Abstract
A punctate, cutaneous application of capsaicin or histamine by means of a cowhage spicule elicits itch accompanied by pricking/stinging, burning and typically one or more areas of dysesthesia (alloknesis, hyperalgesia, hyperknesis). When applied over a wider and deeper area of skin by means of intradermal injection, histamine evokes the same sensory effects but capsaicin evokes pain and hyperalgesia with allodynia instead of alloknesis. To examine the sensory effects of the spatial spread, depth and amount of capsaicin and histamine, we applied different amounts of capsaicin or histamine by intradermal injection or by single vs. multiple spicules within a circular cutaneous region of ~5 mm. Subjects rated the perceived intensity of itch, pricking/stinging and burning for 20 min. Histamine injections or multiple spicules of capsaicin or histamine that resulted in a greater area of flare than a single spicule of each chemical evoked no greater magnitudes of sensation or areas of dysesthesia. Capsaicin injections elicited a dose-dependent increase in the magnitude of nociceptive sensations, areas of dysesthesia and flare. However, there was little or no itch; and allodynia replaced alloknesis. Yet, hyperalgesia was typically accompanied by hyperknesis. We conclude that the pruritic sensory responses produced by capsaicin/histamine spicules and histamine injections may be due to activation of common nerve fibers, possibly different from those mediating the flare, and that capsaicin injections may activate additional fibers whose effects mask the sensory effects of fibers mediating itch and alloknesis but not hyperknesis.
Introduction
Capsaicin is commonly thought to be algogenic and histamine, pruritic. When injected into the skin in humans, capsaicin evoked pain and histamine, itch [28; 29]. The skin surrounding the injection site was reported as tender after capsaicin (with allodynia to stroking and hyperalgesia to pricking) but itchy after histamine (with alloknesis to stroking and hyperknesis to pricking) [1; 27; 28]. In these studies the subjects were neither instructed to rate any nociceptive sensations that may have accompanied the itch to histamine nor any itch or hyperknesis that might have occurred in response to capsaicin. In addition, the relative magnitudes of pruritic and nociceptive sensations could not be compared, as they were not rated using the same psychophysical scale of magnitude.
In other studies, histamine or capsaicin was applied in a more punctate manner to the skin and the subjects were asked to judge both itch and nociceptive sensations using the same rating scale. A small amount of capsaicin contained in a chemically-soaked, heat-inactivated cowhage spicule produced a dominant sensation of itch, accompanied by lesser magnitudes of burning and pricking/stinging [26]. The same qualities of itch and nociceptive sensations were elicited when histamine was applied by means of a spicule. In addition, the types of hypersensitivity in the skin surrounding a capsaicin spicule were similar to those surrounding a spicule of histamine, namely alloknesis, hyperknesis, and hyperalgesia. Thus, capsaicin and histamine applied in a punctate manner to the skin elicited the same qualities of sensation and the same alterations in sensations evoked by mechanical stimulation of the surrounding skin. Histamine therefore evoked the same qualities of sensation and dysesthesias whether injected or applied by spicule. But for capsaicin, injection evoked pain and allodynia whereas spicule application elicited itch and alloknesis.
It is unclear if itch or nociceptive sensations evoked by capsaicin or histamine show spatial summation. For example, it has been shown that varying the diameter of a capsaicin-soaked filter paper applied to the skin resulted in an enhanced frequency, but not intensity, of itch [6]. However, increasing the number of stimuli applied on the skin enhanced the intensity of perceived irritation, suggesting spatial summation of capsaicin-induced pain. Spatial summation of itch induced by histamine is rarely documented [30]. The present study compared the sensory effects of histamine and capsaicin delivery by spicules and by hypodermic injection using the same rating scale. The possibility that a spread of chemical beyond that produced by a single spicule might lead to spatial summation of itch or nociceptive sensations or alter the quality of the predominant sensation produced, was tested by comparing the sensory effects of a single vs. multiple spicules delivered within a region of skin comparable in size to that of a bleb produced by an injection.
METHODS AND MATERIALS
2.1 Subjects
Ten healthy subjects: 8 females and 2 males participated in the single vs. multiple spicule experiment. For experiments involving intradermal injections of chemical, fifteen subjects participated: 7 females and 8 males. The latter were also tested with a single spicule of capsaicin and histamine to compare, with the same scale, their ratings of sensations evoked by single spicule, injections of chemical or brief heat stimuli. The limited sample of males vs. females was not intended to be adequate to determine possible effects of gender on the sensory effects evoked by capsaicin or histamine.
Subjects reporting a history of dermatological, neurological, immunological or cardiac disorders were excluded. In addition, subjects were required to refrain from taking anti-histamines and/or analgesics at least 24hrs prior to an experiment. All protocols were approved by the Yale University Human Investigation Committee.
2.2 Preparation and administration of injections and spicules
Solutions for injection of capsaicin and histamine were prepared in extracellular fluid (ECF) containing, in sterile water for injection: 135 mM sodium chloride, 3 mM potassium chloride, 1 mM sodium phosphate dibasic, 1 mM magnesium sulfate heptahydrate, 1.2 mM calcium chloride dihydrate, and adjusted to a pH of 7.4 with 8.4% sodium bicarbonate. Unlike normal saline, ECF has a composition comparable to that of bodily fluids. Additionally, the initial discomfort associated with an injection of ECF alone was significantly lower than that of a saline injection (mean peak rating of pricking/stinging of 3.0 ± 0.9, n=15 vs.13.4 ± 3.2, n=12, p< 0.01, unpaired t-test).
Histamine dihydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in a vehicle of sterile ECF. Each subject received, in the volar forearm, a single 10 μl injection of the vehicle alone or the vehicle containing histamine (ug): 0.001, 0.01, 0.1, 1 or 10. Capsaicin (Sigma-Aldrich, St. Louis, MO) was dissolved in ethanol first and then titrated into individual vials to obtain the desired dose [28]. The ethanol was evaporated and the chemical was dissolved in the required volume of ECF containing 7% Tween 80 to obtain the final concentration. Solutions were allowed to mix using an ultrasonic bath for at least 1 hr. Each subject received a single 10 μl injection of ECF + 7% Tween 80 and 10 μl of each solution containing: 0.001, 0.01, 0.1, 1 and 10 μg of capsaicin. The solutions were tested in ascending order of concentration. The chemicals (histamine or capsaicin) were injected as superficially as possible by inserting the needle approximately parallel to the skin such that the opening at the tip was (usually) visible through the skin. Typically, each arm was tested two or three times with subsequent injections made well outside the area of dysesthesias produced by a prior injection. No two consecutive injections were made on the same arm.
