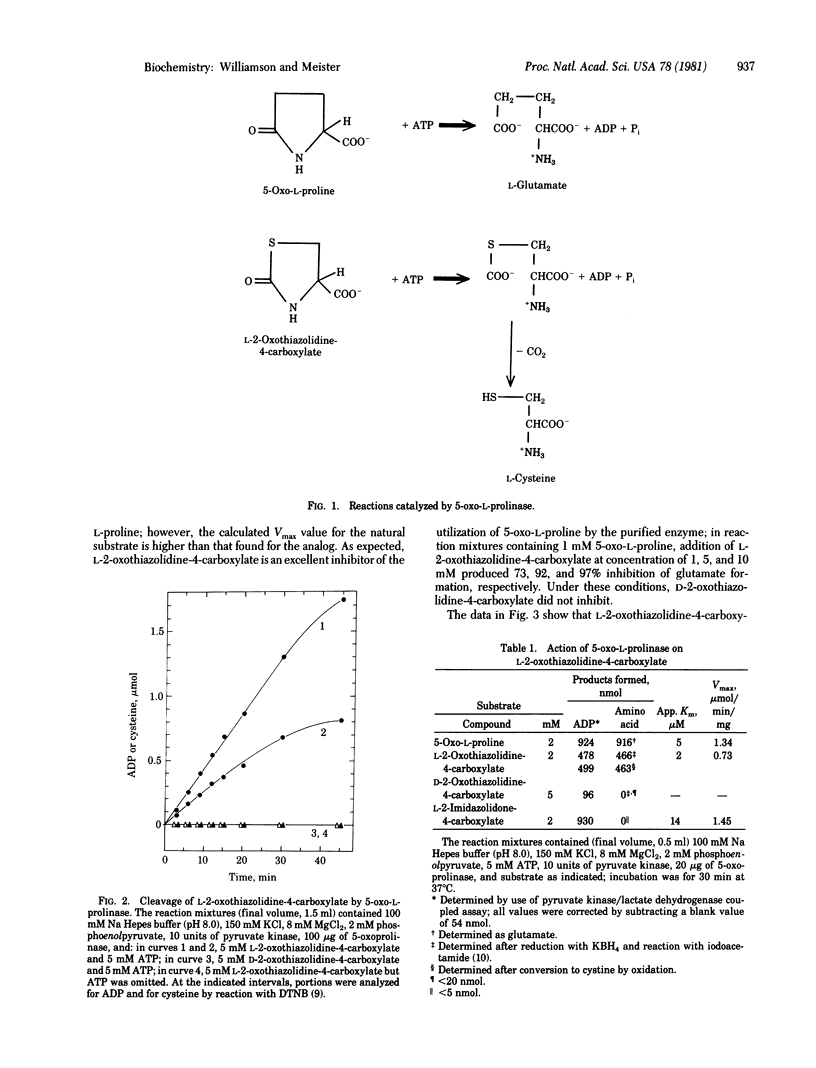
Abstract
5-Oxo-L-prolinase, the enzyme that catalyzes the conversion of 5-oxo-L-proline to L-glutamate coupled to the cleavage of ATP to ADP and Pi, also acts on L-2-oxothiazolidine-4-carboxylate (an analog of 5-oxoproline in which the 4-methylene moiety is replaced by sulfur) and ATP to yield cysteine and ADP. The enzyme, which exhibits an affinity for the analog similar to that for the natural substrate, is inhibited by the analog in vitro and in vivo. L-2-oxothiazolidine-4-carboxylate thus serves as a potent inhibitor of the gamma-glutamyl cycle at the step of 5-oxoprolinase. Administration of L-2-oxothiazolidine-4-carboxylate to mice that had been depleted of hepatic glutathione led to restoration of normal hepatic glutathione levels. Since L-2-oxothiazolidine-4-carboxylate is an excellent substrate of the enzyme, it may serve as an intracellular delivery system for cysteine and thus has potential as a therapeutic agent for conditions in which there is depletion of hepatic glutathione.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Mansuy D., Beaune P., Cresteil T., Lange M., Leroux J. P. Evidence for phosgene formation during liver microsomal oxidation of chloroform. Biochem Biophys Res Commun. 1977 Nov 21;79(2):513–517. doi: 10.1016/0006-291x(77)90187-5. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pohl L. R., Bhooshan B., Whittaker N. F., Krishna G. Phosgene: a metabolite of chloroform. Biochem Biophys Res Commun. 1977 Dec 7;79(3):684–691. doi: 10.1016/0006-291x(77)91166-4. [DOI] [PubMed] [Google Scholar]
- RUBINSTEIN D., KANICS L. THE CONVERSION OF CARBON TETRACHLORIDE AND CHLOROFORM TO CARBON DIOXIDE BY RAT LIVER HOMOGENATES. Can J Biochem. 1964 Nov;42:1577–1585. doi: 10.1139/o64-169. [DOI] [PubMed] [Google Scholar]
- Sekura R., Meister A. Glutathione turnover in the kidney; considerations relating to the gamma-glutamyl cycle and the transport of amino acids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2969–2972. doi: 10.1073/pnas.71.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H., Hartman S. P., Weinhouse S. Formation of carbonyl chloride in carbon tetrachloride metabolism by rat liver in vitro. Cancer Res. 1979 Oct;39(10):3942–3947. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Van Der Werf P., Griffith O. W., Meister A. 5-Oxo-L-prolinase (L-pyroglutamate hydrolase). Purification and catalytic properties. J Biol Chem. 1975 Sep 10;250(17):6686–6692. [PubMed] [Google Scholar]
- Van Der Werf P., Stephani R. A., Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the gamma-glutamyl cycle. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Stephani R. A., Orlowski M., Meister A. Inhibition of 5-oxoprolinase by 2-imidazolidone-4-carboxylic acid. Proc Natl Acad Sci U S A. 1973 Mar;70(3):759–761. doi: 10.1073/pnas.70.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf P., Meister A. The metabolic formation and utilization of 5-oxo-L-proline (L-pyroglutamate, L-pyrrolidone carboxylate). Adv Enzymol Relat Areas Mol Biol. 1975;43:519–556. doi: 10.1002/9780470122884.ch7. [DOI] [PubMed] [Google Scholar]


