43 have shown that the dye is incorporated into clusters of synaptic vesicles in neuroterminals of motoneurons and synapses of hippocampal neurons (21, 28, 29). To determine whether a FM dye-labeled punctum is a cluster of GnRH-1 neurosecretory vesicles, GnRH-1 neurons treated with FM1-43Fx in experiment 2 were fixed right after the 5-min K+ challenge and immunostained with a GnRH-1 antibody and the synaptic vesicle marker, VAMP, antibody. As shown in Fig. 4, FM1-43Fx puncta (Fig. 4, green) located in the somata and neuroprocesses were GnRH-1 immunopositive (Fig. 4, red), and they were also VAMP immunopositive (Fig. 4, blue). The merged picture of all three labels suggests that the FM1-43Fx-labeled puncta (Fig. 4, arrowheads) were indeed synaptic vesicles containing the GnRH-1 peptide.
FM1-43Fx puncta in somata and neuroprocesses colabel GnRH-1 and Bassoon
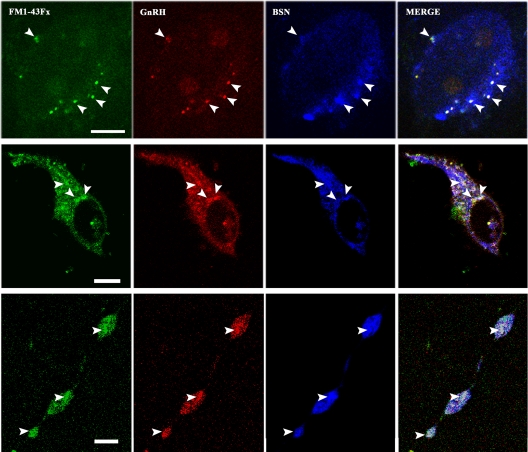
Bassoon is a large protein localized at the neurosecretory active zone (26). If the puncta in GnRH-1 neurons are located at the neurosecretory active zone, puncta labeled with FM dye may coexpress Bassoon. To examine this possibility, GnRH-1 neurons treated with FM1-43Fx in experiment 2 were fixed right after the 5-min K+ challenge and immunostained with a GnRH-1 antibody and the active zone marker, Bassoon, antibody. As shown in Fig. 5, FM1-43Fx puncta (Fig. 5, green) located in the somata and neuroprocesses were GnRH-1 immunopositive (Fig. 5, red), and they were also Bassoon immunopositive (Fig. 5, blue). Varicocities on neuroprocesses were also both GnRH-1 and Bassoon positive (Fig. 5, bottom panel). The merged picture of all three labels (30) suggests that the FM1-43Fx-labeled puncta containing the GnRH-1 peptide were indeed located at the active zone of exocytosis.
Fig. 5.
Colocalization of FM1-43Fx-labeled puncta, GnRH-1, and the marker for the active zone, Bassoon (BSN), indicates GnRH-1 secretory vesicles in the soma and neuroprocess located at the active sites of exocytosis. Representative cases of a GnRH-1 soma (top and middle panels) and one neuroprocess with three varicosities (bottom panel) are shown. All cultures were fixed immediately after the second 5-min infusion of 56 mm K+ (see Materials and Methods) and subjected to double immunocytochemical staining with GnRH-1 and Bassoon antibodies. A single plane confocal microscope picture indicates that puncta (arrowheads) labeled with FM dye (green) were also immunopositive with GnRH-1 (red) and Bassoon (blue). White spots in the merged pictures of the three signals indicate colocalization of FM1-43Fx, GnRH-1, and Bassoon. Scale bar, 10 μm.
Similar to the results from the K+ challenge, several puncta colabeled with GnRH and Bassoon were seen with the veratridine challenge (Fig. 6, top panels). In contrast, there was almost no colabeling of puncta with Bassoon, when the veratridine-induced challenge was applied in the presence of TTX (Fig. 6, bottom panels).
Fig. 6.
