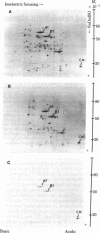
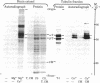
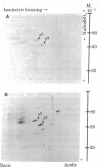
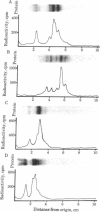
Abstract
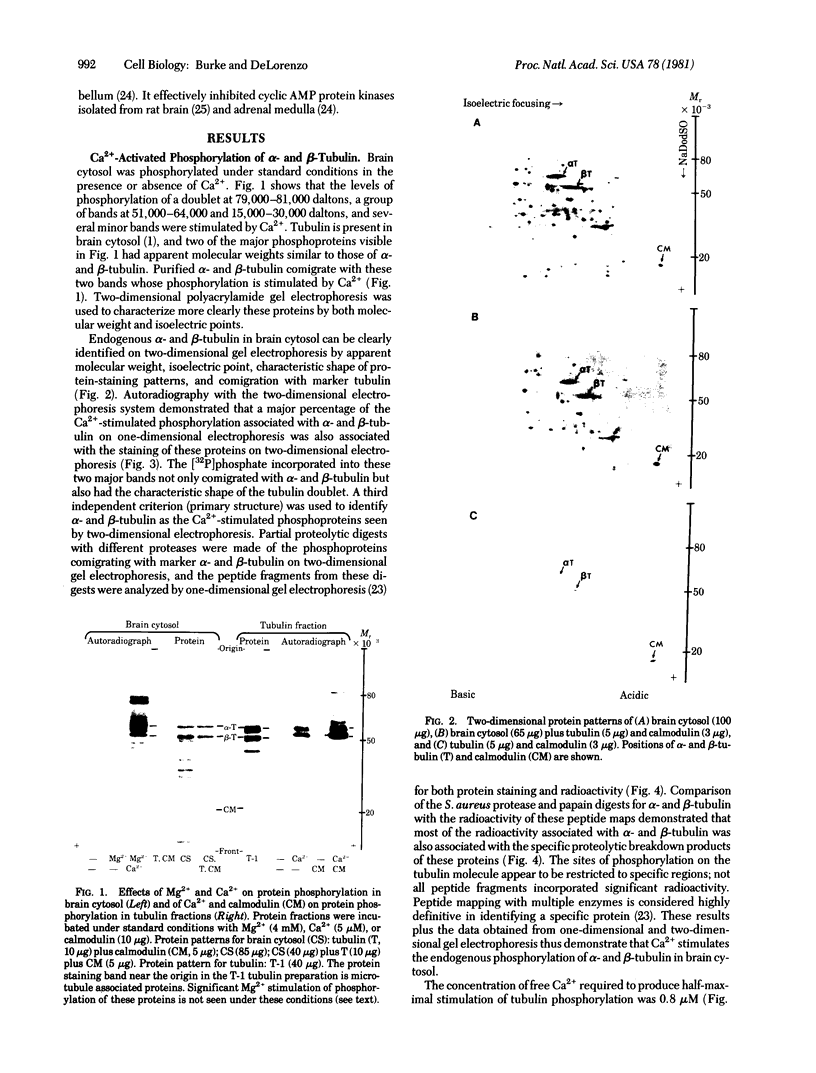
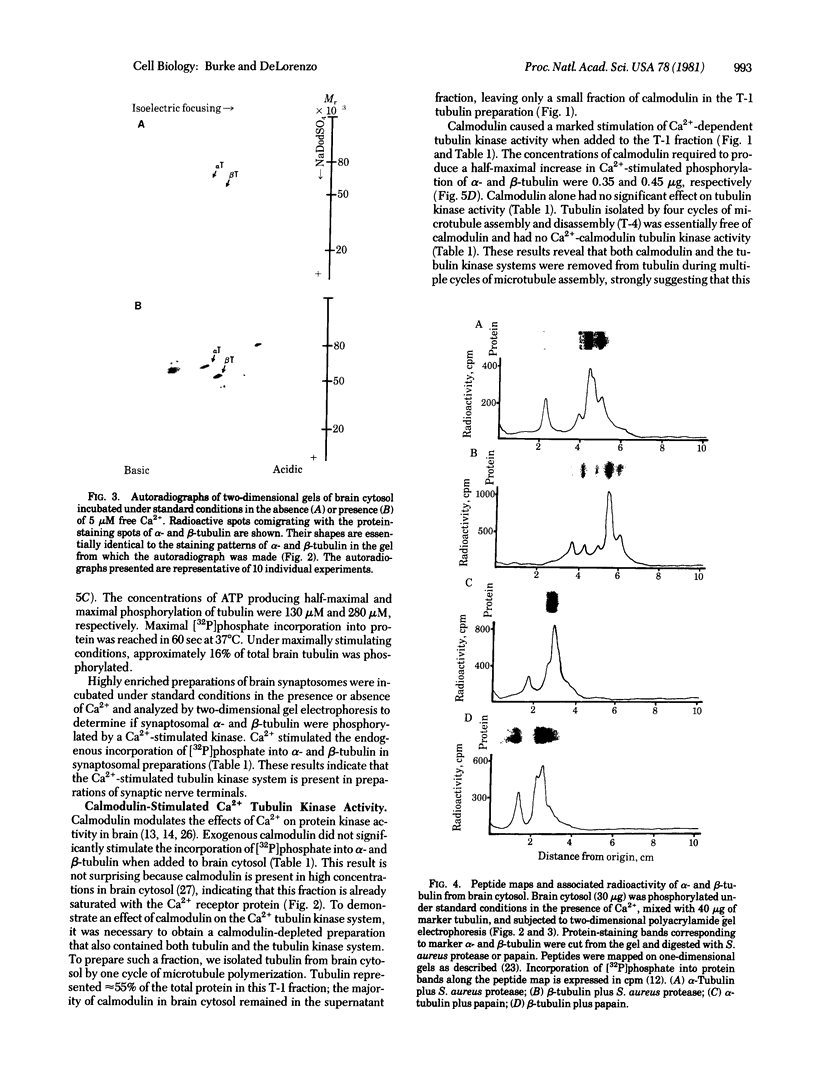
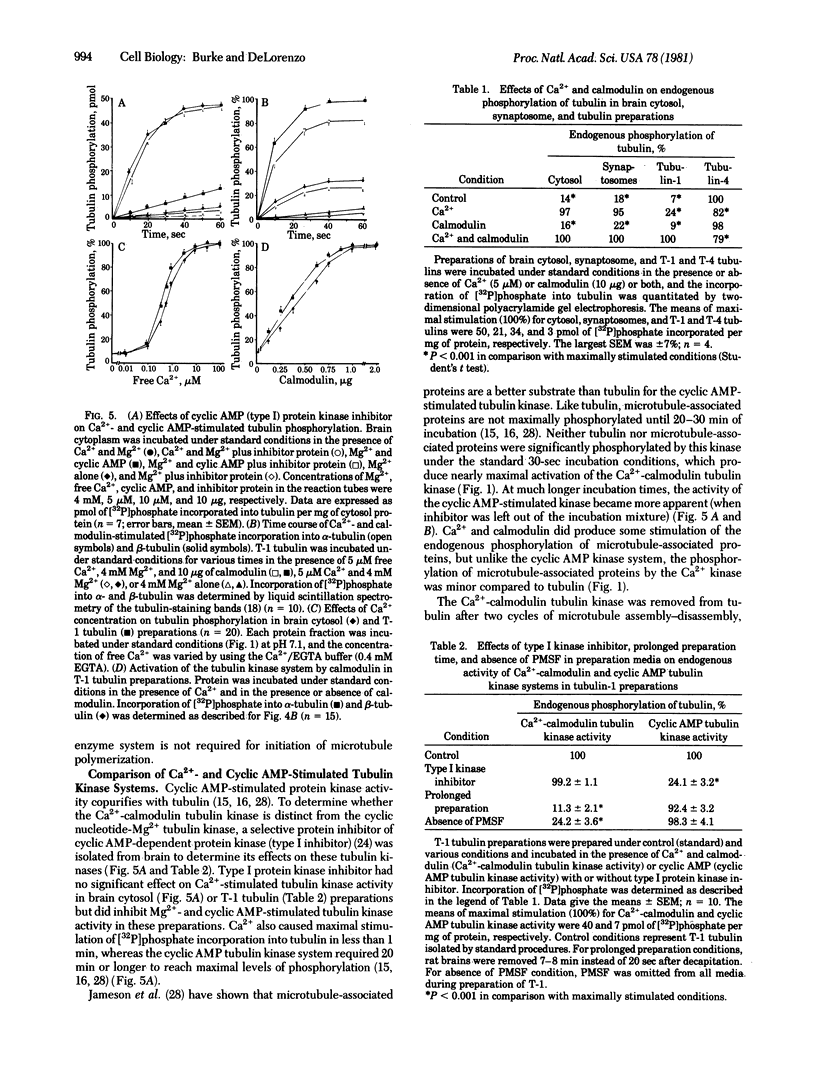
Ca2+ plays a major role in the functional use of tubulin in brain and other tissues. It activates an endogenous tubulin kinase system in brain cytosol, tubulin, and presynaptic nerve terminal fractions prepared from rat brain. Activation of the Ca2+ tubulin kinase system was modulated by the Ca2+ receptor protein calmodulin. The concentrations of Ca2+ and calmodulin required to produce a half-maximal stimulation of the tubulin kinase were 0.8 microM and 0.4 micrograms, respectively. Ca2+ -calmodulin tubulin kinase activity was very unstable after death, and procedures were developed to stabilize the activity of this enzyme system. Evidence is presented demonstrating that the Ca2+ -calmodulin tubulin kinase system is distinct from the previously described cyclic AMP-Mg2+ tubulin kinase. The results suggest that Ca2+- and calmodulin-stimulated phosphorylation of tubulin may be a major biochemical mechanism modulating some of calcium's effects on tubulin and may play a significant role in mediating some of calcium's actions on cell functions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blitz A. L., Fine R. E. Muscle-like contractile proteins and tubulin in synaptosomes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4472–4476. doi: 10.1073/pnas.71.11.