Abstract
Approximately 35% of follicular thyroid carcinomas harbor a chromosomal translocation that results in expression of a paired box gene 8-peroxisome proliferator-activated receptor γ gene (PPARγ) fusion protein (PPFP). To better understand the oncogenic role of PPFP and its relationship to endogenous PPARγ, we generated a transgenic mouse model that combines Cre-dependent PPFP expression (PPFP;Cre) with homozygous deletion of floxed Pten (PtenFF;Cre), both thyroid specific. Although neither PPFP;Cre nor PtenFF;Cre mice develop thyroid tumors, the combined PPFP;PtenFF;Cre mice develop metastatic thyroid cancer, consistent with patient data that PPFP is occasionally found in benign thyroid adenomas and that PPFP carcinomas have increased phosphorylated AKT/protein kinase B. We then tested the effects of the PPARγ agonist pioglitazone in our mouse model. Pioglitazone had no effect on PtenFF;Cre mouse thyroids. However, the thyroids in pioglitazone-fed PPFP;PtenFF;Cre mice decreased 7-fold in size, and metastatic disease was prevented. Remarkably, pioglitazone caused an adipogenic response in the PPFP;PtenFF;Cre thyroids characterized by lipid accumulation and the induction of a broad array of adipocyte PPARγ target genes. These data indicate that, in the presence of pioglitazone, PPFP has PPARγ-like activity that results in trans-differentiation of thyroid carcinoma cells into adipocyte-like cells. Furthermore, the data predict that pioglitazone will be therapeutic in patients with PPFP-positive carcinomas.
Approximately 35% of follicular thyroid carcinomas harbor a t(2,3)(q13;p25) chromosomal translocation that fuses paired box gene 8 (PAX8) with the peroxisome proliferator-activated receptor γ gene (PPARG), resulting in the production of a PAX8-PPARγ fusion protein (PPFP) (1). This fusion protein is composed of the majority of PAX8 followed by the fully intact PPARγ1, and its expression is driven by the strong PAX8 promoter.
The transcription factor PAX8 is essential for thyroid development as evidenced by the phenotypes observed in humans with PAX8 mutations (2) and Pax8 null mice (3). In the mature thyroid, PAX8 induces the expression of many thyroid-specific genes such as those encoding thyroglobulin, thyroid peroxidase, and the sodium iodide symporter (4–6).
PPARγ is a member of the nuclear hormone receptor family of transcription factors and is the master regulator of adipogenesis. Exogenous PPARγ agonists such as the thiazolidinediones pioglitazone and rosiglitazone increase insulin sensitivity and hence are commonly used to treat type 2 diabetes mellitus. In the thyroid, PPARγ is expressed at very low levels, and its function, if any, has not been elucidated.
PPFP is a presumed oncoprotein. Studies have shown that expression of exogenous PPFP in thyroid cell lines increases proliferation, decreases apoptosis, and increases colony formation in soft agar (7, 8). Transient cotransfection data indicate that PPFP can exert dominant negative activity over PPARγ (1). Because there is evidence that PPARγ may have antitumor properties (8–12), it is hypothesized that PPFP induces thyroid cancer by inhibiting the activity of endogenous PPARγ. However, this hypothesis seems inconsistent with the observation that PPFP thyroid cancers have increased expression of PPARγ inducible genes such as aquaporin 7 (AQP7) and angiopoietin-like 4 (ANGPTL4) (13, 14). Also, transient transfection of PPFP into thyroid cells confirmed that PPFP has PPARγ-like activity on the AQP7 and ANGPTL4 promoters (13). Thus, the functional relationship between PPFP and PPARγ, as well as the role of PPFP in thyroid oncogenesis, is poorly understood.
Follicular thyroid carcinomas also occur as part of Cowden syndrome. Patients with this syndrome inherit one defective allele of the gene phosphatase and tensin homolog (PTEN) (15). PTEN is a phosphoinositide phosphatase that negatively regulates the downstream phosphorylation and activation of AKT/protein kinase B and hence functions as a tumor suppressor. Loss or reduction of PTEN expression, or increased phosphorylation of AKT by other mechanisms, also is commonly observed in sporadic follicular thyroid carcinomas (16), including those expressing PPFP (17).
Here, we describe the first transgenic mouse model of PPFP thyroid carcinoma. The model includes thyroid-specific expression of PPFP at a level comparable with that observed in patients, combined with thyroid-specific deletion of Pten; neither alone is tumorigenic. We found that, in the absence of an exogenous ligand, some PPARγ target genes are induced and others are repressed in the PPFP mouse thyroids, but that in the presence of pioglitazone, a broad array of PPARγ target genes is induced. Remarkably, this leads to a unique proadipogenic antitumor response and predicts that pioglitazone will be therapeutic in patients with PPFP thyroid carcinomas.
Materials and Methods
Generation of transgenic mice
PPFP (17) with three myc epitopes at the N terminus was ligated to the NotI and NheI sites of the vector pClex (18). In the resulting plasmid, the CAG promoter drives transcription of a floxed enhanced green fluorescent protein (eGFP) cDNA followed by PPFP, such that expression of PPFP is conditional upon Cre-mediated excision of eGFP. A 6.6-kb KpnI fragment containing the above expression cassette was used to generate transgenic mice by the University of Michigan Transgenic Animal Core Facility. Purified DNA was microinjected into fertilized eggs obtained by mating FVB/N female mice with FVB/N male mice. This transgene has been registered in the Mouse Genome Informatics database as Tg(CAG-EGFP,-PAX8/PPARG)1Rjk (MGI:4942191).
Animal husbandry
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan. All mice were on an FVB/N background. Transgenic mice in which the thyroid peroxidase promoter drives expression of Cre recombinase, and mice in which exon 5 of both Pten alleles is flanked by loxP sites (PtenFF) were as described (19, 20). Mice with thyroid-specific expression of PPFP (PPFP;Cre) were generated by mating mice heterozygous for PPFP with mice that were either heterozygous or homozygous for thyroid peroxidase promoter that drives expression of Cre recombinase. Mice with thyroid-specific expression of PPFP and homozygous deletion of Pten (PPFP;PtenFF;Cre), as well as mice with just thyroid-specific homozygous deletion of Pten (PtenFF;Cre), were generated by mating mice of genotypes PPFP;PtenFF x PtenFF;CreCre. Tail DNA genotyping was performed as described (17). Pten gene excision in thyroid DNA was assessed by three primer PCR using primers TCCCAGAGTTCATACCAGGA, AATCTGTGCATGAAGGGAAC, and GCAATGGCCAGTACTAGTGAAC, which yields product sizes of 511 bp for wild type, 682 bp for floxed, and 288 bp for excised Pten.