TTX blocks the veratridine-induced puncta increase. Confocal images (green, FM1-43Fx; red, GnRH-1; blue, Bassoon (BSN); and white, all merged) of clustered GnRH-1 neurons treated with veratridine alone (top panel) and veratridine in the presence of TTX (bottom panel). Note that although several puncta were observed in a cluster of 5 GnRH-1 neurons with veratridine alone, only one punctum was barely seen in a cluster of five GnRH-1 neurons with veratridine plus TTX, indicating the veratridine-induced puncta increase was blocked by TTX. Scale bar, 10 μm.
Non-GnRH neurons differ from GnRH neurons
As we described previously (10), a small number of cultures from the GnRH-1 migratory pathway in the terminal nerve region in older fetuses contains non-GnRH neurons, in addition to GnRH-1 neurons. This was the case in cultures obtained from one fetus at E38. Interestingly, unlike GnRH-1 neurons in the same culture, GnRH-1-negative neurons were neither labeled with FM1-43Fx (Fig. 7, bottom panels) nor did they respond to high K+ (data not shown). Subsequently, we obtained cultures of non-GnRH neurons from the telencephalic vesicles (31) and conducted similar experiments to that described with GnRH-1 neurons. Again, non-GnRH neurons were not labeled with FM1-43Fx and did not respond to high K+ (data not shown).
Discussion
In this study, we were able to visualize the neurosecretory process of primate GnRH-1 neurons with FM1-43. We found that in GnRH-1 neurons, 1) the puncta are labeled with FM1-43 not only in the neuroterminals but also in the somata and neuroprocesses; 2) the intensity and size of puncta increase with exposure to depolarization signals, such as high K+ and veratridine; 3) the veratridine-induced changes in puncta are blocked by the fast Na+ channel blocker, TTX; 4) FM1-43-labeled puncta carrying GnRH-1 peptide are a cluster of secretory vesicles, because they are also labeled with VAMP; and 5) GnRH-1 secretory vesicles in the somata and neuroprocesses appear to locate at the active sites of exocytosis, because colocalization of FM1-43-labeled puncta, GnRH-1, and Bassoon, was observed. Altogether, GnRH-1 neurons release the decapeptide not only from the neuroterminal but also the soma and neuroprocess, upon the arrival of depolarization stimuli.
FM1-43 does not cross the plasma membrane, and therefore, FM1-43 labeling of the vesicular membrane requires exo/endocytotic events (32, 33). The presence of puncta in GnRH-1 neurons without any external stimuli indicates that GnRH-1 neurons are active and may be indicative of spontaneous peptide release. This is consistent with our observation that cultured primate GnRH-1 neurons exhibit spontaneous intracellular calcium oscillations, which synchronize at approximately 60-min intervals (34) and release the decapeptide in a pulsatile manner at approximately 60-min intervals (23). Moreover, an increase in the number and size of puncta by depolarization stimuli and the absence of the depolarization-induced changes by a blocker in this study are parallel to our previous observations that exposure of GnRH-1 neurons to high K+ and veratridine results in GnRH-1 release and that the veratridine-induced GnRH-1 release is blocked by TTX (23). Therefore, the changes in the puncta intensity are a good indicator of neurosecretion from GnRH-1 neurons.
A few reports available to date suggest dendritic or somatic release of GnRH-1 and salmon GnRH (GnRH-3). GnRH-1 immunopositive LDCV are present in neuroprocesses without synaptic specification in the rat brain (22, 35), GnRH-3-positive LDCV are found in close proximity to the soma membrane of adult teleost terminal GnRH-3 neurons (36), and changes in FM1-43 puncta occur with high K+ in teleost terminal GnRH-3 neurons (37). In this study, we examined primate GnRH-1 neurons using FM dye labeling of secretory vesicles combined with immunostaining of the vesicular marker proteins, VAMP, and the secretory active zone marker, Bassoon. Our observations that immunoreactive GnRH-1 colocalize with immunoreactive VAMP and Bassoon over FM1-43-labeled puncta indicate that vesicles containing GnRH-1 are present at the active zone for exo/endocytosis. In general, depolarization of neurons results in a rise of intracellular Ca2+ concentrations, leading to synaptic vesicles docking at the active zone and subsequently fusing to the plasma membrane, followed by neurotransmitter release from the active zone into the synaptic cleft (26, 38). Bassoon and Piccolo are major proteins localized at the active zone (25, 26). Upon depolarization stimuli, the changes in puncta size occurred repeatedly at the same spot in this study, indicating that exo/endocytosis occurs at specific sites, where the vesicles dock and fuse to release GnRH-1, rather than a random site. Collectively, the results of this study indicate that the somata and neuroprocesses (dendrites) of GnRH-1 neurons are equipped with the neurosecretory machinery.
