Abstract
Several lines of research have now suggested the controversial hypothesis that the central noradrenergic system acts to exacerbate depression as opposed to having an antidepressant function. If correct, lesions of this system should increase resistance to depression, which has been partially but weakly supported by previous studies. The present study reexamined this question using two more recent methods to lesion noradrenergic neurons in mice: intraventricular (ivt) administration of either the noradrenergic neurotoxin, DSP4, or of a dopamine-β-hydroxylase-saporin immunotoxin (DBH-SAP ITX) prepared for mice. Both agents given 2 weeks prior were found to significantly increase resistance to depressive behavior in several tests including acute and repeated forced swim, tail suspension and endotoxin-induced anhedonia. Both agents also increased locomotor activity in the open field. Cell counts of brainstem monoaminergic neurons, however, showed that both methods produced only partial lesions of the locus coeruleus and also affected the dorsal raphe or ventral tegmental area. Both the cell damage and the antidepressant and hyperactive effects of ivt DSP4 were prevented by a prior i.p. injection of the NE uptake blocker, reboxetine. The results are seen to be consistent with recent pharmacological experiments showing that noradrenergic and serotonergic systems function to inhibit active behavior. Comparison with previous studies utilizing more complete and selective LC lesions suggest that mouse strain, lesion size or involvement of multiple neuronal systems are critical variables in the behavioral and affective effects of monoaminergic lesions and that antidepressant effects and hyperactivity may be more likely to occur if lesions are partial and/or involve multiple monoaminergic systems
Keywords: noradrenergic system, lesion, DSP4, DBH-SAP immunotoxin, depression, motor activity
1. INTRODUCTION
Brainstem noradrenergic neurons are thought to be intimately involved in the control of affective processes. Numerous drugs that affect these neurons produce changes in mood and depressive behavior whereas stressors that produce depression are associated with increases in the firing rate of neurons of the locus coeruleus (LC), the main noradrenergic nucleus of the brain (Nemeroff, 2002; West et al., 2009; Goddard et al., 2010; Itoi and Sugimoto, 2010). Furthermore, post mortem assays of the LCs of depressed patients or animal models have revealed a number of biochemical and morphological changes consistent with chronic increases in functional activity (Bissette et al., 2003; Karolewicz et al., 2008; Kitayama et al., 2008). These data have challenged the classical hypothesis, based on earlier pharmacological studies, which posited that depression results from reductions of central noradrenergic activity (Wong et al., 2000; Gold and Chrousos, 2002).
A partial resolution of this question in the direction of a causative effect of increased noradrenergic activity in depression was advanced by Weiss and colleagues. These authors showed that temporary local inactivation of LC neurons in rats by the α2-adrenoceptor agonist, clonidine, produced marked and immediate anti-immobility effects in the forced swim test whereas activation of LC cells with an α2-antagonist produced the reverse effect (Simson et al., 1986; Weiss et al., 1986). They subsequently showed that the firing rates of LC neurons were significantly higher in the stressed depressed animals than in controls (Simson and Weiss, 1988) and that virtually every clinically effective antidepressant drug reduced these firing rates in a time dependent manner consistent with their antidepressant actions (Szabo and Blier, 2001; West et al., 2009).
The above findings of Weiss et al, have been confirmed in mice in a series of studies by our group. We have shown that 4th ventricular infusion of 6-fluoronorepinephrine (6FNE), a full agonist for inhibitory α1- and α2-adrenergic receptors at the LC and one which acutely inhibits virtually every neuron in the mouse LC (Stone et al., 2009), produced marked antidepressant effects in a variety of antidepressant screens and disinhibition of a wide range of motivated behaviors in these animals (Stone et al., 2003a; 2004; 2009; 2011a) The antidepressant screens included acute and repeated forced swim stress, tail suspension and endotoxin-induced anhedonia, while the motivated behaviors included wheel running, cage escape and an operant appetitive response. Conversely, blockade of these LC receptors with either central or peripheral administration of α-adrenoceptor antagonists caused intense activation of virtually every LC neuron, exacerbated the above depressive-behaviors and produced marked behavioral inactivity in these same tests (Stone et al., 2009).
If central noradrenergic neurons are involved in mediating behavioral depression rather than antidepressant effects, then it would be expected that lesions of these neurons would produce chronic reductions in depressive behavior. Several groups have, in fact, reported weak antidepressant effects in the forced swim test in animals previously administered the noradrenergic neurotoxin, DSP4, systemically (Plaznik et al., 1988; Semba and Takahashi, 1988; Harro et al., 1999) though others have failed to find this effect (Esposito et al., 1987). In an effort to determine whether more definitive results could be obtained using alternate lesioning techniques, we have experimented with two more recent methods, one involving intraventricular (ivt) rather than ip administration of DSP4 and the second, ivt administration of a dopamine-β-hydroxylase-saporin immunotoxin (DBH-SAP ITX). For DSP4, ivt administration was chosen over the ip.route for two reasons: First, we attempted to induce a selective central lesion because there is evidence that central and peripheral noradrenergic systems have differing behavioral functions with peripheral α1-receptors mediating behavioral inhibitory effects (Yang et al., 1990) while brainstem α1-receptors mediate the above disinhibitory ones (Stone et al., 2002; 2003b). Secondly it was expected that central administration of this neurotoxin would have a greater ability to destroy LC neurons as opposed to only affecting axons and might therefore produce more dramatic behavioral results (Jonsson et al., 1981). Since ivt DSP4 represented a novel lesioning method, we attempted to replicate its effect with an independent method, ivt DBH-SAP ITX. As the commercially available preparation of this ITX proved to be toxic to our mice, we synthesized and tested a new ITX designed specifically for this species (see Methods).