Pioglitazone was delivered to the mice through their food at a concentration of 200 parts per million in irradiated Purina rodent diet no. 5001 (Research Diets, Inc., New Brunswick, NJ). All mice were fed ad libitum.
Thyroid ultrasound examinations were performed using a VisualSonics Vevo 770 high-resolution in vivo microimaging system (VisualSonics, Toronto, Canada) as described (17). Transverse images were taken in the plane of greatest thyroid size, and the thyroid area was determined. To validate thyroid area as a measure of size, we correlated this value determined just before killing with thyroid weight in 50 mice and achieved r2 = 0.97. Therefore, ultrasound measurements before and after pioglitazone therapy were used to determine changes in thyroid size.
Immediately before euthanasia, mice were anesthetized with ketamine/xylazine, and blood was obtained for measurement of serum T4 and T3. In general, one thyroid lobe was dissected en bloc with the trachea and placed in formalin for paraffin embedding and histological analyses, and the other thyroid lobe was dissected free of the trachea and surrounding tissues, frozen in liquid nitrogen, and used for the isolation of RNA, DNA, or protein. For oil red O staining, the thyroid lobes with trachea were frozen in optimal cutting temperature embedding media. The lungs were placed in formalin for paraffin embedding and histological analyses.
Serum T4 and T3 assays
T3 concentrations were determined in 50-μl aliquots of sera using RIA kit no. 06B-254215, and T4 concentrations were determined in 25-μl aliquots of sera using RIA kit no. 06-B254029 (MP Biomedicals, Solon, OH).
Histology and immunohistochemistry (IHC)
Ten percent buffered formalin fixed paraffin-embedded thyroid glands and lungs were cut into 5-μm sections, deparaffinized, and subjected to antigen retrieval using Retrieve-All 1 (Covance, Madison, WI). Histology was assessed by hematoxylin and eosin (H&E) staining. To evaluate for the presence of lung metastases, 30 slides with two sections per slide were prepared per mouse, and slides 1, 10, 20, and 30 were stained with H&E. IHC was performed using the Vectastain Elite ABC kit for rabbit IgG (no. PK-6101; Vector Laboratories, Inc., Burlingame, CA) for PAX8 (1:50, no. 10336-1-AP; Proteintech Group, Chicago, IL), thyroglobulin (1:4000, no. A0251; Dako Cytomation, Carpinteria, CA), adiponectin (Adipoq) (0.75 μg/ml, no. PRS3553; Sigma-Aldrich, St. Louis, MO), perilipin 1 (Plin1) (1:75, no. 3470; Cell Signaling Technology, Beverly, MA), and fatty acid binding protein 4 (FABP4) (1:100, no. 3544; Cell Signaling Technology). The M.O.M. Mouse IgG kit (no. BMK-2201; Vector Laboratories, Inc.) and protocol were used for IHC for myc (1:1000, clone 4A6 no. 05-724; Millipore, Bedford, MA) and thyroid transcription factor (TTF)-1 (1:1000, clone 8G7G3/1 no. M3575; Dako Cytomation). Cleaved caspase-3 staining was performed according to the protocol included with the product kit (no. 8120; Cell Signaling Technology). The DeadEnd Colorimetric Apoptosis Detection System (G7130; Promega, Madison, WI), an IHC version of the terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) assay, was performed according to the manufacturer's protocol. All sections were counterstained lightly with hematoxylin. Oil red O staining was performed on 10-μm frozen sections.
Quantitative real-time PCR (qPCR)
Total RNA isolation, RT, and RT-qPCR were performed as previously described (17). The PCR primer sequences are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Target gene expression was normalized to phosphoglycerate kinase 1. The PCR was performed on an Applied Biosystems StepOne Plus using the instrument default protocol (Applied Biosystems, Foster City, CA), and the ΔΔCt method within the StepOne software was used for calculations. Amplicons were intron spanning except for PPFP, in which case control qPCR using mock RT reactions confirmed the lack of contaminating genomic DNA.
Immunoblotting
Thyroid protein was isolated used T-Per reagent (Thermo Fisher Scientific, Waltham, MA). Forty micrograms of protein were loaded per lane of a sodium dodecyl sulfate 15% polyacrylamide gel. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane and probed for intact and cleaved caspase-3 (1:400, antibody no. 9665; Cell Signaling Technology) followed by peroxidase-tagged goat antirabbit IgG (1:25,000, no. sc-2301; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Detection was with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Digital images were captured on a Bio-Rad Fluor-S MAX Multi-Imager, and band intensities were quantified using Bio-Rad Quantity One software (Bio-Rad, Hercules, CA).
Statistical analysis
Statistical analysis of gene expression used ANOVA followed by the Newman-Keuls test, with P < 0.05 considered significant. Serum T4 and T3 levels were compared with levels in wild-type mice by ANOVA followed by Dunnett's test, with P < 0.05 considered significant.