The heat-inactivated spicules were filled with a solution of either histamine (10 mg/ml) or capsaicin (200 mg/ml) as previously described [26]. Briefly, inert spicules were soaked in 10 mg/ml histamine solution made in distilled water or 200 mg/ml capsaicin solution prepared in 80% ethanol. The spicules were observed under a microscope as they filled. Once filled, the spicules were pulled out of the solution and transferred on a filter paper placed in a Petri dish and allowed to dry. The tip of a spicule was applied to the skin by means of fine forceps, using a stereo microscope. We estimated that the theoretical amount of chemical contained in a single histamine spicule was 0.3 ng and for a single capsaicin spicule, 6 ng. Alternatively, a group of spicules was applied by means of a “spicule inserter” (Si) (Fig. 1, in [13]). The Si was prepared from a semi-circular, cut-off Weck-Cel ophthalmic surgical spear, 5 mm wide upon which 25 chemically-soaked spicules were mounted parallel/adjacent to each other and secured by means of nail polish. Two Si’s were applied towards each other to a small area of skin such that the tips of 20 to 30 spicules were inserted at an oblique angle of < 30° within a circular region of ~ 5 mm, similar to the diameter of an injection bleb. Not all of the spicules mounted on the Si’s became inserted into the skin upon application. An average of 24.7 ± 1.5 capsaicin spicules and 29.6 ± 1.5 histamine spicules became inserted. Thus, the theoretical calculations of the average amount of chemical applied via multiple spicules would be approximately 25 × 6 ng/spicule or 0.15 μg of capsaicin and 30 × 0.3 ng/spicule or 9 ng of histamine.
Figure 1.
Time course of the perceived intensity of itch and nociceptive sensations evoked by single vs. multiple spicules containing capsaicin or histamine. A-F, The mean ratings of itch, pricking/stinging and burning obtained from 10 subjects are plotted for successive intervals of 30 s after spicule application. For clarity, the SEM is plotted every 5 min starting with the peak rating for each quality. On the right vertical axis, the locations of 3 of the verbal descriptors are shown in correspondence with the ratings of perceived intensity indicated on the left vertical axis (see section 2.7 for further details).
2.3 Rating the intensity of sensation
Each subject on different occasions received injections of one or more concentrations of histamine (or ECF only) and capsaicin (or ECF + 7%Tween) and/or chemically soaked spicules (histamine or capsaicin). In a previous study it was determined that inactive spicules produced no sensations [26]. Prior to testing, the subjects were instructed in the use of the generalized Labeled Magnitude Scale (gLMS) [2; 7] to rate the perceived intensity of itch, pricking/stinging and burning evoked by a given stimulus. Itch was described as a sensation that evokes a desire to scratch. Pricking/stinging was defined as sharp, well-localized sensation that was either intermittent (pricking) (as a “prick” from a needle) or continuous (stinging) similar to being stung by an insect. Burning was described as a sensation that is most often associated with thermal burns and sunburns but may also be evoked by other stimuli such as skin abrasions, strong cold or chemical irritants. The subjects were told that these sensations may or may not be accompanied by a thermal sensation and might or might not be painful. The subjects were also instructed to rate only the sensations and not the “stimulus” and that some stimuli may produce no sensations. They were asked to disregard the initial prick experienced at moment of stimulus application.
The gLMS was presented to the subject on a computer screen and consisted of a vertical, thermometer-like scale that had labels consisting of “no sensation”, “barely detectable”, “weak”, “moderate”, “strong”, “very strong”, and “strongest imaginable sensation of any kind” spaced quasi-logarithmically along its length. The subject was instructed to rate the intensity of itch, pricking/stinging and burning relative to the “strongest imaginable sensation of any kind” placed at the top of the scale. Subjects were prompted to make ratings for each of the three qualities every 30 seconds following application of the stimulus. Ratings were recorded for up to a maximum of 20 minutes or until all sensations were rated zero three times consecutively. The ratings were acquired and recorded on the computer using a customized program (Dapsys 7.0, Dr. Brian Turnquist, Bethel University, St. Paul, Minnesota).
All sensations rated as equal to, or greater, than “Barely detectable” on the scale were included in analyses. An opaque cover was used to hide the injection or spicule insertion site to prevent the subject from observing any skin reaction associated with an injection.
2.5 Cutaneous dysesthesias
When all the sensations from the injected solution or the inserted spicule/s had disappeared or 20 minutes had elapsed, the borders of regions exhibiting the following areas of hypersensitivities (dysesthesias) to mechanical stimuli were mapped and marked on the skin: 1) Alloknesis, defined as itch evoked by stroking the skin with a cotton swab that was mounted on a coping-saw blade that exerted approximately 100 mN of compressional force; or allodynia, defined as a feeling of burning or tenderness in response to the same stroking stimulus; 2) Hyperknesis, defined as enhanced itch to pricking the skin with a von Frey filament having a tip diameter of 50 μm and exerting a bending force of 20 mN; the filament produced an initial transient prickle or pricking pain sensation followed by a sensation of itch. The subjects were asked to disregard the pricking sensation and attend only to the itch. 3) Hyperalgesia, defined as enhanced pricking pain to a von Frey filament with tip diameter of 200 μm and exerting a bending force of 200 mN. In response to each of these two von Frey filaments (one evoking itch, the other pain), the subjects were instructed to judge only the intensity of the sensation and not the geometric features of the stimulus itself such as how “sharp” it was.
The borders of each dysesthesia were mapped using a stimulus of ~1s applied as a short stroke with the swab or indentation with the filament proceeding from outside the anticipated area towards the chemical application site along 8-10 radial paths. To facilitate accurate mapping of the dysesthetic area the subject was occasionally asked to compare dysesthetic sensations with the normal sensations evoked by an application of the mapping stimulus to the opposite arm. The subjects were instructed not to look at their arms during application of either the test stimulus or mapping of dysesthesias.