2. METHODS AND MATERIALS
2.1. Subjects
All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and were approved by the New York University Langone School of Medicine IUCAC. Swiss Webster male mice (Taconic), 8–10 weeks old, were subjects. The animals were housed singly with nesting material for 5 d prior to experiments in standard size polycarbonate mouse cages (12.5 × 17 x 28 cm) at a room temperature of 22 ± 1o C under a 12 hr light/dark cycle (lights on 0500 hr). Food and water were available ad libitum.
2.2. DSP4 lesions
Mice were pretreated with the adrenergic receptor blocking agent, labetalol, 10 mg, kg, i.p., to block potential sympathetic hyperactivity to the lesion. After 1 h they were anesthetized with pentobarbital (70 mg/kg) and stereotaxically infused with either saline (sham lesion) or 75 µg of DSP4 in 350 nl of saline in the 4th cerebral ventricle (−5.9 mm to Bregma, 1 mm lateral, 3.8 mm ventral to skull surface, approached at a 13.7° angle to the sagittal suture). A serotonin reuptake inhibitor was not employed as pilot experiments with fluoxetine, 5 mg/kg, i.p. given 1 h prior to infusion, showed interference with the production of noradrenergic lesions. The decision was made, therefore, to permit the toxin to lesion both noradrenergic and serotonergic neurons. Some of the mice, however, were pretreated for 1 h with the NE uptake inhibitor, reboxetine, 10 mg/kg, i.p. in an attempt to selectively block the uptake of the neurotoxin into noradrenergic neurons. All animals were given 2 weeks to recover prior to the start of all behavioral tests which lasted 2 further weeks.
2.3. DBH-SAP ITX lesions
The ITX was synthesized as per a modification of the procedure of Wiley and colleagues (Wrenn et al., 1996) in which the crosslinker N-succinimidyl 3 (2-pyridyldithio)-propionate (SPDP) was used to link each molecule of a polyclonal rabbit anti-dopamine-β-hydroxylase antibody (Chemicon AB1585) to two molecules of saporin after chemical transformation of the latter's amino to sulfhydryl groups with Traut's reagent (Picklo et al., 1994; Traut et al., 1973). Pentobarbital anesthetized animals were stereotaxically infused 1 µl of the preparation containing 0.8 µg of protein in the lateral cerebral ventricle. Control animals were infused with the same quantity of a non-targeted saporin control molecule (goat IgG-SAP, Advanced Targeting Systems). A recovery period of 3–5 weeks prior to testing was used to allow retrograde transport of the ITX to the LC and neuronal degeneration.
2.4. Tests for depressive-like behaviors
These tests included the tail suspension, repeated forced swim and endotoxin-induced anhedonia models. The tail suspension and repeated forced swim tests were conducted in the same groups of lesioned and non-lesioned animals in a randomized and balanced order with an interval of at least 4 d of rest interposed between the two. One week after the completion of these, all of these animals were subjected to an open field test to assess locomotor activity. The endotoxin-anhedonia test was conducted on an independent group of lesioned and non-lesioned animals.
2.5. Tail suspension test
The procedure of Steru et al. (1985) was used. Control and lesioned animals were individually taped by the tail 72 cm above a padded platform for 6 min during which time they were videorecorded. Recordings were rated blind for immobility times.
2.6. Repeated forced swim (RFS) procedure
This modification of the Porsolt forced swim test was used in the place of the more standard chronic mild stress procedure because it produces a similar responsiveness to chronic, but not acute, antidepressant treatment in a much briefer time period (5 d as opposed to 4 weeks) and is also significantly milder than the latter procedure (Stone and Lin, In press). Control and lesioned animals were swum individually for 15 min/day in rat tub cages (24 × 43 × 23 cm, w x h x l) filled with 13 cm high tepid water (32–34°C) on 4 consecutive days and given a fifth test swim 1–3 d later. The animals were videotaped from above on 5th swim (test) and recordings were rated blind for duration of immobility and distance swum (quadrants of tank entered).