The physiological significance of peptide release from the cell soma and neuroprocess in GnRH-1 neurons is multifold. First, this could be important for pulsatile release of GnRH-1. Pulsatile release of GnRH-1 is a consequence of synchronized activity among GnRH-1 neurons. Synchronized burst firing of multiple GnRH-1 neurons with periodical peptide release has been shown in cultured embryonic mouse GnRH-1 neurons (39). A recent study by Campbell et al. (40) with visualization of dendritic trees of individual mouse GnRH-1 neurons by biocytin filling further illustrate that there are considerable dendro-dendritic interactions, including juxtapositon of dendrites from scattered GnRH-1 cell bodies. Our observation in this study showing dendritic release of GnRH-1 peptide provides a communication tool among multiple GnRH-1 neurons. Second, GnRH-1 neurosecretion from the soma and dendrites may play a role in communication with other neurons and glia. For example, direct application of GnRH-1 into brain areas, such as the central gray and ventromedial hypothalamic nucleus, where GnRH-1 somata are not present, results in lordosis behavior (41, 42). It is possible that GnRH-1 release from dendritic arbors in the central gray and ventromedial hypothalamic nucleus may participate in communication with other neurons and glia for tuning of reproductive behavior.
Although this is the clearest report to date on soma-dendritic release of GnRH, exocytosis from the soma and neuroprocess is not limited to GnRH neurons. Release of oxytocin and vasopressin from the magnocellular neurons in the rat hypothalamus has been described with ultrastructural observations using immunocytochemistry, membrane capacitance, or microdialysis methods with immunoassay (43–47). Substance P release from rat dorsal root ganglion neurons is reported with changes in membrane capacitance and immunoblotting (48) or photoconversion of FM1-43 with electron microscopy (EM, 49). Somatic or dendritic release of biogenic amines and amino acid neurotransmitters, such as dopamine from rat substantia nigra neurons with EM, amperometric measurements with carbon microelectrodes, immunocytochemistry with confocal imaging (50–53), norepinephrine release from the rat sympathetic ganglia with EM (54, 55), and release of γ-aminobutyric acid and glutamate from the rat olfactory bulb, and mitral cells, cortical pyramidal neurons, hippocampal neurons, and Purkinje cells (56–59), have also been reported. Considering these universal features of the capacity of the soma and dendrites to release neuropeptides and neurotransmitters, and the potential importance of somato-dendritic release for synchronized activity among GnRH-1 neurons as discussed above, the present finding provides a new insight into coordinated neurosecretion. Further experiments and similar observations of somatic and/or dendritic release in mature GnRH neurons in vivo are needed to prove this hypothesis.
In vitro neuronal maturation recapitulates in vivo maturation (60, 61). Rhesus monkey GnRH-1 neurons migrate in the hypothalamus 2 wk after originating from the nasal placode and start to stimulate gonadotropes by E50 (31, 62). Using the same culture system as this study, we have shown that primate GnRH-1 neurons become fully mature 2–3 wk in vitro (10). In contrast, non-GnRH neurons appear to take a longer time to mature. Despite the fact that non-GnRH neurons obtained from the telencephalon or GnRH-1 migratory pathway from E36–E38 embryos were cultured for at least 2 wk, they were neither labeled with FM1-43 nor did they respond to high K+. This difference between non-GnRH neurons and GnRH-1 neurons mirrors our previous observation that non-GnRH neurons rarely exhibit spontaneous calcium oscillations (34). We interpreted those differences to mean that non-GnRH neurons are still immature, based on the following reports describing the developmental events in mouse neural stem cells and human embryonic stem cells: neural stem cells obtained from E14 mice are morphologically differentiated on 7 d in vitro, but they require more than 22 d in vitro to attain matured electrophysiological properties (63), and it takes 7–10 wk for human embryonic stem cells to exhibit mature patterns of action potentials after differentiation to neuroprogenitor cells (64). In the same article, the authors also conclude that maturation of embryonic stem cells to neurons are parallel to maturational changes in the central nervous system in human embryos. Although early embryonic developmental events in rhesus fetuses occur at slightly earlier gestational days than those in humans (65, 66), it is reasonable to believe that non-GnRH neurons are still nonfunctional after 2–4 wk in cultures.