Results
Development of mice with thyroid-specific PPFP expression and Pten deletion
The strong and ubiquitously active CAG promoter was used to drive transcription of a floxed eGFP cDNA followed by myc-tagged PPFP, such that PPFP expression requires Cre-mediated deletion of eGFP. By breeding these mice with mice in which the thyroid peroxidase gene promoter drives Cre, we created mice in which thyroidal expression of PPFP is 23 ± 13-fold greater than endogenous PPARγ as assessed by RT-qPCR (mean ± sd, n = 14). Thus, the level of PPFP expression in these mice (denoted PPFP;Cre) is comparable with that in patients with thyroid cancer, in which PPFP is typically expressed 10- to 50-fold above normal endogenous PPARγ (13). By 6 months of age, PPFP;Cre mice uniformly develop thyroid hyperplasia (Fig. 1), but no tumors are observed. However, in patients, PPFP is occasionally expressed in benign follicular adenomas (21, 22), strongly suggesting the PPFP by itself is insufficient to cause carcinoma. Given that PPFP thyroid carcinomas have increased phosphorylated AKT (17), we generated PPFP;Cre mice that also have thyroid-specific homozygous deletion of floxed Pten alleles (PPFP;PtenFF;Cre) and compared these with mice that just have thyroid-specific homozygous deletion of Pten (PtenFF;Cre). At 5 months of age, the mice were killed for evaluation. Pten excision in thyroid DNA was confirmed by PCR (Supplemental Fig. 1). The gross anatomies of the PtenFF;Cre and PPFP;PtenFF;Cre thyroids were distinctly different (Fig. 2A). PtenFF;Cre thyroids were enlarged in a smooth and symmetric manner, consistent with diffuse hyperplasia. In contrast, the PPFP;PtenFF;Cre thyroids were larger still, irregularly shaped, and multinodular. Histologically, the PtenFF;Cre thyroids were homogeneous in appearance and hyperplastic with abundant colloid (Fig. 2B). In contrast, the PPFP;PtenFF;Cre thyroids were more cellular with far less colloid.
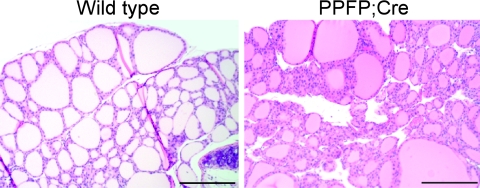
Fig. 1.
H&E staining of a representative wild-type FVB/N mouse thyroid (left panel) and a PPFP;Cre mouse thyroid (right panel) that demonstrates mild hyperplasia. Scale bars, 200 μm.
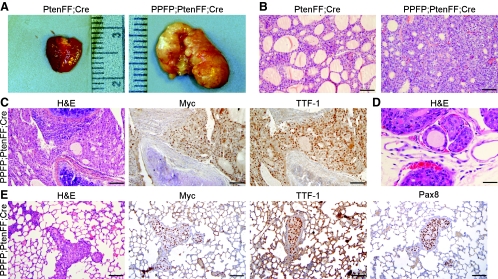
Fig. 2.
Abnormal thyroid morphology in PtenFF;Cre and PPFP;PtenFF;Cre mice with PPFP;PtenFF;Cre mice developing invasive, metastatic carcinoma. A, Thyroid gross anatomy. PtenFF;Cre thyroids are enlarged in a smooth and symmetrical manner. PPFP;PtenFF;Cre thyroids are larger, irregularly shaped, and multinodular. The rulers are metric. B, Histological analysis by H&E staining. PtenFF;Cre thyroids are hyperplastic with substantial colloid, whereas PPFP;PtenFF;Cre thyroids are more cellular with less colloid. C, Thyroid carcinoma invasion of the trachea as seen by H&E staining. The thyroid cancer cells immunostain for myc-PPFP and TTF-1. D, H&E stain demonstrating local vascular invasion. E, Lung metastases of thyroid origin confirmed by the presence of the thyroid markers myc-PPFP, TTF-1, and PAX8. Scale bars, 100 μm.
PPFP;PtenFF;Cre mice develop invasive thyroid carcinoma
The PPFP;PtenFF;Cre thyroids were locally invasive into the adjacent soft tissues and trachea (Fig. 2C). By IHC, these cells express myc-tagged PPFP and TTF-1 (Nkx2–1), confirming they are thyroid cells. In addition to the soft tissue invasion, these tumors invaded the local vasculature (Fig. 2D).
The PPFP;PtenFF;Cre mice also developed metastatic disease in their lungs (Fig. 2E) and soft tissues (data not shown). The lung is the most common site of metastatic disease in patients with follicular thyroid carcinoma. IHC demonstrated that the lung metastases in PPFP;PtenFF;Cre mice express myc-PPFP, TTF-1, and PAX8, consistent with their origin as thyroid cancer cells (Fig. 2E). These findings were independent of gender. TTF-1 staining also was observed in the normal lung parenchyma, as expected.
Thus, PPFP;PtenFF;Cre mice develop thyroid cancer with invasion of the trachea and local vasculature, as well as distant metastases. In contrast, PtenFF;Cre mice develop benign thyroid hyperplasia with no evidence of local invasion or distant metastases.
The PPARγ agonist pioglitazone induces a lipogenic antitumor response in PPFP;PtenFF;Cre mice
The functional relationship between PPFP and endogenous PPARγ is not well understood, because there is evidence that PPFP can exert dominant negative activity over PPARγ (1), as well as evidence that it can have PPARγ-like activity (13, 14). Two of the most highly induced genes in human PPFP cancers, AQP7 and ANGPTL4, are induced by PPARγ in adipocytes. Therefore, we were interested in determining whether these genes are induced in PPFP;PtenFF;Cre thyroids. By RT-qPCR, we found that Angptl4 was induced 8.3-fold and Aqp7 was borderline-induced 1.3-fold, relative to expression in PtenFF;Cre thyroids (see figure 5 below). This suggests that, similar to the human disease, PPFP may have PPARγ-like activity in this mouse model of thyroid carcinoma. These findings prompted us to test the effects of the PPARγ agonist pioglitazone in these mice.
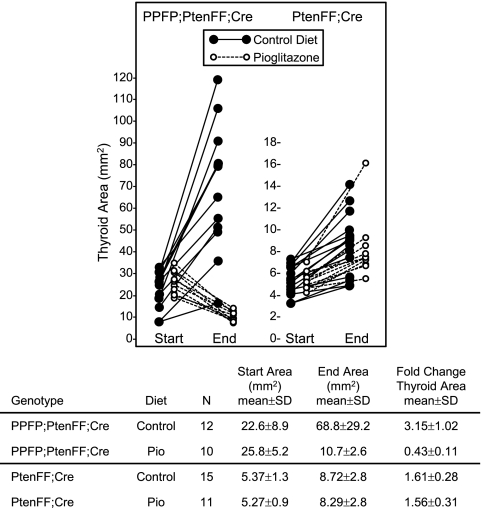
PPFP;PtenFF;Cre mice and PtenFF;Cre control mice between the ages of 2 and 2.5 months were fed a control chow diet or pioglitazone chow for 10 wk. Thyroid size was measured by ultrasound at the start and end of the 10-wk treatment, after which the animals were euthanized for further evaluation. Figure 3 shows the thyroid size in each mouse at the start and end of the study. The PPFP;PtenFF;Cre thyroids grew approximately 3-fold when the mice were fed the control diet but decreased in size by over 50% in response to pioglitazone. Therefore, the thyroids exposed to pioglitazone ended up only 15% the size of the control diet thyroids in the PPFP;PtenFF;Cre mice. In contrast, pioglitazone had no effect on thyroid size in the PtenFF;Cre mice. At the end of the study, the pioglitazone-treated PPFP;PtenFF;Cre thyroids were virtually the same size as the PtenFF;Cre thyroids, indicating that 10 wk of pioglitazone almost completely reversed the growth stimulating effect of PPFP expression.