It has been argued that the immobility in forced swim experiments like the above is not an authentic depression analogue because the animals have learned to conserve their energy by becoming immobile and are simply exhibiting an adaptive acquired response (Nishimura et al., 1988). Arguing against this, however, are the findings that the performance of passive responses is likely to be aversive and associated with negative mood and physiological signs of stress (Matsuda et al., 1996; Mercado et al., 2005). The RFS procedure in mice has also been shown to produce activation of the paraventricular nucleus of the hypothalamus and one that shows minimal habituation with repeated swims (Stone et al., 2007). Furthermore, the immobility occurring in both mice in the RFS and in rats in extinction experiments with the Morris water maze is reversed by chronic antidepressant treatment (Schulz et al., 2007; Stone et al., 2007).
2.7. Sweetened milk intake after endotoxin (DSP4 only)
Intake of a sweetened milk solution made from 1 part condensed milk to three parts tap water was used to assess hedonic behavior both at baseline and 24 h after endotoxin treatment in a 2 × 2 DSP4 × Endotoxin experimental design. Nondeprived sham and DSP4 lesioned mice were first tested for baseline intakes in three 30 min tests over a period of one week in their home cages and then matched on intake levels within each group and randomly assigned to vehicle and endotoxin subgroups. The subgroups received, respectively, i.p. injections of lipopolysaccharide (E. coli 0127:B8), 400 ug/kg, or distilled water, and were tested for 30 min milk intake 24 hr later (Frenois et al., 2007). It has been shown that the initial sickness behavior caused by this dose of endotoxin abates by 24 hr leaving a persisting affective disturbance in the form of both anhedonia and increased immobility to tail suspension (Frenois et al., 2007).
2.8. Motor activity
Control and lesioned animals were placed individually in the center of the field (46 × 46 × 33 cm clear Plexiglas) and permitted to explore undisturbed for 1 h. Videorecordings were rated for quadrants of the field entered per 15 min intervals.
2.9. Immunohistochemistry
After completion of the behavioral experiments (4–5 weeks post lesion), all animals were terminally anesthetized with a combination of isoflurane and urethane (2.2 g/kg, i.p.) and perfused intracardially with saline (25 ml) followed by 4% paraformaldehyde (100 ml) for subsequent immunohistochemistry and histological examination of the injection cannula tract with respect to the 4th ventricle. Sucrose-infiltrated brains (30% for 48 h) were sectioned at 35 µ in a cryostat and immunostained with a chicken anti-TH antibody (Novus Biologicals, 1:5000) for tyrosine hydroxylase (LC, A1/C1, A2, VTA) or with a rabbit anti- 5HT antibody (Immunostar 1:5000) for 5-HT (DR). The entire LC (Bregma, −5.20 to − 5.80 mm), A1/C1 (−6.36 to −7.0), A2 (−8.24 to −8.30) and DR (−4.04 to −5.20) whereas every fifth section of the VTA between −3.28 to −3.88 mm were processed. Primary antibodies were detected with fluorescent secondary antibodies (goat-anti-chicken Alexa 594 or goat anti-rabbit Alexa 488, both at 1:500, Invitrogen). To avoid biased counting, the full 2 dimensional areas of each nucleus/region in all of its sections were counted for labeled cells showing over 3X background fluorescence either manually (LC) or automatically (all other nuclei) by ImageJ at magnifications of 40X or 100X (Stone et al., 2009). This counting method is more rapid than standard stereological procedures but is equally effective at eliminating biased sampling (Stone et al., 2007). Correlations between cell counts obtained at magnifications of either 40 or 100X with those at 400X were above r = 0.9.
2.10. HPLC determination of brain monoamine and metabolite concentrations
HPLC measures were made only in the DSP4 study to ascertain if the ivt neurotoxin produced axotomy of the dorsal noradrenergic bundle like the systemic neurotoxin in addition to its possible effect on LC cell number. Animals were anesthetized with isoflurane and euthanized by decapitation for harvesting of brains. The frontal cortex (extending to 2 mm caudal to the frontal pole) and the hypothalamus (extending between Bregma −3.5 to −1mm, lateral ± 1 mm, and dorsally to the roof of the 3rd ventricle) were dissected over ice and immediately frozen on Dry Ice for subsequent HPLC determinations of NE, 5-HT, DA, 4-methoxy-3-hydroxyphenylglycol (MHPG), 5-hydroxyindoeacetic acid (5HIAA) and homovanillic acid (HVA) by previously described methods (Dunn, 1988)
2.11. Statistics
Most of the DSP4 experiments involved only 3 groups, a vehicle, DSP4 and reboxetine+DSP4 group. As preliminary experiments indicated that the reboxetine preinjection, by itself, did not affect any of the behavioral and immunohistochemical measures obtained 2–4 weeks later, this group was omitted from the design. In each of these 3 group experiments, the behavioral and biochemical data were analyzed statistically by making two planned Bonferroni-corrected comparisons (t-tests) of the vehicle versus the DSP4 group and the vehicle versus the reboxetine+DSP4 group. The endotoxin-anhedonia experiment was analyzed with a 2 × 2 factorial ANOVA while open field DSP4 experiment was with by a 3 × 4 Treatment X Interval repeated measures ANOVA. Both ANOVAs were followed by 2 corrected planned comparisons. In the experiments using DBH-SAP ITX, which involved only two groups, all of the comparisons were made by independent t-test, excepting the open field study which was analyzed with a repeated measures ANOVA.