Once the dysesthetic areas were mapped, the borders of any skin reaction were marked on the skin. Such a reaction, if it occurred, consisted of a wheal (raised edematous region) and/or an area of erythema. The application of an inert spicule occasionally produced a small area of local redness of only 1-2 millimeters at the application site. To be included in the analyses, the size of the wheal from an injection was required to have a minimum area of 0.6 cm2 (bigger than the size of the bleb produced by an injection). The wheal from a spicule had to exceed a diameter of 2 mm. To be included in analyses as a “neurogenic flare”, the area of erythema was arbitrarily required to be greater than 1 cm2. The areas of dysesthesias and skin reactions were photographed with a digital camera and subsequently measured by means of a computer.
2.6 Thermal stimulation
In a separate test, subjects were presented with thermal stimuli (43°C, 47°C, 51°C) delivered to nine previously marked regions along the length of the volar forearm. The purpose was to relate the magnitude of heat evoked sensations, commonly studied in psychophysical studies of pain in humans, to those obtained in response to chemical stimuli. The heat stimuli were delivered by means of a Peltier contact thermode of 1 cm2 that maintained each stimulus temperature within 0.1°C of the desired value by means of electronic circuitry and feedback from a thermocouple at the skin-thermode interface [12]. Each stimulus was a trapezoidal waveform that began with a rapid increase (19 °C/s) in temperature from a base temperature of 38°C, to a desired plateau temperature that was maintained for 5 seconds, followed by a rapid decrease back to the base. Each region on the skin and the corresponding temperature stimulus to be tested were selected pseudo randomly such that each temperature stimulus was delivered three times in total and each test region was stimulated only once. At the termination of each stimulus, the subject was instructed to rate the evoked maximal perceived intensity of any evoked itch, pricking/stinging and burning sensation.
2.7 Data analyses
Each rating of a subject was saved as a number (not visible to the subject) corresponding to the position of the cursor on a linear scale from 0 (“no sensation”) at the bottom of the scale to 100 (“strongest sensation imaginable of any kind”) at the top and the positions of the other labels were 1 for barely detectable, 6 for weak, 17 for moderate, 35 for strong, and 53 for very strong [2; 7].
Data were analyzed after performing a logarithmic (log) transformation. A value of 1.0 was added to each sensory rating to avoid the presence of any zeros prior to executing the logarithmic transformation. For each sensory quality, logarithmic values of the following variables were calculated: the area under the curve (AUC); the peak magnitude of the sensation, and the total duration of the sensation - from the first non-zero value (rated ≥ Barely detectable) to the first of the three consecutive zeros, or until 20 min had elapsed.
Student’s t-test was used to determine significance of differences between single and multiple spicule evoked sensations and areas of dysesthesias. A linear regression line was computed between the amount of injected capsaicin or histamine and the mean value of each of the variables. A variable was determined to be dependent on the concentration of the chemical if the slope of the regression line was significantly different from zero. To study the effect of concentration on the areas of dysesthesias and skin reaction, linear regression lines were computed to determine the effects of concentration of capsaicin or histamine on the mean log areas of dysesthesias and skin reaction. Analysis of variance with repeated measures (RMANOVA) was performed to test the effect of heat stimuli on the intensity of evoked itch and nociceptive sensations. Data from single and multiple spicules (or different concentrations of chemical injected) were combined to calculate the correlation between peak magnitudes of itch and the areas of dysesthesias and flare for the data sets using the Pearson coefficient.
Logistic regression analyses (followed by Bonferroni correction for multiplicity) were performed to determine the significance of the occurrence of a given dysesthesia and the concentration of the capsaicin or histamine injection. Fischer’s exact test was performed to determine the significance of the occurrence of a given dysesthesia for single and multiple spicules. Image J software (National Institute of Health) was used to measure the areas of dysesthesias and skin reaction marked in the digitized pictures of the skin tested with injections or spicules(s).
GraphPad Prism 4.03 software (GraphPad Software, San Diego, CA) was used to perform all calculations. Data are presented as means ± SEMs. The probability criterion for statistical significance was set at p < 0.05.
3. Results
3.1 Itch and nociceptive sensations evoked by one or multiple spicules of capsaicin
Itch was evoked in all ten subjects tested with single and multiple spicules of capsaicin. The mean ratings of the peak magnitude of each sensory quality were plotted as a function of time (Fig. 1A-C). Analyses of these intensity-time profiles revealed only one difference between ratings obtained for single and multiple spicules: The mean AUC for itch ratings was significantly lower in response to multiple spicules (p = 0.04). Otherwise, there were no significant differences between ratings for the two groups in the mean peak magnitude and mean total duration of itch or in the mean peak magnitude, total duration and AUC for pricking/stinging and burning. Thus, as the number of spicules was increased from 1 to as many as 30, distributed within a few millimeters (~5 mm) of skin, there was no increase (spatial summation) in the magnitude of itch or nociceptive sensation; rather there was an overall suppression of itch without a change in the intensity of nociceptive sensations.
3.2 Itch and nociceptive sensations evoked by one or multiple spicules of histamine
We compared the incidence, magnitude, and duration of sensations produced by single versus multiple histamine spicules. The profiles of perceived intensity vs. time were obtained for itch, pricking/stinging and burning produced under the two experimental conditions (Fig 1, D-F). Each subject reported itch when tested with single or multiple histamine spicules. Itch was the dominant sensory quality for 90% of subjects and its magnitude or duration was not significantly different between the two groups. There were no significant differences for single vs. multiple spicules in the mean log values of the AUC, peak magnitude, and total duration of itch.
Additionally, in comparison to one spicule, multiple spicules did not significantly evoke an increase in the incidence, magnitude, or duration of pricking/stinging and burning. Thus, no summation of either itch or nociceptive sensations was observed as the number of histamine spicules was increased from 1 to as many as 30 within a localized region of the skin.
The log AUC (p=0.007) and log peak magnitude (p=0.01) but not the log total duration (p = 0.31) of itch produced by a single histamine spicule was significantly greater than the itch produced by a single capsaicin spicule. We believe that these apparent differences are unlikely to be functionally significant based on the results of an earlier study with a larger sample of subjects (n=21) that obtained comparable ratings of itch for single spicules of capsaicin and histamine at the doses presently used [26].