Fig. 3.
Pioglitazone reverses PPFP;PtenFF;Cre mouse thyroid gland enlargement but does not affect thyroid growth in PtenFF;Cre mice. Cross sectional thyroid gland area was measured in PPFP;PtenFF;Cre and PtenFF;Cre mice by ultrasound before and after a 10-wk treatment with pioglitazone or control diet. Each circle represents one mouse. Start area end area and fold change were determined for each experimental group. The thyroid cross-sectional area in wild-type mice is 1.9 ± 0.5 mm2.
Local soft tissue/tracheal invasion was seen in 11 of 12 PPFP;PtenFF;Cre control diet mice vs. one of 10 pioglitazone-treated mice (P = 0.0002, Fisher exact test). Vascular invasion was seen in five of 12 PPFP;PtenFF;Cre control diet mice vs. zero of 10 treated with pioglitazone (P = 0.03). Seven of 12 PPFP;PtenFF;Cre mice on the control diet developed distant metastases (three lung, three sc, one lung and sc) vs. zero of 10 on pioglitazone (P = 0.005). No local soft tissue/tracheal invasion, vascular invasion, or metastases was observed in any of the PtenFF;Cre mice.
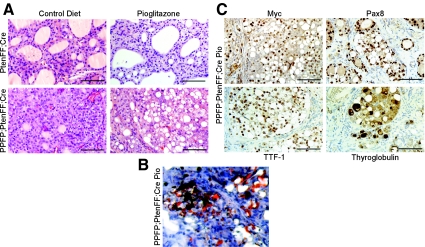
Histologically, the PtenFF;Cre thyroids demonstrated benign, diffuse hyperplasia with large amounts of colloid that was unaffected by pioglitazone treatment (Fig. 4A). Given the dramatic response of the PPFP;PtenFF;Cre thyroids to pioglitazone, we expected their histological appearance to resemble that of the PtenFF;Cre mice. Surprisingly, the pioglitazone-treated PPFP;PtenFF;Cre thyroids were very abnormal; in fact, more abnormal than the thyroids from the control diet mice of the same genotype. The major distinguishing feature of these thyroids was the accumulation of large amounts of what appeared to be intracellular lipid (Fig. 4A, lower right), which was confirmed by oil red O staining (Fig. 4B) and IHC for perilipin-1 (see figure 6 below). These data suggest that pioglitazone caused an adipogenic response in the PPFP;PtenFF;Cre thyroids.
Fig. 4.
Pioglitazone causes a lipogenic response in PPFP;PtenFF;Cre mouse thyroids. A, H&E staining of PtenFF;Cre and PPFP;PtenFF;Cre thyroids with and without 10 wk of pioglitazone treatment displays lipid accumulation in the thyroid cells of pioglitazone-fed PPFP;PtenFF;Cre mice (lower right panel). B, Oil red O staining of lipid within the thyroid of a pioglitazone-fed PPFP;PtenFF;Cre mouse. C, Myc-PPFP, PAX8, TTF-1, and thyroglobulin IHC was performed to confirm the thyroid cell origin of lipid containing cells in PPFP;PtenFF;Cre mice fed pioglitazone. Scale bars, 100 μm.
To ensure that these lipid-laden cells are thyroidal in origin, we performed IHC for myc-tagged PPFP, PAX8, TTF-1, and thyroglobulin (Fig. 4C). All markers were clearly expressed in the cells with lipid droplets.
Pioglitazone causes an adipogenic response in PPFP;PtenFF;Cre thyroids
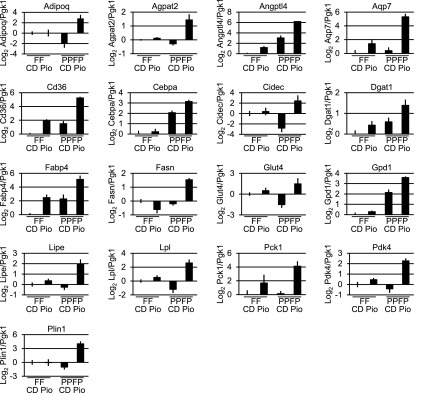
To further evaluate the lipogenic response to pioglitazone, we performed RT-qPCR for 17 adipocyte PPARγ target genes on thyroid RNA from PtenFF;Cre and PPFP;PtenFF;Cre mice fed control or pioglitazone chow (Fig. 5). In the PtenFF;Cre thyroids, pioglitazone induced the expression of Angptl4, Cd36, and Fabp4, suggesting that endogenous PPARγ is sufficient to elicit a significant change in expression of these genes. In contrast, pioglitazone repressed fatty acid synthase (Fasn), and expression of the remaining 13 genes was unchanged. To evaluate the possible PPARγ-like activity of PPFP, we first compared gene expression in the PPFP;PtenFF;Cre thyroids vs. the PtenFF;Cre thyroids, both on control diet. Five of the 17 genes were induced by PPFP (Angptl4, Cd36, Cebpa, Fabp4, and Gpd1), three were repressed (Adipoq, Cidec, and Glut4), and nine were unchanged. This suggests that in the absence of an exogenous ligand, PPFP may have either PPARγ-like activity or may antagonize PPARγ, depending on the target gene. Two additional comparisons were made to assess the activity of PPFP in the presence of pioglitazone. First, a comparison of the PPFP;PtenFF;Cre thyroids vs. the PtenFF;Cre thyroids, both on pioglitazone, revealed that PPFP induced 15 of the 17 PPARγ target genes (all but Cidec and Glut4). Second, a comparison of the PPFP;PtenFF;Cre thyroids on pioglitazone vs. the same genotype on control diet revealed that all 17 genes were induced. Thus, in the presence of this exogenous ligand, PPFP appears to be broadly PPARγ like.