3. RESULTS
3.1. Histology
The effects of ivt DSP4 and DBH-SAP ITX on the number of cells of the mouse LC, DR, VTA, A1/C1, A2 and A7 are shown in Table 1. Photomicrographs of the LC, DR and VTA of representative animals are shown in Fig. 1. Monoamine and metabolite levels in the frontal cortex and hypothalamus obtained in separate groups of DSP4 lesioned and sham lesioned mice are shown in Table 2.
Table 1.
| LC | DR | VTA | A1C1 | A2 | A7 | |
|---|---|---|---|---|---|---|
| Sham lesion (12) | 997.2±113.1 | 1514.4±176.3 | 667.8±158.7 | 69.3±22.9 | 78.1±12.8 | 27.1±6.9 |
| DSP4 (13) | 693.4±89.4* | 915.6±147.5* | 446.9±76 | 72.2±17.2 | 58.7±9.2 | 14.1±2.0 |
| Rebox + DSP4 (6) | 1039.1±193.4 | 1536.7±147.5 | 420.9±31.1 | 89.7±22.6 | 60.2±13.5 | 45.8±11.6 |
| Control conjugate (8) | 692.4±21.4 | 1147.6±235.6 | 598.2±3.0 | 103.8±4.8 | 91.1±10.2 | 25.1±8.3 |
| DBH-SAP ITX (6) | 304.5±48.0**** | 957.3±240.7 | 422.0±32.7** | 74.2±19.2 | 62.2±21.2 | 25.6±4.5 |
Mean cell counts and SEMs for monoaminergic nuclei in control and lesioned mice. N’s are shown in parentheses in groups. N’s fof A7 in sham and DSP4 groups were 6 and 10.
p < 0.05
< 0.01
< 0.001 versus respective control group.
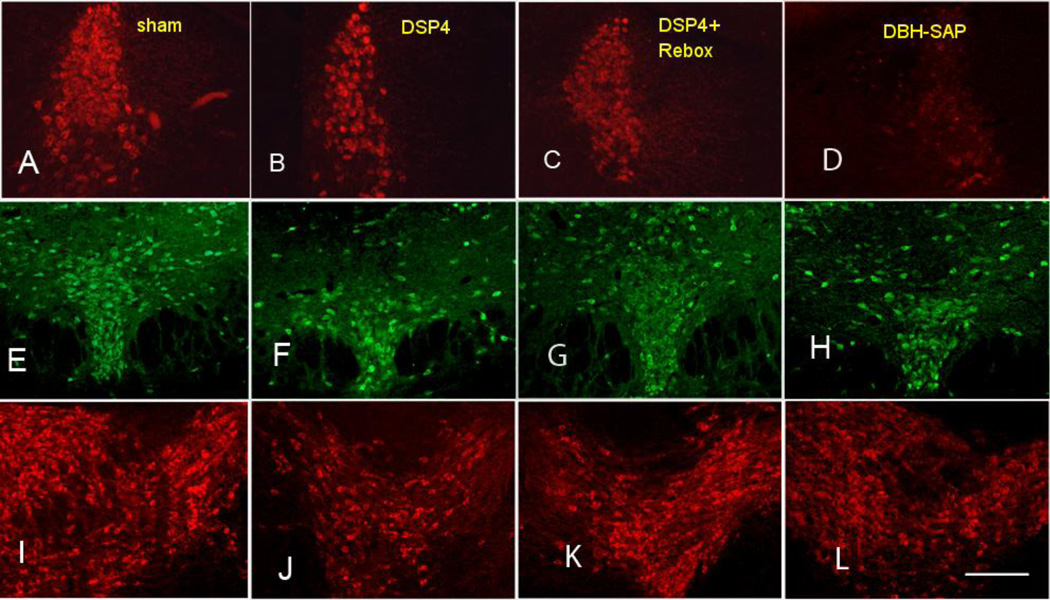
Figure 1.
Photomicrographs of the LC (A–D), DR (E–H) and VTA (I–L) in representative mice from the sham (A,E,I), DSP4 (B,F,J), Reboxetine + DSP4 (C,G,K) and DBH-SAP (D,H,L) treated experimental groups. All photographed at 40X. Bar is 500 µm.