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J. Antagonistic action of diphenylhydantoin and calcium on the level of phosphorylation of particular rat and human brain proteins. Brain Res. 1977 Sep 23;134(1):125–138. doi: 10.1016/0006-8993(77)90930-1. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Emple G. P., Glaser G. H. Regulation of the level of endogenous phosphorylation of specific brain proteins by diphenylhydantoin. J Neurochem. 1977 Jan;28(1):21–30. doi: 10.1111/j.1471-4159.1977.tb07704.x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D. Calcium-dependent phosphorylation of synaptic vesicle proteins and its possible role in mediating neurotransmitter release and vesicle function. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1036–1043. doi: 10.1016/s0006-291x(77)80082-x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J. Role of calmodulin in neurotransmitter release and synaptic function. Ann N Y Acad Sci. 1980;356:92–109. doi: 10.1111/j.1749-6632.1980.tb29603.x. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain tubulin and protein kinase activity. J Biol Chem. 1974 Mar 10;249(5):1398–1406. [PubMed] [Google Scholar]
- Feit H., Barondes S. H. Colchicine-binding activity in particulate fractions of mouse brain. J Neurochem. 1970 Sep;17(9):1355–1364. doi: 10.1111/j.1471-4159.1970.tb06870.x. [DOI] [PubMed] [Google Scholar]