Fig. 5.
Pioglitazone causes an adipogenic response in PPFP;PtenFF;Cre thyroids as evidenced by increased expression of PPARγ-regulated adipocyte genes. RT-qPCR was performed for 17 adipocyte PPARγ target genes on thyroid RNA from PtenFF;Cre mice (FF) on control diet (CD) or pioglitazone diet (Pio) and PPFP;PtenFF;Cre mice (PPFP) on control diet or pioglitazone diet. Results were normalized to phosphoglycerate kinase 1 expression and presented on a Log2 scale, with the PtenFF;Cre CD value normalized to 1 (0 on a Log2 scale). Significance was determined by Newman-Keuls test (see Results).
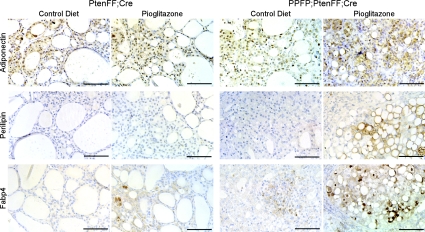
To confirm the increase in gene expression at the protein level, we performed IHC for adiponectin, perilipin-1, and Fabp4. As can be seen in Fig. 6, all three proteins were detected at levels proportional to their RNA in the four experimental mouse groups. Within the PPFP;PtenFF;Cre pioglitazone-treated thyroids, protein expression was highest in the lipid-containing cells (Fig. 6, right panels). In adipocytes, perilipin-1 forms a protein coat that surrounds the lipid droplets, and it shows the same localization in the lipid-laden thyroid cells. These data confirm the thyroid cell adipogenic response to pioglitazone in PPFP;PtenFF;Cre mice.
Fig. 6.
Adiponectin, perilipin-1, and Fabp4 protein expression mirror mRNA expression changes in PtenFF;Cre and PPFP;PtenFF;Cre mouse thyroids. Thyroid sections from PtenFF;Cre and PPFP;PtenFF;Cre mice receiving the pioglitazone or control diet were analyzed by IHC for adiponectin, perilipin-1, and Fabp4. Scale bars, 100 μm.
The decrease in thyroid size in pioglitazone-fed PPFP;PtenFF;Cre mice reflects an increase in apoptosis
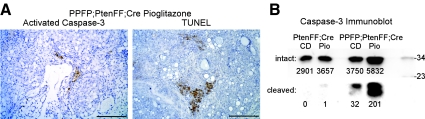
The dramatic response of the PPFP;PtenFF;Cre thyroids to pioglitazone suggests that this drug increases the rate of cell death in this genotype. To evaluate this, IHC was performed for activated caspase-3, and DNA fragmentation was assessed by an IHC-based TUNEL assay. Activated caspase-3 and TUNEL staining were negative in the PPFP;PtenFF;Cre and PtenFF;Cre control diet thyroids, as well as the PtenFF;Cre pioglitazone diet thyroids (data not shown). However, activated caspase-3 was detected in the PPFP;PtenFF;Cre pioglitazone diet thyroids at one to five cells per field using a ×10 objective, with occasional larger areas of staining (Fig. 7A). TUNEL staining also was readily detected in the pioglitazone diet PPFP;PtenFF;Cre thyroids (Fig. 7A). Increased activated caspase-3 in this group was confirmed by immunoblot (Fig. 7B).
Fig. 7.
Pioglitazone induces apoptosis in PPFP;PtenFF;Cre mouse thyroids. A, Active, cleaved caspase-3 and TUNEL staining demonstrated by IHC. Scale bars, 200 μm. B, Immunoblot demonstrating caspase-3 activation in thyroids from pioglitazone-treated PPFP;PtenFF;Cre mice. Each lane represents pooled thyroid protein from four mice, 10 μg of protein per thyroid. The membrane was cut at the 23-kDa marker, and the upper and lower portions were immunostained separately with an antibody that recognizes both intact (30 kDa) and active cleaved (17 and 19 kDa) caspase-3, so that the exposure of the latter could be adjusted to allow detection of relatively weak bands. The true signal intensity is given below each band. The two images were reunited to create this figure. CD, Control diet; Pio, pioglitazone. Molecular mass markers are shown at the right.
PPFP;PtenFF;Cre mice are hyperthyroid and pioglitazone normalizes thyroid function
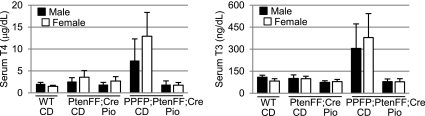
Control diet PPFP;PtenFF;Cre mice were hyperthyroid with serum T4 and T3 levels averaging approximately 6- and 3.5-fold above those in wild-type mice (Fig. 8). Although a 6-fold elevation of T4 would be considered marked hyperthyroidism in a clinical setting, this change is modest relative to the approximately 100-fold increase in thyroid size in the PPFP;PtenFF;Cre mice. In any case, pioglitazone eliminated the elevations in serum T4 and T3. The serum levels of T4 and T3 in PtenFF;Cre mice did not differ significantly from those in wild-type mice. To determine whether the mouse genotype or serum thyroid hormone levels affected expression of thyroid marker genes, we measured by RT-qPCR the expression of Pax8 (using primers that do not detect PPFP), thyroid peroxidase (Tpo), solute carrier family 5 (sodium iodide symporter), member 5 (Slc5a5), and thyroglobulin (Tg) in the four groups of mice (Supplemental Table 2). On average, expression of these genes was about 2-fold lower in PPFP;PtenFF;Cre thyroids than PtenFF;Cre thyroids, consistent with the former being less differentiated. However, the expression did not differ in hyperthyroid (control diet) vs. euthyroid (pioglitazone) PPFP;PtenFF;Cre mice. Similarly, pioglitazone did not affect the expression of PPFP.
Fig. 8.