Table 2.
| CORTEX | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NE | DA | 5-HT | MHPG | DOPAC | HVA | 5-HIAA | MHPG/NE | DOPAC/DA | HVA/DA | 5-HIAA/5-HT | |
| IVT VEH (N=6) | 0.254 ±0.011 |
1.045 ±0.113 |
0.985 ±0.063 |
0.558 ±0.031 |
0.170 ±0.006 |
0.264 ±0.019 |
0.710 ±0 951 |
2.032 ±0.112 |
0.162 ±0.028 |
0.218 ±0.016 |
0.679 ±0.071 |
| IVT DSP4 (N=11) | 0.217 ±0.024 |
1.C52 ±0.176 |
0.840 ±0.071 |
0.467 ±0.098 |
0.145 ±0.019 |
0.236 ±0.031 |
0.637 ±0.102 |
1.768 ±0.314 |
0.175 ±0.0429 |
0246 ±0.050 |
0.772 ±0.181 |
| HYPOTHALAMUS | |||||||||||
| IVT VEH | 0.645 ±0.086 |
0.346 ±0.117 |
0.887 ±0.229 |
0894 ±0.116 |
0.094 ±0.030 |
0.153 ±0.036 |
1.369 ±0.140 |
1.474 ±0.220 |
0.311 ±0.082 |
0.535 ±0.111 |
1.139 ±0.065 |
| IVT DSP4 | 0.693 ±0.075 |
0.387 ±0.082 |
1.205 ±0.089 |
0875 ±0.130 |
0.086 ±0.011 |
0.155 ±0.026 |
1.173 ±0.193 |
1.229 ±0.101 |
0.249 ±0.036 |
0.491 ±0.074 |
0.987 ±0.114 |
Contents of major monoamines and metabolites in frontal cortex and hypothalamus of mice previously injected ivt with saline or 75 ug dsp4. Concentrations are ng per mg of tissue.
With respect to effects on cell numbers, DSP4 produced significant reductions in the LC (−30.4 ± 12.9%, t23 = 2.31, p < 0.02), DR (−39.6 ± 9.7%, t23 = 2.62, p < 0.05) and a borderline reduction in A7 (−47.9 ± 7.5%, t14 = 2.23, p < 0.1). All of these effects were prevented by pre-injection of reboxetine. DBH-SAP ITX caused a significant reductions in the LC (−56.1 ± 6.9%, t12 = 7.06, p < 0.001) and VTA (−29.4 ± 5.5%, t12 = 2.64, p = 0.05) but failed to significantly affect the DR (−16.6 ± 25.1%). The ITX may have also damaged