3.3 Cutaneous dysesthesias evoked by spicules of capsaicin and histamine
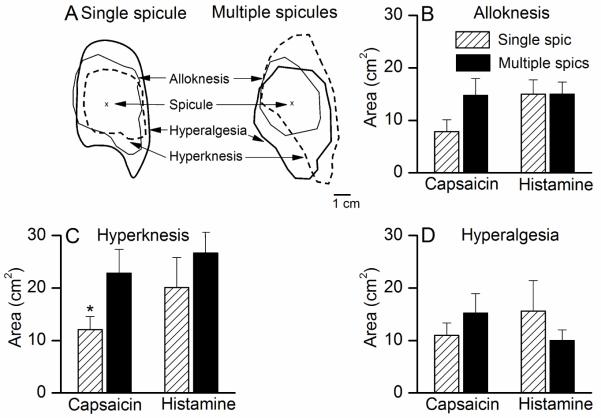
Areas of one of more types of cutaneous dysesthesia often developed within the skin surrounding the site of a spicule application (Fig. 2A).
Figure 2.
The areas of dysesthesia evoked by single vs. multiple spicules containing capsaicin or histamine. A, Typical areas of dysesthesias evoked by single and multiple capsaicin spicules in a subject. B-D, The mean areas of alloknesis, hyperknesis, and hyperalgesia evoked by each chemical delivered in single vs. multiple spicules.
For capsaicin, single and multiple spicules produced areas of alloknesis, hyperalgesia and hyperknesis but no allodynia (Fig 2, B-D). The incidence of occurrence of each type of dysesthesia did not differ for single vs. multiple spicules (50 vs. 40% for alloknesis, 70 vs. 70% for hyperalgesia, and 80 vs. 60% for hyperknesis, p = 0.1, Fischer’s exact test). The mean areas of alloknesis and hyperalgesia were not significantly different for single vs. multiple spicules (Student’s t-test, p = 0.06 and p = 0.2, respectively). The mean area of hyperknesis was significantly smaller for the single spicules when compared with multiple spicules (Student’s t-test, p = 0.02). Thus, the incidences and areas of dysesthesias (except for hyperknesis) were the same for single and multiple spicule delivery.
For histamine, the incidence of occurrence (p=0.1, Fischer’s exact test) and the mean areas of alloknesis, hyperknesis and hyperalgesia were not significantly different for single and multiple spicules (Student’s t-test, p>0.05, Fig 2B-D). For example, the mean area (and incidence of occurrence) of alloknesis for a single spicule was 15 ± 2.7 cm2 (70%) and for multiple spicules was 15 ± 2.3 cm2 (80%). Allodynia was never reported with histamine spicules (single or multiple).
3.4 Skin reactions evoked by spicules of capsaicin and histamine
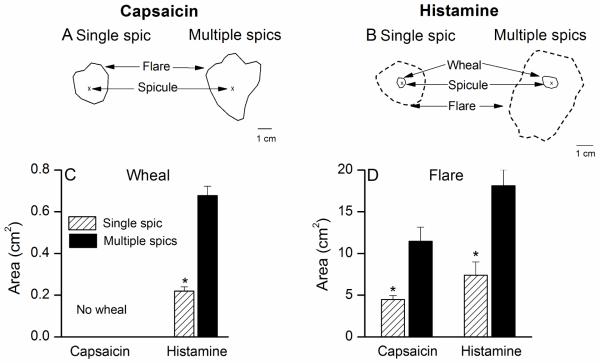
Neither single nor multiple spicules of capsaicin resulted in a wheal. A single capsaicin spicule commonly produced no visible skin reaction (Fig 3A). The percentage of total subjects tested that exhibited a flare with a single capsaicin spicule was 20%. In contrast, 80% of subjects tested with multiple capsaicin spicules exhibited a flare. The mean area of flare for the multiple spicules was significantly greater than that accompanying a single capsaicin spicule, (Student’s paired t-test, p= 0.0008). No significant correlation (p = 0.18) existed between the peak magnitudes of itch and the areas of flare evoked by capsaicin spicules.
Figure 3.
Areas of wheal and flare produced by single vs. multiple spicules of capsaicin or histamine. Typical areas of wheal or flare accompanying the sensations evoked by single vs. multiple spicules of capsaicin (A) and histamine (B) in a subject. Unlike histamine, capsaicin did not elicit a wheal. C, The mean area of wheal was significantly greater for multiple than a single spicule of histamine. D, The mean area of flare evoked by histamine or capsaicin was significantly larger for multiple spicules than for the single spicule.
In contrast to capsaicin, histamine in a single spicule or multiple spicules was capable of eliciting a wheal along with a flare (Fig 3B). The incidence of wheal and flare was significantly greater for multiple spicules than for single spicule (100% vs. 30% for wheal and 100% vs. 90% for flare). The mean areas of the wheal and flare were each significantly greater in response to multiple spicules than to a single spicule (Student’s paired t-test, p<0.001, Fig 3 C-D). Additionally, the areas of wheal and flare were not correlated with the peak magnitude of itch evoked by single/multiple histamine spicules.
The increased incidence and area of wheal and/or flare with multiple vs. single spicules of histamine and capsaicin provide evidence that more chemical was delivered. Thus, the lack of spatial summation of sensory effects produced by increasing the number of spicules was not due to the absence of increased chemical delivery to the skin. That is, different mechanisms govern the skin reactions and sensory effects of chemical application by spicule.
3.5 Itch and nociceptive sensations evoked by injections of capsaicin
An intradermal injection of capsaicin, regardless of the amount, produced predominantly pricking/stinging and burning and seldom itch in the 15 subjects tested (Fig 4A-C). Nociceptive sensations peaked instantaneously after the injection and rapidly decreased within a minute for the lower amounts of capsaicin (0.001-0.1 μg), but slowly over time for the higher doses (1-10 μg). In the few instances when itch was experienced, it was reported as transient, typically lasting less than a minute. Four subjects reported a transient itch following the injection of the vehicle. After injection of 0.001, 0.01, 0.1, 1 and 10 μg, 2, 3, 6, 2 and 1 subjects, respectively, reported such an itch. The linear regression lines plotted for amount injected and the mean log of peak magnitude (p = 0.7), duration (p = 0.2) and AUC (p = 0.7) of itch had slopes that did not significantly differ from zero (Table 1).