In this study, we were able to visualize the neurosecretory process using FM1-43 or FM1-43Fx in primate GnRH-1 neurons and found that both somata and neuroprocesses have active zones for neurosecretion, where GnRH-1 peptide-containing vesicles appear to aggregate. The method used here provides a tool for studying the mechanism of neurosecretion in real time, such that we should be able to assess whether neurosecretion occurs from GnRH-1 neurons at the time of synchronization of intracellular calcium oscillations by simultaneous recording of exo/endocytosis with FM1-43 and changes in intracellular calcium levels. The higher amplitude of the calcium peak is consistently observed with synchronization of GnRH-1 neurons (34). Although presently we do not know the proportion of GnRH-1 peptide released from the soma and neuroprocess when compared with the amount released from the neuroterminal, this study clearly indicates soma-dendritic release of the decapeptide and describes a method that will be an important tool for further investigation of GnRH-1 neurosecretion.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants HD15433 and HD11355 and was possible to perform by NIH supports (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 4014
- E
- Embryonic day
- EM
- electron microscopy
- FM1-43Fx
- fixable form of FM1-43
- LDCV
- large dense core vesicle
- TTX
- tetrodotoxin
- VAMP
- vesicle-associated membrane protein.
References
- 1. Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. 1970. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 87:850–853 [DOI] [PubMed] [Google Scholar]
- 2. Clarke IJ, Cummins JT. 1982. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111:1737–1739 [DOI] [PubMed] [Google Scholar]
- 3. Gearing M, Terasawa E. 1988. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- 4. Moenter SM, Caraty A, Karsch FJ. 1990. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- 5. Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. 1985. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41:473–531 [DOI] [PubMed] [Google Scholar]
- 6. Knobil E, Hotchkiss J. 1988. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill J. eds. The physiology of reproduction. New York: Raven Press; 1971–1994 [Google Scholar]
- 7. Yen SS. 1980. The polycystic ovary syndrome. Clin Endocrinol 12:177–207 [DOI] [PubMed] [Google Scholar]
- 8. Marshall LA, Monroe SE, Jaffe RB. 1988. Physiologic and therapeutic aspects of GnRH and its analogs. In: Martini L, Ganong WF. eds. Frontiers in neuroendocrinology. New York: Raven Press; 239–278 [Google Scholar]
- 9. Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. 1993. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- 10. Kurian JR, Keen KL, Terasawa E. 2010. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology 151:5359–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwan EP, Gaisano HY. 2005. Glucagon-like peptide 1 regulates sequential and compound exocytosis in pancreatic islet β-cells. Diabetes 54:2734–2743 [DOI] [PubMed] [Google Scholar]
- 12. Neill JD, Frawley LS. 1983. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology 112:1135–1137 [DOI] [PubMed] [Google Scholar]
- 13. Gilon P, Ravier MA, Jonas JC, Henquin JC. 2002. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes 51(Suppl 1):S144–S151 [DOI] [PubMed] [Google Scholar]
- 14. Kilic G, Angleson JK, Cochilla AJ, Nussinovitch I, Betz WJ. 2001. Sustained stimulation of exocytosis triggers continuous membrane retrieval in rat pituitary somatotrophs. J Physiol 532:771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. 2007. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans 35:1236–1242 [DOI] [PubMed] [Google Scholar]
- 16. Ludwig M, Pittman QJ. 2003. Talking back: dendritic neurotransmitter release. Trends Neurosci 26:255–261 [DOI] [PubMed] [Google Scholar]
- 17. Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. 2008. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol 4:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gähwiler BH, Sandoz P, Dreifuss JJ. 1978. Neurones with synchronous bursting discharges in organ cultures of the hypothalamic supraoptic nucleus area. Brain Res 151:245–253 [DOI] [PubMed] [Google Scholar]