the A2 (−31.7 ± 23.2%) and A1 (−28.5 ± 18.5%) nuclei although these reductions also failed to reach significance. With respect to monoamine and metabolite levels, DSP4 failed to significantly alter any of the levels in either the frontal cortex or hypothalamus although small nonsignificant decreases were found for both NE (−14.6 ± 9.5%) and 5HT (−14.7 ± 7.3%) and for the ratio of MHPG/NE (−13.0 ± 17.8%) in the cortex. The effects of DBH-SAP on monoamine levels were not examined.
3.2. DSP4 behavioral effects
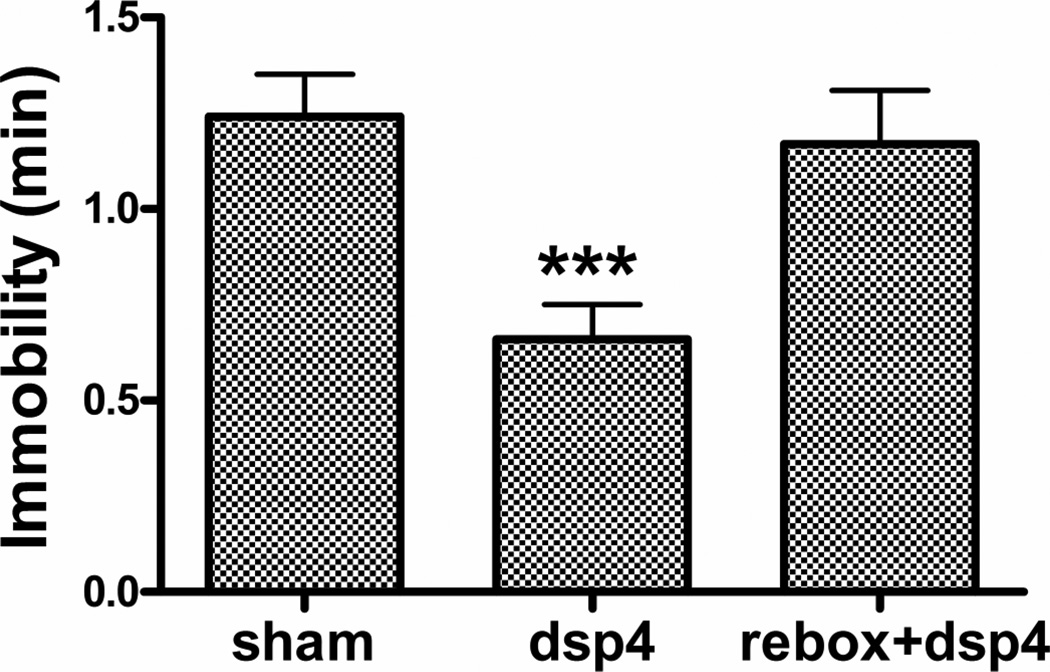
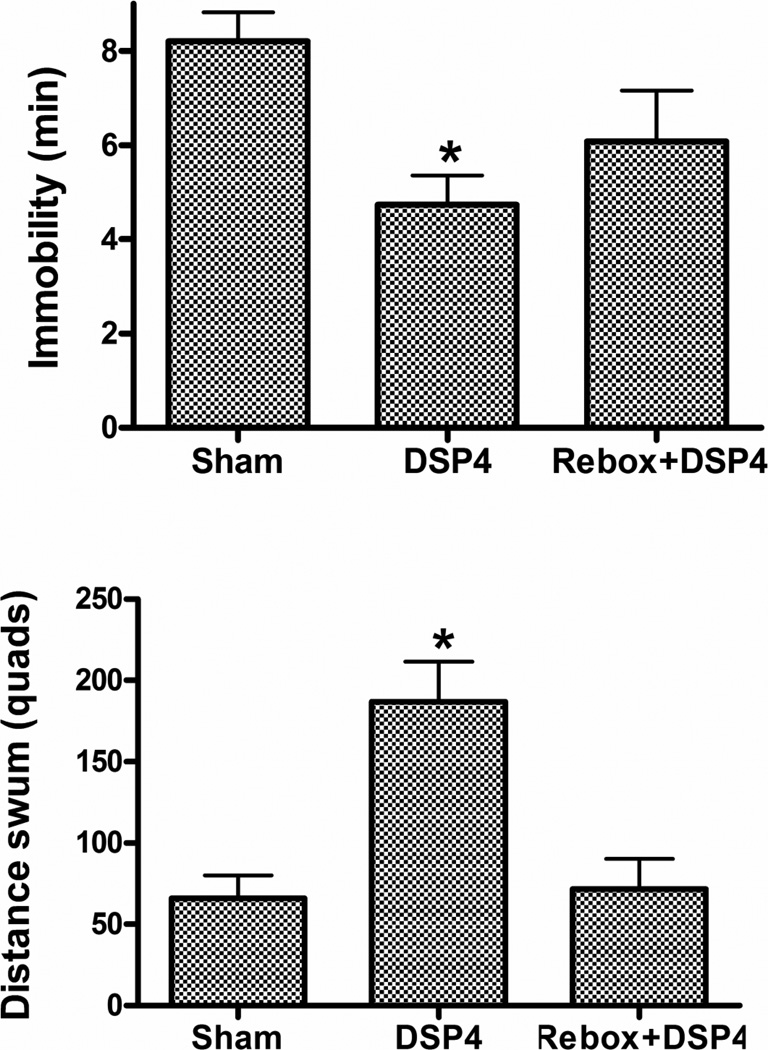
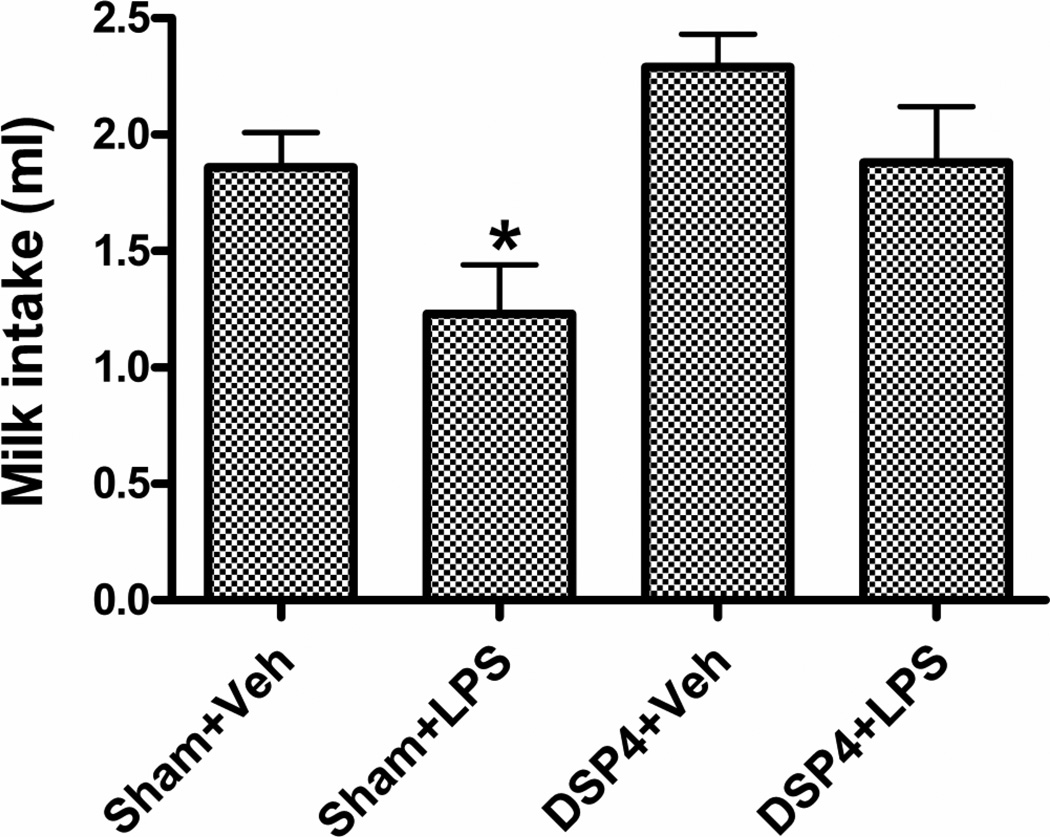
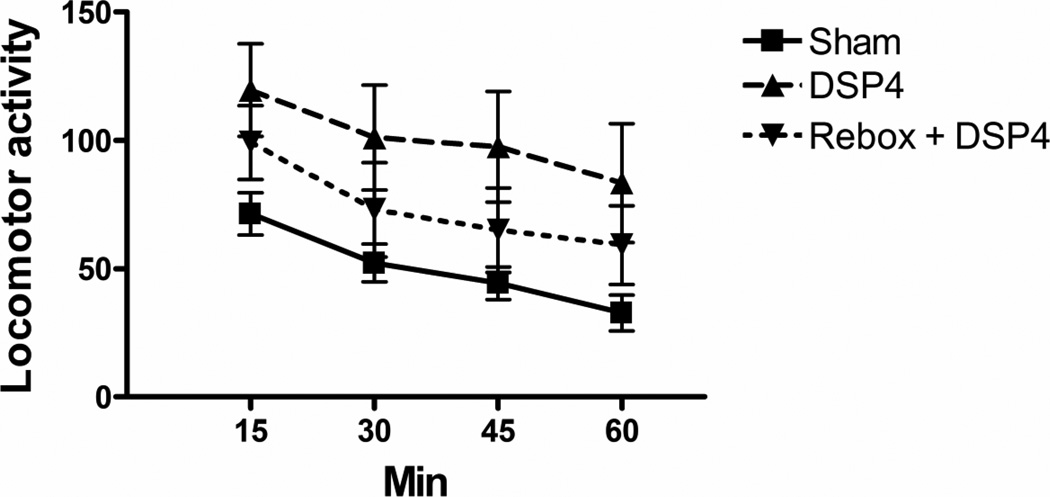
TST: A significant decrease in immobility was found in the DSP4 (t23 = 2.49, p < 0.05) but not the reboxetine + DSP4 group (t16 = 1.44, NS) (Fig. 2). Repeated forced swim (RFS): DSP4 produced a significant decrease in immobility (t23 = 4.42, p < 0.0002) accompanied by a significant increase in distance swum (t23 = 6.95, p < 0.0001) (Fig. 3). Both effects were prevented by reboxetine pretreatment (immobility, t16 = 0.75, NS; distance, t16 = 0.26, NS). Sweetened milk intake after endotoxin (LPS) pretreatment: Thirty-min milk intakes at 24 h post vehicle or LPS in the sham- or DSP4-treated animals are shown in Fig 4. Because of the complexity of this experiment, reboxetine pre-injections were omitted. A 2 × 2 (DSP4 x LPS) factorial ANOVA revealed a significant overall increase of intake in the DSP4 treated groups (F1,56 = 8.11, p < 0.01) and a statistically significant overall decrease after LPS treatment (F1,56 = 7.73, p < 0.01) with no