Figure 4.
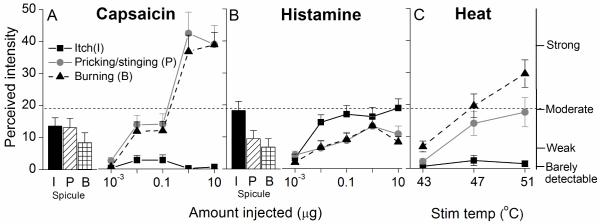
Time course of the perceived intensity of itch and nociceptive sensations evoked by different concentrations of injected capsaicin or histamine. Mean ratings of the perceived intensity of itch, pricking/stinging and burning obtained from 15 subjects are plotted for successive intervals of 30 s after injection. The inset in panel A indicates the amount of chemical injected corresponding to each curve in the figure. The SEM is plotted every 5 min starting with the peak rating for each quality. On the right vertical axis, the locations of 4 of the verbal descriptors are shown in correspondence with the numerical ratings of perceived intensity indicated on the left vertical axis.
Table 1.
Summary of the effects of the amount of injected capsaicin and histamine on sensation and skin reactions. The data are mean log value and S.E.M. for each measure of sensory quality, type of dysesthesia, flare and wheal for the highest amount (10 μg) of capsaicin and histamine injected. The results of a linear regression of the mean log measure of sensory effect (or skin reaction) onto different amounts of chemical injected (μg) are presented as the slope and standard error, S.E. of the regression line
| Peak Mag |
Itch Duration |
AUC | Peak Mag |
Pricking/ Duration |
stinging AUC |
Peak Mag |
Burning Duration |
AUC | ALD/ ALK |
Areas of HKN |
dysesthesia HLG |
(log cm2) Flare |
Wheal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsaicin | ||||||||||||||
| HighestDose | ||||||||||||||
| Mean | x | x | x | 1.5 | 1.1 | 2.3 | 1.6 | 1.1 | 2.3 | 1.3 | 1.4 | 1.5 | 1.5 | x |
| SEM | 0.07 | 0.09 | 0.08 | 0.05 | 0.05 | 0.06 | 0.07 | 0.06 | 0.09 | 0.04 | ||||
| Regression line slope1 | ||||||||||||||
| slope | 0.026 | −0.087 | 0.003 | 0.2* | 0.32* | 0.26* | 0.24* | 0.34* | 0.27* | 0.23* | 0.29* | 0.33* | 0.4* | x |
| SE | 0.091 | 0.06 | 0.009 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.07 | 0.04 | 0.04 | 0.04 | |
| Histamine | ||||||||||||||
| Highest Dose | ||||||||||||||
| Mean | 1.2 | 0.9 | 1.9 | 1.03 | 0.6 | 1.6 | 1 | 0.6 | 1.6 | 1.2 | 1.4 | 1.3 | 1.7 | 0.48 |
| SEM | 0.08 | 0.06 | 0.07 | 0.09 | 0.1 | 0.07 | 0.06 | 0.13 | 0.05 | 0.08 | 0.03 | 0.07 | 0.06 | 0.04 |
| Regression line slope1 | ||||||||||||||
| slope | 0.091* | 0.15* | 0.087* | 0.081* | 0.081* | 0.043* | 0.088* | 0.072 | 0.038 | 0.11* | 0.155 | 0.174 | 0.295 | 0.24 |
| SE | 0.026 | 0.028 | 0.02 | 0.027 | 0.037 | 0.021 | 0.029 | 0.04 | 0.02 | 0.027 | 0.023 | 0.03 | 0.023 | 0.011 |
an asterisk if the slope was significantly different from zero at p < 0.05. Mag (peak rating of sensory magnitude), AUC (area under the rating curve), areas of ALD/ALK (allodynia or alloknesis), HKN (hyperknesis), and HLG (hyperalgesia). No means were obtained for itch in response to 10 μg capsaicin. Capsaicin produced ALD but no ALK and no wheal; histamine produced AKN but no ALD. “x” indicates no response.
The residuals of these regressions did not reveal any remarkable deviations from the regression model.
For each nociceptive sensory quality and response measure (e.g. peak magnitude), the mean log response was plotted against the amount of capsaicin injected. Linear regression analyses demonstrated a significant concentration-dependent increase in the peak magnitude (p< 0.0001), total duration (p< 0.0001) and AUC (p<0.0001) of pricking/stinging as indicated by the slopes of the regression line that were significantly different from zero (Table 1). Eleven subjects reported experiencing pricking/stinging with the vehicle injection. Only seven subjects reported a transient pricking/stinging with the lowest amount of capsaicin (0.001 μg). Fourteen subjects reported experiencing this sensation at each of the other capsaicin concentrations (0.01-10 μg). Thus, the number of subjects experiencing pricking/stinging increased with the amount of injected capsaicin.
Similar to pricking/stinging, the mean of log peak magnitude (p<0.0001), log total duration (p< 0.0001) and log AUC (p<0.0001) of burning exhibited concentration dependence in the linear regression analyses (Table 1). The vehicle alone produced a burning sensation in six of the total fifteen subjects tested. Five subjects reported burning with the lowest capsaicin concentration of 0.001 μg and all subjects reported experiencing burning at concentrations ≥ 1 μg. Thus, as with pricking/stinging, the incidence, intensity and duration of burning were dependent on dose.
3.6 Itch and nociceptive sensations evoked by injections of histamine
Each dose of histamine evoked itch accompanied by lesser sensations of pricking/stinging and burning in all subjects tested. The mean peak magnitude of each quality of sensation evoked by injection increased rapidly to a peak within the first minute followed by a slow decrease over time (Fig. 4D-F). Whereas the greatest mean peak magnitudes of sensation evoked by injection of capsaicin were rated as “strong” those elicited by histamine approached the category of “moderate.” Six subjects reported itch with the vehicle alone and nine subjects, with 0.001 μg of histamine. But this dose and the vehicle did not produce significantly different peak magnitudes of itch (p > 0.05, Student’s t-test). All subjects reported itch with concentrations greater than 0.01 μg. In nine subjects the vehicle alone evoked pricking/stinging but with a mean peak magnitude less than that produced by ≥ 0.1 μg of histamine. Each dose of histamine elicited pricking/stinging in most (11 to13) subjects.