PPFP;PtenFF;Cre mice are hyperthyroid and normal thyroid function is regained through pioglitazone treatment. T4 (left) and T3 (right) levels were measured in the various groups of mice. Males (M) and females (F) were analyzed separately, because wild-type females tend to have slightly lower thyroid hormone levels. WT, Wild-type mice; CD, control diet; Pio, pioglitazone diet. Error bars denote sd. The number of mice per group ranged from four to 11.
Discussion
Approximately 35% of follicular thyroid carcinomas contain a t(2,3)(q13;p25) chromosomal translocation that fuses the genes encoding PAX8 and PPARγ, resulting in the expression of PPFP (1). Although this was one of the first identified examples of a carcinoma-associated chromosomal translocation, the biology of PPFP in thyroid neoplasia remains poorly understood. The functional relationship between PPFP and PPARγ in regulating gene expression is unclear, and hence the role of PPFP in the development and progression of this cancer remains enigmatic. Progress has been limited by a lack of suitable model systems. Although transient and stable transfections of PPFP into various cell lines have provided insight (7, 8), there are no available cell lines from human PPFP cancers, nor has there been an animal model of this disease. Here, we report the development of the first mouse model of PPFP-associated thyroid carcinoma and show a remarkable therapeutic benefit of the PPARγ agonist pioglitazone on this cancer.
In this mouse model, PPFP expression is Cre dependent and driven by the strong CAG promoter, resulting in a level of expression within the thyroid comparable with that found in patients with PPFP thyroid carcinomas. Despite this, PPFP expression by itself in mice results in mild thyroid hyperplasia but no tumors by 6 months of age, strongly suggesting that PPFP is insufficient to cause carcinoma. This finding is consistent with data from patients, because PPFP has been identified occasionally in apparently benign adenomas (21, 22). The specific additional events that are required for the development of PPFP cancers are not known, but it is known that these cancers express high levels of activated (phosphorylated) AKT (17). Increased phosphorylated AKT is a common finding in follicular thyroid carcinomas in general, and can result from a variety of genetic or epigenetic changes (16). Based upon these data, we reasoned that mice with PPFP expression also may need increased AKT activation to develop thyroid cancer, and we decided to achieve this by the thyroid-specific deletion of Pten, which encodes a phosphatase that is a negative regulator of AKT signaling. Homozygous deletion of Pten had been reported to result only in thyroid hyperplasia or adenomas in mice 8–12 months of age (23), and therefore, it seemed plausible that PPFP expression and loss of PTEN could synergize to cause thyroid carcinomas at a younger age. Additionally, patients with Cowden syndrome inherit one defective PTEN allele and are prone to developing follicular thyroid adenomas and carcinomas, thus demonstrating the clinical relevance of PTEN loss in thyroid carcinogenesis (15).
As predicted, we found that expression of PPFP and homozygous deletion of Pten together resulted in the development of thyroid carcinomas characterized by local invasion of the trachea and vasculature, as well as metastatic disease. By 5 months of age, seven of 12 mice had distant metastases, whereas mice with homozygous deletion of Pten alone only developed benign hyperplasia. The mice in these studies were on a pure FVB/N background, and it is possible that the phenotype could be affected by the background strain.
PPFP;PtenFF;Cre mice differ in two respects from a previous transgenic mouse model combining PPFP expression and Pten deletion, which developed benign hyperplasia but no neoplasia (17). In the previous model, PPFP was expressed only at the same level as endogenous PPARγ, which is approximately 20-fold below the level in our PPFP;PtenFF;Cre mice or patients with PPFP carcinomas. In addition, the previous model used a single allele deletion of Pten, rather than homozygous deletion. Because in the current model neither the appropriately high level of PPFP expression nor homozygous Pten deletion alone was tumorigenic, one can conclude the two synergize to yield thyroid carcinomas.
By 5 months of age, the PPFP;PtenFF;Cre mice developed thyroids approximately 100-fold larger than normal, and this was associated with hyperthyroidism. It is noteworthy that these mice developed distant metastases despite hyperthyroidism, which suppresses TSH secretion and hence removes a growth factor for thyroid cancer. Presumably these thyroid cancers may have been even more aggressive had the mice been euthyroid. Patients with follicular thyroid carcinoma of course have their thyroid glands surgically removed, but they occasionally develop hyperthyroidism when extensive metastatic disease is present.
The standard hypothesized role for PPFP in thyroid cancer is as an antagonist to endogenous PPARγ (1). This fits with cotransfection data demonstrating dominant negative activity of PPFP over PPARγ in reporter gene assays, and it also fits with a substantial body of data suggesting that PPARγ has antitumor properties (9, 11, 12). The antitumor activity of PPARγ and thiazolidinediones is thought to result from a combination of cell growth arrest, induction of apoptosis, and inhibition of cell invasion (24). Many molecular mechanisms appear to underlie these effects, such as decreased expression of cyclins, increased expression of p21 and p27, increased expression of proapoptotic molecules such as BCL2-associated X protein, decreased expression of antiapoptotic molecules such as survivin, increased expression of E-cadherin, and decreased expression of matrix metalloproteinases.
On the other hand, PPFP also has been shown to have PPARγ-like activity (13). Two of the most overexpressed genes in PPFP thyroid carcinomas, AQP7 and ANGPTL4, are induced by PPARγ in adipocytes, and this PPARγ-like activity of PPFP has been confirmed in luciferase reporter assays (13). In addition, PPARγ and thiazolidinediones do not always have antitumor activity. For example, thiazolidinediones increase colon tumor formation in APCMin/+ mice (25, 26). Mutations in PPARG are rarely found in human cancers (27). Furthermore, PPARγ antagonists have antiproliferative effects on a wide variety of cancer cell lines (28), and this effect is likely mediated through PPARγ, because it is attenuated by PPARγ knockdown (29).
With this as background, it was not possible to predict whether pioglitazone would ameliorate or exacerbate thyroid cancer in PPFP;PtenFF;Cre mice. The former turned out to be the case. There are two striking aspects to the therapeutic response that we observed. First, the drug is extremely effective, completely eliminating metastatic disease and decreasing thyroid size 7-fold, to nearly that of the control PtenFF;Cre mice. The response of other rodent cancer models to thiazolidinediones tends to be less dramatic. For example, in a rat model of mammary cancer, pioglitazone reduced the incidence of cancer by only 32% (30). In a mouse model of colon tumorigenesis, pioglitazone decreased the incidence of tumors by 50% (31). In a murine model of mammary cancer, rosiglitazone decreased the number of lung metastases per mouse but did not increase the number of metastasis-free mice or affect primary tumor size (32). In a mouse model of thyroid cancer caused by homozygous knockin mutations in thyroid hormone receptor β, rosiglitazone decreased thyroid growth rate by only 40% (11).