significant LPS x DSP4 interaction (F1,56 = 0.34, NS), Planned comparisons, however, revealed that LPS reduced intake in the sham group (F1,56 = 5.76, p < 0.02) to a greater extent than in the DSP4 group (F1,56 = 2.36, NS). Motor activity (open field): The effect of DSP4 with and without reboxetine preinjection on open field locomotor activity is shown in Fig 5. A one-way ANOVA for repeated measures on the Interval variable showed a significant main effect of Treatment (F2,28 = 6.01, p < 0.01) with no interaction between Treatment and Interval. Planned comparisons revealed significantly greater activity, compared to the sham group, in the DSP4 (F1,28 = 11.42, p < 0.005) but not in the reboxetine + DSP4 treated animals (F1,28 = 0.47, NS).
Figure 2.
Effect of ivt. DSP4 infusion with and without reboxetine pre-injection on immobility in tail suspension test conducted 2–4 weeks post lesion. N = 12 (Veh), 13 (DSP4), 6 (Reboxetine + DSP4). * p < 0.05 versus Vehicle (Bonferroni-corrected t-test).
Figure 3.
Effect of ivt. DSP4 infusion with and without reboxetine preinjection on performance in the 5th swim of mice given 4 prior daily forced swims (RFS) at 2–4 weeks post lesion. N’s as in Fig. 2. * p < 0.0002 versus Vehicle group.
Figure 4.
Effect of prior ivt. DSP4 infusion on intakes of sweetened milk in mice treated 24 h earlier with endotoxin (LPS, 400 µg/kg, i.p.). N = 17–18. * p < 0.02.
Figure 5.
Effect prior ivt. DSP4 infusion with and without reboxetine preinjection on 1 h locomotor activity in an open field. N’s as in Fig. 2. The DSP4 group differed from the Vehicle at p < 0.005 by ANOVA contrast.