For each sensory quality and response parameter, the mean of the log sensory response was plotted against the amount of histamine injected. Linear regression analyses revealed a significant concentration-dependent increase in itch, pricking/stinging and burning (Table 1). The slopes were significantly different from zero for the mean log peak magnitude (itch, p <0.0001, pricking/stinging, p =0.009), mean log total duration (itch, p <0.0001, pricking/stinging, p <0.001) and mean log AUC (itch, p < 0.0001, pricking/stinging, p = 0.003). The number of subjects reporting burning increased overall from 6 at the lowest dose of histamine to 14 and 13 at the two highest. The mean log peak magnitude (p< 0.0001), mean log duration (p< 0.01) and mean log AUC (p=0.0026) of burning also demonstrated a significant dependence on the amount of histamine injected (Table 1).
3.7 Cutaneous dysesthesias evoked by injections of capsaicin and histamine
The intradermal injection of capsaicin resulted in hyperknesis, hyperalgesia, and allodynia but never alloknesis. The incidence of occurrence of each of these dysesthesias was dependent on the amount of capsaicin injected (p < 0.001, logistic regression analyses). The percentage of total subjects reporting allodynia with 0.1 μg of capsaicin was 13%, with 1 μg was 87% and with 10 μg was 100%. The percentage of total subjects reporting hyperknesis with 0.01 μg of capsaicin was 27%, with 0.1 μg, 7%, with 1 μg, 67%, and with 10 μg, was 80%. All subjects experienced hyperalgesia after injections of 1 and 10 μg. The percentage of total subjects reporting hyperalgesia increased from 13% for 0.001 μg to 53% with both 0.01 and 0.1 μg.
For those subjects reporting a given dysesthesia, regression analyses were used to determine the significance of the relation between the area of dysesthesia and the amount of capsaicin injected. For each dysesthesia, the slope of the regression line for the mean log area vs. the amount injected of capsaicin injected had a slope significantly different from zero (p < 0.01) (Table 1). That is, the areas of allodynia, hyperknesis, and hyperalgesia each significantly increased with the concentration of capsaicin. The mean areas of hyperalgesia but not hyperknesis reported with 10 μg of capsaicin were significantly greater than the areas of these dysesthesias reported with multiple capsaicin spicules (40.2 ± 4.9 cm2 vs. 15.2 ± 3.7 cm2for hyperalgesia, p=0.004, 22.8 ± 4.5 cm2 vs. 28.4 ± 4.1 cm2 for hyperknesis, p=0.4, Students’ t-test).
Additionally, a significant correlation was observed between the peak magnitude of pricking/stinging or burning and the areas of allodynia, hyperknesis and hyperalgesia.
The intradermal injection of histamine resulted in hyperknesis, hyperalgesia, and alloknesis but never allodynia. The incidence of occurrence of each of these dysesthesias was dependent on the amount of histamine injected (p < 0.02, logistic regression analyses). For example, the incidence of alloknesis increased from 20% with 0.001 μg to 73% with 1 μg of histamine. The percentage of subjects reporting hyperknesis (and hyperalgesia) increased from 53% (and 60%) with 0.001 μg of histamine to 93% (and 87%) with 10 μg.
For subjects exhibiting a given dysesthesia, the mean of log area of dysesthesia was obtained in response to different amounts of histamine (Table 1). For alloknesis, hyperknesis, and hyperalgesia, the slope of the regression line (mean log area vs. amount injected) was significantly different from zero (p < 0.0001). The mean areas of alloknesis, hyperknesis and hyperalgesia reported with 10 μg of histamine were not significantly different than the areas of dysesthesias reported with multiple histamine spicules (19.3 ± 3.2 cm2 vs. 15 ± 2.3 cm2 for alloknesis, p=0.3; 25.4 ± 2.1 cm2 vs. 26.7 ± 4.8 cm2 for hyperknesis, p=0.3, and 24.4 ± 4.2 cm2 vs. 10 ± 2 cm2 for hyperalgesia, p=0.24, Students’ t-test).
Additionally, peak magnitudes of itch, pricking/stinging and burning correlated with the areas of alloknesis, hyperknesis and hyperalgesia.
3.8 Skin reactions evoked by injections of capsaicin and histamine
Although injections of histamine and capsaicin often evoked a flare, only histamine elicited a wheal. For both chemicals, the incidence of the flare significantly increased with the amount injected (p < 0.001, logistic regression analyses). The incidence of a flare with each chemical increased from 20% with 0.001 μg to 100% with ≥ 0.1 μg. For histamine, the incidence of a wheal increased from 47% with 0.01 μg to 100% at the three highest doses (0.1-10 μg).
For both capsaicin and histamine, there was a significant relation between the area of the flare and the amount of chemical injected (Table 1). For histamine, there was a significant increase in the area of the wheal with the amount injected.
Using correlation analyses, a significant relation was found between the area of the flare evoked by different amounts of injected capsaicin and both the peak magnitudes of pricking/stinging and burning. For histamine, there was no significant correlation between the area of wheal and the peak magnitude of itch. There was, however, a weak but significant relation between the peak magnitudes of itch and the area of flare.
3.9 Itch and nociceptive sensations evoked by heat stimuli; comparisons with chemically evoked sensations
In a separate experiment, using the same scale used to rate chemically evoked sensations, subjects rated the qualities and magnitude of sensations evoked by a nociceptive heat stimulus. The heat stimuli elicited pricking/stinging and burning but little or no itch (Fig. 5C). The number of subjects reporting itch in response to thermal stimuli of 43°, 47° and 51 °C, were 3, 4 and 3 respectively. There was no significant difference in the mean peak itch evoked by each thermal stimulus (p = 0.5, RMANOVA). Pricking/stinging and burning were the predominant sensations elicited by each thermal stimulus. A significant increase in the mean peak pricking/stinging and burning was observed with increasing temperature of the thermal stimulus (RMANOVA, p < 0.001).
Figure 5.