- Jameson L., Frey T., Zeeberg B., Dalldorf F., Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980 May 27;19(11):2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Gray C. J. Tryptophanyl and carboxylic acid residues in the active centre of glucoamylase I from Aspergillus niger. Carbohydr Res. 1976 Jul;49:361–370. doi: 10.1016/s0008-6215(00)83153-5. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. I., Walters B. B., Mughal S. Immunohistochemical demonstration of tubulin associated with microtubules and synaptic junctions in mammalian brain. J Neurocytol. 1975 Dec;4(6):733–744. doi: 10.1007/BF01181633. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochs S. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Systems of material transport in nerve fibers (axoplasmic transport) related to nerve function and trophic control. Ann N Y Acad Sci. 1974 Mar 22;228(0):202–223. doi: 10.1111/j.1749-6632.1974.tb20511.x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Calcium and cAMP as interrelated intracellular messengers. Ann N Y Acad Sci. 1975 Jun 30;253:789–796. doi: 10.1111/j.1749-6632.1975.tb19247.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978 Feb 2;271(5644):478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soifer D. Enzymatic acitivity in tubulin preparations: cyclic-AMP dependent protein kinase activity of brain microtubule protein. J Neurochem. 1975 Jan;24(1):21–33. doi: 10.1111/j.1471-4159.1975.tb07623.x. [DOI] [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K., Mickey D. L., Story M. The calibration and use of a calcium ion-specific electrode for kinetic studies of mitochondrial calcium transport. Anal Biochem. 1979 Mar;93(2):430–441. doi: 10.1016/s0003-2697(79)80175-x. [DOI] [PubMed] [Google Scholar]