Secondly, the therapeutic response to pioglitazone in PPFP;PtenFF;Cre thyroids is strongly adipogenic, as assessed by induction of a broad array of adipocyte PPARγ target genes and the accumulation of large lipid droplets within the remaining thyroid cells. An adipogenic response to thiazolidinediones has not been previously reported in animal models of cancer. In general, thiazolidinedione-treated tumor histologies are either similar to those of control animals (31) or show increased apoptotic cell death (11). The only finding somewhat similar to ours was lipid accumulation when a normal murine breast epithelial cell line and a breast cancer cell line in culture were exposed to thiazolidinediones (33). However, there was no induction of the classic adipocyte marker Fabp4, which is induced 7.5-fold in pioglitazone-exposed PPFP;PtenFF;Cre thyroids, and an adipogenic response has not been observed after thiazolidinedione treatment of animal models of breast cancer.
We speculate that these unique qualitative and quantitative aspects of the response to pioglitazone in our mice are due to the fact that the drug target is PPFP, not PPARγ. Perhaps the PAX8 portion of PPFP endows this fusion protein with unique properties, or perhaps the fact that PPFP is expressed at high levels accounts for the response. In the absence of pioglitazone, PPFP appears to have mixed pro- and anti-PPARγ effects, because five of 17 adipocyte PPARγ target genes were induced, three were repressed, and nine were unchanged. However, in the presence of pioglitazone, PPFP is strongly PPARγ like, with at least 15 of the 17 target genes being induced, several more than 10-fold. Thus, pioglitazone causes a trans-differentiation-type effect, through which the thyroid cells become adipocyte like. We speculate that the antitumor response is intimately tied to this trans-differentiation effect; as the thyroid tumor cells become more like mature adipocytes, their malignant character declines. Nonthiazolidinedione PPARγ ligands have been identified that are poorly adipogenic yet retain the property of improving insulin sensitivity (34). It will be of interest to determine whether such ligands have antitumor and/or proadipogenic effects in our model of PPFP thyroid carcinoma.
In general, clinical trials of thiazolidinediones in cancer have been disappointing. A trial of rosiglitazone administration to 20 patients with advanced thyroid carcinomas showed no significant benefit (only one patient had follicular thyroid carcinoma and its PPFP status was not reported) (35). Similarly, a phase 2 trial of rosiglitazone in liposarcoma demonstrated no benefit (36). Our results predict that pioglitazone will be beneficial in PPFP-expressing metastatic thyroid cancers. Should this be confirmed, it would likely reflect the fact that the drug target is PPFP rather than PPARγ. In addition to the use of pioglitazone or other exogenous PPARγ ligands as therapeutics for PPFP cancers, it is of interest to consider the role of endogenous PPARγ ligands. These ligands are not chemically well defined but are thought to be fatty acid/prostaglandin metabolites (37–40). It is possible that dietary changes could alter the quantity or repertoire of endogenous PPARγ ligands and thus could affect the development or progression of PPFP-expressing thyroid carcinomas.
Supplementary Material
Acknowledgments
We thank Mark Deming, Supervisor, Pathology Imaging, for photography of the mouse thyroid glands and the Transgenic Animal Model Core of the University of Michigan Biomedical Research Core Facilities for preparation of the transgenic mice.
This work was supported by National Institutes of Health (NIH) Grant R01CA151842 and by the following core facilities: the Cell and Molecular Biology Core of the Michigan Diabetes Research and Training Center NIH Grant P60DK020572, the University of Michigan Comprehensive Cancer Center NIH Grant P30CA046592, and the University of Michigan Center for Organogenesis.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANGPTL4
- Angiopoietin-like 4
- AQP7
- aquaporin 7
- eGFP
- enhanced green fluorescent protein
- FABP4
- fatty acid binding protein 4
- Fasn
- fatty acid synthase
- H&E
- hematoxylin and eosin
- IHC
- immunohistochemistry
- PAX8
- paired box gene 8
- PPARG
- peroxisome proliferator-activated receptor γ gene
- PPFP
- PAX8-PPARγ fusion protein
- PTEN
- phosphatase and tensin homolog
- qPCR
- quantitative real-time PCR
- TTF
- thyroid transcription factor
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling.