Comparison of the perceived intensity of itch and nociceptive sensations produced by histamine, capsaicin, and heat. For applications of capsaicin (A) and histamine (B), the mean peak magnitude of itch, pricking/stinging, and burning (I, P, and B, respectively) are plotted separately for the responses to a single spicule (shown as histograms) and the responses to differing amounts injected. C: Mean perceived intensity of each sensory quality evoked by heat stimulus of 5 s duration at different temperatures. The dashed horizontal line indicates that the mean perceived intensity of itch did not exceed a rating of moderate whereas nociceptive sensations approached or exceeded ratings of “strong” in response to the higher amounts of capsaicin or temperature of heat.
The ratings of sensations evoked by heat were compared with those elicited by chemical stimuli. The mean peak magnitude of pricking/stinging and burning evoked by 47 °C approximately equaled “moderate” on the scale (Fig. 5C). This corresponded to the same mean peak rating of itch elicited by injections of 0.1 to 10 μg of histamine (Fig. 5B) and approximately the mean peak magnitude of pricking/stinging evoked by injections of 0.01 and 0.1 μg of capsaicin (Fig. 5A). Higher mean peak ratings of burning were obtained in response to the stimulus of 51 °C (between “moderate” and “strong”) and capsaicin injections of 1 and 10 μg (“strong” or slightly greater) (Fig. 5C and A, respectively). Thus, the more intense algesic heat and capsaicin stimuli evoked greater magnitudes of nociceptive sensations than the most intense pruritic stimuli.
4. Discussion
We have for the first time compared the itch and nociceptive sensations evoked by capsaicin and histamine applied by spicule(s) and injected at different doses and heat stimuli of different temperatures. It is therefore possible to estimate equivalencies in the perceived intensity for different intensities of the three types of stimuli. This has not been possible in previous studies of a single type of stimulus and/or sensory modality.
The pain sometimes reported as accompanying an injection of histamine might be interpreted as acting to decrease itch [1]. However, previous studies were typically not designed to study the perceived intensity of specific qualities of nociceptive sensations (pricking/stinging and burning) that accompany the itch induced by an injection of histamine. These qualities may not necessarily be “painful”, i.e. “hurt,” but nevertheless are nociceptive in quality. In the present study, we found that the intensity and duration of itch induced by injections and spicule applications of histamine were comparable. There was no inverse relationship between the magnitude of these nociceptive sensations (or the presence and area of hyperalgesia) and the magnitude of itch as previously demonstrated for spicules alone [13; 26]. Thus, the nociceptive sensations evoked by the injection of histamine or the application of spicules apparently do not act to reduce itch.
Our intradermal injections of chemicals delivered up to 30,000 times the amount in a single spicule. For histamine, the magnitudes and qualities of sensations and dysesthesias were not substantially different for the two methods of application, though the areas of dysesthesia, wheal and flare increased with the amount of chemical injected; but the sensory effects of capsaicin differed according to how it was applied. When administered by one or multiple spicules, capsaicin evoked itch accompanied by lesser nociceptive sensations, and sometimes alloknesis. But when injected, capsaicin evoked nociceptive sensations without itch and produced allodynia but not alloknesis. Surprisingly, hyperknesis was evoked by injections of capsaicin and grew in area, as did the other dysesthesias, with the amount injected. Hyperknesis was not tested, or was overlooked, in previous studies of sensory effects produced by the injection of capsaicin [4; 12].
The peripheral sensory neurons activated by pruritic chemicals are nociceptive afferents with C- or A-fibers that can be classified as mechanosensitive (MSA) or mechano-insensitive (MIAs). In humans, iontophoresis of histamine [22] or an intradermal injection of prostaglandin E2 [17], activated C-fiber MIAs (C-MIAs) but not C- MSAs and the responses corresponded to simultaneously recorded sensations of itch. Histamine, delivered by spicule, failed to activate the few C-MSA tested in human [18] but has yet to be tested by spicule or intradermal injection in MIAs. It is possible that MIAs responsive to iontophoresis of histamine (some of which also respond to injection of capsaicin) [23] would also be activated by, and contribute to the sensory effects of, histamine delivered by intradermal injection or by spicule or to capsaicin delivered by spicule. To our knowledge, MIAs and MSAs have not been tested with capsaicin spicules in humans.
In the monkey, subpopulations of C-MSAs and some A-fiber MSAs (A-MSAs) responded to injections of histamine with a time course roughly matching the time course of itch and nociceptive sensations we have presently obtained [9; 20]. In addition, some C-MSAs tested were also activated by spicules containing either histamine or capsaicin both in the monkey [18] and the mouse [15]. Additionally, in monkeys, C-MSAs, classified by their responses to rapidly applied heat as quickly adapting or slowly adapting fibers (QCs and SCs, respectively), exhibited different responses to native cowhage spicules [9] and to spicules containing histamine or capsaicin [18]. The QCs had lower heat thresholds and responded with more discharges than SCs to each type of spicule. Thus, the QC-MSAs may play an important role in mediating the itch and nociceptive sensations evoked by cowhage and capsaicin or histamine spicules. It is also possible, though not tested, that one or more types of MIAs in animals may be activated by and contribute to the sensory effects of, capsaicin applied by spicule or histamine applied by spicule or intradermal injection..
The amount, the area, and the depth of delivery may determine the quality of the major sensation produced by capsaicin. In comparison with a single spicule, multiple spicules increased the spatial spread and amount of capsaicin applied yet evoked the same itch and nociceptive sensations. A lack of spatial summation of itch and nociceptive sensations within a local region of skin has been previously shown for cowhage [13]. But nociceptive sensations replaced itch when capsaicin was injected, possibly due to activation of “nociceptive specific” nerve endings terminating deeper within the skin.
The strong pricking/stinging and burning sensations mediated by injections of capsaicin may not be served by MSAs as these are weakly activated or immediately desensitized [3; 9; 11]. Rather, capsaicin evoked nociceptive sensations are likely to be encoded by a subpopulation of MIAs, some responsive to heat [3; 11; 19; 23; 24]
An intradermal injection of capsaicin in CKO mice lacking the vesicular glutamate transporter type 2 (VGLUT2) mainly in Nav1.8 expressing nociceptors produced enhanced hind paw scratching of the injected cheek, signifying itch [14], whereas in wild type mice it elicited pain behavior exhibited by forelimb wiping of the cheek [25]. Thus, capsaicin may activate pruriceptive nociceptors mediating itch, hyperknesis, alloknesis and hyperalgesia as well as nociceptors specifically mediating pain, allodynia and hyperalgesia. In addition, we hypothesize that activity in the latter type of “nociceptive specific” fibers may act centrally to mask the itch and alloknesis but not the hyperknesis resulting from activity in the pruriceptive fibers. Thus, histamine- and capsaicin-evoked hyperknesis can co-exist with hyperalgesia. In contrast, we never observed any co-mingling of areas of alloknesis and allodynia. Brull and colleagues [4] previously found that an injection of histamine evoked no itch if applied within an area of allodynia resulting from a prior injection of capsaicin. Thus, the implication was that a state of allodynia blocked both the sensation of itch and alloknesis.