- 19. Ludwig M. 1995. Functional role of intrahypothalamic release of oxytocin and vasopressin: consequences and controversies. Am J Physiol 268:E537–E545 [DOI] [PubMed] [Google Scholar]
- 20. Betz WJ, Mao F, Bewick GS. 1992. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci 12:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henkel AW, Lübke J, Betz WJ. 1996. FM1-43 dye ultrastructural localization in and release from frog motor nerve terminals. Proc Natl Acad Sci USA 93:1918–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buma P, Roubos EW. 1986. Ultrastructural demonstration of nonsynaptic release sites in the central nervous system of the snail Lymnaea stagnalis, the insect Periplaneta americana, and the rat. Neuroscience 17:867–879 [DOI] [PubMed] [Google Scholar]
- 23. Terasawa E, Keen KL, Mogi K, Claude P. 1999. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- 24. Annaert WG, Becker B, Kistner U, Reth M, Jahn R. 1997. Export of cellubrevin from the endoplasmic reticulum is controlled by BAP31. J Cell Biol 139:1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. tom Dieck S, Sanmartí-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kämpf U, Fränzer JT, Stumm M, Garner CC, Gundelfinger ED. 1998. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol 142:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. 2001. The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci 58:94–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hida Y, Ohtsuka T. 2010. CAST and ELKS proteins: structural and functional determinants of the presynaptic active zone. J Biochem 148:131–137 [DOI] [PubMed] [Google Scholar]
- 28. Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. 2001. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci 24:637–643 [DOI] [PubMed] [Google Scholar]
- 29. Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. 2001. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1-43 photoconversion. Proc Natl Acad Sci USA 98:12748–12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Danneman PJ, White WJ, Marshall WK, Lang CM. 1988. An evaluation of analgesia associated with the immobility response in laboratory rabbits. Lab Anim Sci 38:51–57 [PubMed] [Google Scholar]
- 31. Quanbeck C, Sherwood NM, Millar RP, Terasawa E. 1997. Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol 380:293–309 [PubMed] [Google Scholar]
- 32. Betz WJ, Ridge RM, Bewick GS. 1993. Comparison of FM1-43 staining patterns and electrophysiological measures of transmitter release at the frog neuromuscular junction. J Physiol Paris 87:193–202 [DOI] [PubMed] [Google Scholar]
- 33. Cochilla AJ, Angleson JK, Betz WJ. 1999. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci 22:1–10 [DOI] [PubMed] [Google Scholar]
- 34. Terasawa E, Schanhofer WK, Keen KL, Luchansky L. 1999. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buma P. 1988. Synaptic and nonsynaptic release of neuromediators in the central nervous system. Acta Morphol Neerl Scand 26:81–113 [PubMed] [Google Scholar]
- 36. Oka Y, Ichikawa M. 1991. Ultrastructure of the ganglion cells of the terminal nerve in the dwarf gourami (Colisa lalia). J Comp Neurol 304:161–171 [DOI] [PubMed] [Google Scholar]
- 37. Abe H, Oka Y. 2009. Primary culture of the isolated terminal nerve-gonadotrophin-releasing hormone neurones derived from adult teleost (dwarf gourami, Colisa lalia) brain for the study of peptide release mechanisms. J Neuroendocrinol 21:489–505 [DOI] [PubMed] [Google Scholar]
- 38. Landis DM, Hall AK, Weinstein LA, Reese TS. 1988. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron 1:201–209 [DOI] [PubMed] [Google Scholar]
- 39. Constantin S, Caraty A, Wray S, Duittoz AH. 2009. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology 150:3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell RE, Gaidamaka G, Han SK, Herbison AE. 2009. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA 106:10835–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakuma Y, Pfaff DW. 1979. Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol 237:R285–R290 [DOI] [PubMed] [Google Scholar]
- 42. Moss RL, Foreman MM. 1976. Potentiation of lordosis behavior by intrahypothalamic infusion of synthetic luteinizing hormone-releasing hormone. Neuroendocrinology 20:176–181 [DOI] [PubMed] [Google Scholar]