References
- 1. Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA. 2000. PAX8-PPARγ1 fusion oncogene in human thyroid carcinoma. Science 289:1357–1360 [DOI] [PubMed] [Google Scholar]
- 2. Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A, Fenzi G, Grüters A, Busslinger M, Di Lauro R. 1998. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet 19:83–86 [DOI] [PubMed] [Google Scholar]
- 3. Mansouri A, Chowdhury K, Gruss P. 1998. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90 [DOI] [PubMed] [Google Scholar]
- 4. Esposito C, Miccadei S, Saiardi A, Civitareale D. 1998. PAX 8 activates the enhancer of the human thyroperoxidase gene. Biochem J 331(Pt 1):37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R. 1999. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol 19:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fabbro D, Pellizzari L, Mercuri F, Tell G, Damante G. 1998. Pax-8 protein levels regulate thyroglobulin gene expression. J Mol Endocrinol 21:347–354 [DOI] [PubMed] [Google Scholar]
- 7. Au AY, McBride C, Wilhelm KG, Jr, Koenig RJ, Speller B, Cheung L, Messina M, Wentworth J, Tasevski V, Learoyd D, Robinson BG, Clifton-Bligh RJ. 2006. PAX8-peroxisome proliferator-activated receptor γ (PPARγ) disrupts normal PAX8 or PPARγ transcriptional function and stimulates follicular thyroid cell growth. Endocrinology 147:367–376 [DOI] [PubMed] [Google Scholar]
- 8. Gregory Powell J, Wang X, Allard BL, Sahin M, Wang XL, Hay ID, Hiddinga HJ, Deshpande SS, Kroll TG, Grebe SK, Eberhardt NL, McIver B. 2004. The PAX8/PPARγ fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARγ inhibition. Oncogene 23:3634–3641 [DOI] [PubMed] [Google Scholar]
- 9. Fujisawa T, Sugiyama M, Tomimoto A, Wada K, Endo H, Takahashi H, Yoneda K, Yoneda M, Inamori M, Saito S, Terauchi Y, Kadowaki T, Tsuchiya N, Nakagama H, Nakajima A. 2008. Inhibition of peroxisome proliferator-activated receptor γ promotes tumorigenesis through activation of the β-catenin/T cell factor (TCF) pathway in the mouse intestine. J Pharmacol Sci 108:535–544 [DOI] [PubMed] [Google Scholar]
- 10. Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. 2007. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25:1476–1481 [DOI] [PubMed] [Google Scholar]
- 11. Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY. 2006. PPARγ insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-κB signaling pathway. Oncogene 25:2736–2747 [DOI] [PubMed] [Google Scholar]
- 12. Park JW, Zarnegar R, Kanauchi H, Wong MG, Hyun WC, Ginzinger DG, Lobo M, Cotter P, Duh QY, Clark OH. 2005. Troglitazone, the peroxisome proliferator-activated receptor-γ agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid 15:222–231 [DOI] [PubMed] [Google Scholar]
- 13. Giordano TJ, Au AY, Kuick R, Thomas DG, Rhodes DR, Wilhelm KG, Jr, Vinco M, Misek DE, Sanders D, Zhu Z, Ciampi R, Hanash S, Chinnaiyan A, Clifton-Bligh RJ, Robinson BG, Nikiforov YE, Koenig RJ. 2006. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARγ translocation. Clin Cancer Res 12:1983–1993 [DOI] [PubMed] [Google Scholar]
- 14. Lacroix L, Lazar V, Michiels S, Ripoche H, Dessen P, Talbot M, Caillou B, Levillain JP, Schlumberger M, Bidart JM. 2005. Follicular thyroid tumors with the PAX8-PPARγ1 rearrangement display characteristic genetic alterations. Am J Pathol 167:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. 1997. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16:64–67 [DOI] [PubMed] [Google Scholar]
- 16. Paes JE, Ringel MD. 2008. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am 37:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diallo-Krou E, Yu J, Colby LA, Inoki K, Wilkinson JE, Thomas DG, Giordano TJ, Koenig RJ. 2009. Paired box gene 8-peroxisome proliferator-activated receptor-γ fusion protein and loss of phosphatase and tensin homolog synergistically cause thyroid hyperplasia in transgenic mice. Endocrinology 150:5181–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. 2006. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20:3161–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kusakabe T, Kawaguchi A, Kawaguchi R, Feigenbaum L, Kimura S. 2004. Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis 39:212–216 [DOI] [PubMed] [Google Scholar]
- 20. Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. 2002. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148–149 [DOI] [PubMed] [Google Scholar]
- 21. Dwight T, Thoppe SR, Foukakis T, Lui WO, Wallin G, Höög A, Frisk T, Larsson C, Zedenius J. 2003. Involvement of the PAX8/peroxisome proliferator-activated receptor γ rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab 88:4440–4445 [DOI] [PubMed] [Google Scholar]
- 22. Marques AR, Espadinha C, Catarino AL, Moniz S, Pereira T, Sobrinho LG, Leite V. 2002. Expression of PAX8-PPARγ 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab 87:3947–3952 [DOI] [PubMed] [Google Scholar]
- 23. Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A. 2007. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res 67:959–966 [DOI] [PubMed] [Google Scholar]
- 24. Okumura T. 2010. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J Gastroenterol 45:1097–1102 [DOI] [PubMed] [Google Scholar]
- 25. Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. 1998. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med 4:1053–1057 [DOI] [PubMed] [Google Scholar]
- 26. Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. 1998. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nat Med 4:1058–1061 [DOI] [PubMed] [Google Scholar]
- 27. Ikezoe T, Miller CW, Kawano S, Heaney A, Williamson EA, Hisatake J, Green E, Hofmann W, Taguchi H, Koeffler HP. 2001. Mutational analysis of the peroxisome proliferator-activated receptor γ gene in human malignancies. Cancer Res 61:5307–5310 [PubMed] [Google Scholar]
- 28. Burton JD, Goldenberg DM, Blumenthal RD. 2008. Potential of peroxisome proliferator-activated receptor γ antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res 2008:494161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaefer KL, Wada K, Takahashi H, Matsuhashi N, Ohnishi S, Wolfe MM, Turner JR, Nakajima A, Borkan SC, Saubermann LJ. 2005. Peroxisome proliferator-activated receptor γ inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res 65:2251–2259 [DOI] [PubMed] [Google Scholar]
- 30. Bojková B, Garajová M, Kajo K, Péc M, Kubatka P, Kassayová M, Kisková T, Orendás P, Ahlersová E, Ahlers I. 2010. Pioglitazone in chemically induced mammary carcinogenesis in rats. Eur J Cancer Prev 19:379–384 [DOI] [PubMed] [Google Scholar]
- 31. Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H. 2003. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 124:361–367 [DOI] [PubMed] [Google Scholar]
- 32. Magenta G, Borenstein X, Rolando R, Jasnis MA. 2008. Rosiglitazone inhibits metastasis development of a murine mammary tumor cell line LMM3. BMC Cancer 8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. 1998. Terminal differentiation of human breast cancer through PPARγ. Mol Cell 1:465–470 [DOI] [PubMed] [Google Scholar]
- 34. Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR, Spiegelman BM. 2010. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466:451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. 2009. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid 19:953–956 [DOI] [PubMed] [Google Scholar]
- 36. Debrock G, Vanhentenrijk V, Sciot R, Debiec-Rychter M, Oyen R, Van Oosterom A. 2003. A phase II trial with rosiglitazone in liposarcoma patients. Br J Cancer 89:1409–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 1995. 15-Deoxy-Δ12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83:803–812 [DOI] [PubMed] [Google Scholar]
- 38. Hallenborg P, Jørgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, Langer T, Furstenberger G, Krieg P, Koppen A, Kalkhoven E, Madsen L, Kristiansen K. 2010. Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor γ activity. Mol Cell Biol 30:4077–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. 2008. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat Struct Mol Biol 15:924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. 1995. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 83:813–819 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.