The neurogenic flare is thought to result from the release of a vasodilatory neuropeptide from the terminals of certain C-MIAs [21]. Itch can be evoked by electrical stimulation and by cowhage without eliciting a flare [8; 10; 13]. As in previous studies [5; 26] we presently found only a weak relationship between the area of flare and the magnitude of sensory effects (sensations and dysesthesias). A punctate application by spicule of the smallest amount of histamine evoked the smallest flare but the greatest sensory effects. Larger amounts of histamine applied by injection increased the area of flare but produced no greater sensory effects than a spicule. Thus, the flare and pruritic sensory effects are governed by different processes. Whether these processes are mediated by different mechanisms within the same type of fiber such as the histamine responsive MIA or a combination of roles played by different fibers including MSAs and MIAs remains to be determined. There was no summation of itch, nociceptive sensations or areas of dysesthesias, except for the capsaicin evoked area of hyperknesis, as the number of spicules was increased within a ~5 mm region of skin. Yet the increase in chemical delivered by multiple as opposed to a single spicule (roughly estimated as 0.009 vs. 0.0003 μg for histamine and 0.15 vs. 0.006 μg for capsaicin – see Sect. 2.2) was sufficient to produce significantly greater areas of wheal for histamine and greater areas of flare for both chemicals. Perhaps the peripheral neural events mediating the hyperknesis and the flare evoked by capsaicin are in some way linked together but separate from those mediating the other effects of these low amounts of chemical.
Within the fixed volume of injected solution, the increase in the amount of histamine delivered resulted in a dose-dependent increase in sensory effects possibly due to the increased activation of the same and new sensory neurons. Though the amount of chemical contained in a single spicule was small, and more spatially restricted than the volume of injectate, the concentration was high and capable of eliciting a suprathreshold sensory effect. Thus, an unanswered question is why an increase in the number of spicules did not necessarily increase the magnitude of sensory effects. One possibility is that the initial discharges of the nerve fiber(s) most strongly activated by histamine act centrally to inhibit central transmission of activity from adjacent fibers, thereby preventing spatial summation [13]. There may also be a local, peripheral effect that acts to suppress the summation of activity from simultaneously activated collaterals of the same parent axon.
We conclude that histamine produces itch and nociceptive sensations whether applied by spicule or injection. Capsaicin produces similar sensory effects when applied by spicule. But we hypothesize that when capsaicin is injected, it activates additional nerve fibers whose activity acts centrally to mask itch, replace alloknesis with allodynia while simultaneously eliciting hyperknesis and hyperalgesia. The central neural mechanisms mediating these dysesthesias and the inhibitory mechanisms hypothesized as blocking spatial summation and acting to reduce itch, while allowing hyperknesis, will require extensive investigation.
Acknowledgements
The authors thank Aaron Altman and Gregory Wirak for technical assistance and Daniel Zelterman for his help with statistical analyses. This study was supported by the NINDS grant P01 NS 047399. The authors report no conflict of interest between the funding source and aims of their experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, Lamotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- [2].Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- [3].Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- [4].Brull SJ, Atanassoff PG, Silverman DG, Zhang J, Lamotte RH. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res. 1999;16:299–303. doi: 10.1080/08990229970366. [DOI] [PubMed] [Google Scholar]
- [5].Darsow U, Ring J, Scharein E, Bromm B. Correlations between histamine-induced wheal, flare and itch. Arch Dermatol Res. 1996;288:436–441. doi: 10.1007/BF02505231. [DOI] [PubMed] [Google Scholar]
- [6].Green BG. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept Psychophys. 1990;48:12–18. doi: 10.3758/bf03205007. [DOI] [PubMed] [Google Scholar]
- [7].Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- [8].Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [9].Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- [13].LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–1443. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma C, Shimada SG, Lamotte RH. Abstract viewer/itinerary planner. Society for Neuroscience; San Diego: 2010. In-vivo responses of cutaneous C-mechanosensitive neurons, and behavior, evoked in the mouse by punctate chemical stimuli that elicit itch and nociceptive sensations in humans. Program No. 584.4. [Google Scholar]
- [16].Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neisius U, Olsson R, Rukwied R, Lischetzki G, Schmelz M. Prostaglandin E2 induces vasodilation and pruritus, but no protein extravasation in atopic dermatitis and controls. J Am. Acad. Dermatol. 2002;47:28–32. doi: 10.1067/mjd.2002.120462. [DOI] [PubMed] [Google Scholar]
- [18].Ringkamp M, Borzan J, Schaefer K, Hartke TV, Meyer RA. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2010. Activation of polymodal nociceptors in monkey by punctate chemical stimulation with histamine and capsaicin. Activation of polymodal nociceptors in monkey by punctate chemical stimulation with histamine and capsaicin, Abstract. Program No. 548.6. [Google Scholar]
- [19].Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci. 2001;21:4460–4468. doi: 10.1523/JNEUROSCI.21-12-04460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schepers RJ, Johanek LM, Hartke TV, Shim B, Borzan J, Meyer RA, Ringkamp M. Abstract viewer/itinerary planner. Society for Neuroscience; Washington DC: 2008. A subpopulation of A nociceptors in monkey is vigorously activated by cowhage spicules. Program No. 170.3. [Google Scholar]
- [21].Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2000;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- [22].Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- [24].Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- [28].Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- [29].Simone DA, Ngeow JY, Whitehouse J, Becerra-Cabal L, Putterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens Res. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- [30].Wahlgren CF, Ekblom A. Two-point discrimination of itch in patients with atopic dermatitis and healthy subjects. Acta Derm Venereol. 1996;76:48–51. doi: 10.2340/00015555764851. [DOI] [PubMed] [Google Scholar]