- 43. Theodosis DT, Poulain DA, Vincent JD. 1981. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience 6:919–929 [DOI] [PubMed] [Google Scholar]
- 44. Pow DV, Morris JF. 1989. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 32:435–439 [DOI] [PubMed] [Google Scholar]
- 45. Ludwig M, Sabatier N, Dayanithi G, Russell JA, Leng G. 2002. The active role of dendrites in the regulation of magnocellular neurosecretory cell behavior. Prog Brain Res 139:247–256 [DOI] [PubMed] [Google Scholar]
- 46. Miyata S, Taguchi K, Maekawa S. 2003. Dendrite-associated opioid-binding cell adhesion molecule localizes at neurosecretory granules in the hypothalamic magnocellular neurons. Neuroscience 122:169–181 [DOI] [PubMed] [Google Scholar]
- 47. Soldo BL, Giovannucci DR, Stuenkel EL, Moises HC. 2004. Ca(2+) and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus. J Physiol 555:699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang LY, Neher E. 1996. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron 17:135–145 [DOI] [PubMed] [Google Scholar]
- 49. Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 2009. 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheramy A, Leviel V, Glowinski J. 1981. Dendritic release of dopamine in the substantia nigra. Nature 289:537–542 [DOI] [PubMed] [Google Scholar]
- 51. Araneda R, Bustos G. 1989. Modulation of dendritic release of dopamine by N-methyl-D-aspartate receptors in rat substantia nigra. J Neurochem 52:962–970 [DOI] [PubMed] [Google Scholar]
- 52. Jaffe EH, Marty A, Schulte A, Chow RH. 1998. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci 18:3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Witkovsky P, Patel JC, Lee CR, Rice ME. 2009. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience 164:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zaidi ZF, Matthews MR. 1997. Exocytotic release from neuronal cell bodies, dendrites and nerve terminals in sympathetic ganglia of the rat, and its differential regulation. Neuroscience 80:861–891 [DOI] [PubMed] [Google Scholar]
- 55. Zaidi ZF, Matthews MR. 1999. Stimulant-induced exocytosis from neuronal somata, dendrites, and newly formed synaptic nerve terminals in chronically decentralized sympathetic ganglia of the rat. J Comp Neurol 415:121–143 [DOI] [PubMed] [Google Scholar]
- 56. Isaacson JS. 2001. Mechanisms governing dendritic γ-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci USA 98:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maletic-Savatic M, Malinow R. 1998. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part I: trans-Golgi network-derived organelles undergo regulated exocytosis. J Neurosci 18:6803–6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morishita W, Kirov SA, Alger BE. 1998. Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1. J Neurosci 18:4870–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Glitsch M, Llano I, Marty A. 1996. Glutamate as a candidate retrograde messenger at interneurone-Purkinje cell synapses of rat cerebellum. J Physiol 497(Pt 2):531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van den Pol AN, Kogelman L, Ghosh P, Liljelund P, Blackstone C. 1994. Developmental regulation of the hypothalamic metabotropic glutamate receptor mGluR1. J Neurosci 14:3816–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moore JP, Jr, Wray S. 2000. Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- 62. Ronnekleiv OK, Resko JA. 1990. Ontogeny of gonadotropin-releasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology 126:498–511 [DOI] [PubMed] [Google Scholar]
- 63. Balasubramaniyan V, de Haas AH, Bakels R, Koper A, Boddeke HW, Copray JC. 2004. Functionally deficient neuronal differentiation of mouse embryonic neural stem cells in vitro. Neurosci Res 49:261–265 [DOI] [PubMed] [Google Scholar]
- 64. Johnson MA, Weick JP, Pearce RA, Zhang SC. 2007. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 27:3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Davignon RW, Parker RM, Hendrickx AG. 1980. Staging of the early embryonic brain in the baboon (Papio cynocephalus) and rhesus monkey (Macaca mulatta). Anat Embryol 159:317–334 [DOI] [PubMed] [Google Scholar]
- 66. Gribnau AA, Geijsberts LG. 1985. Morphogenesis of the brain in staged rhesus monkey embryos. Adv Anat Embryol Cell Biol 91:1–69 [DOI] [PubMed] [Google Scholar]