3.3. DBH-SAP ITX behavioral effects
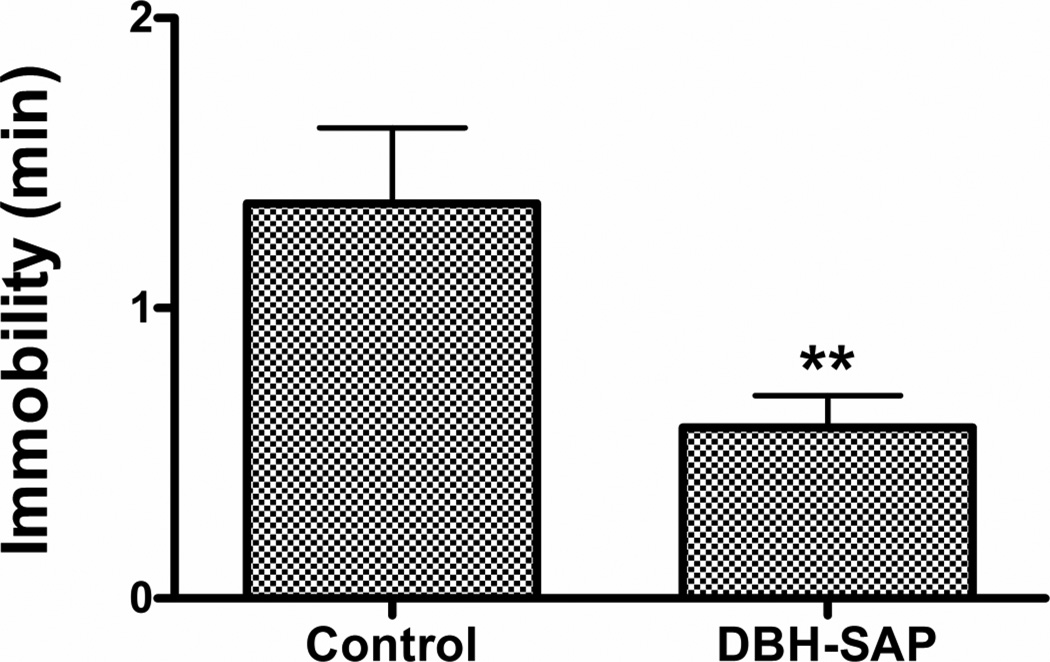
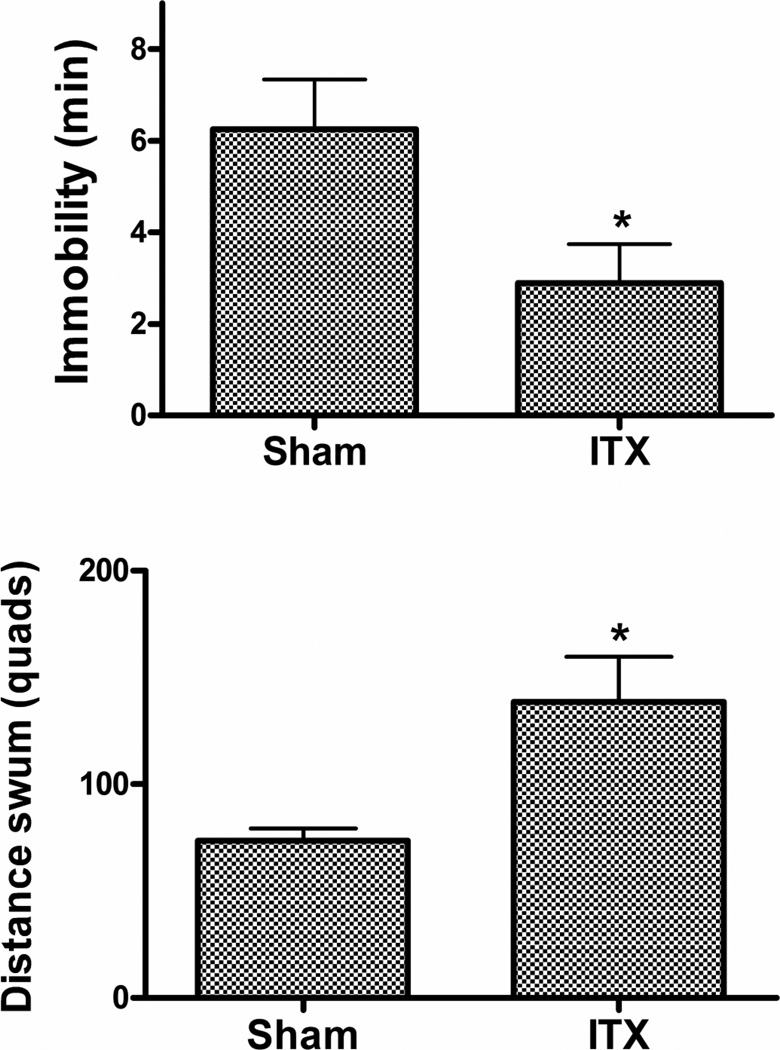
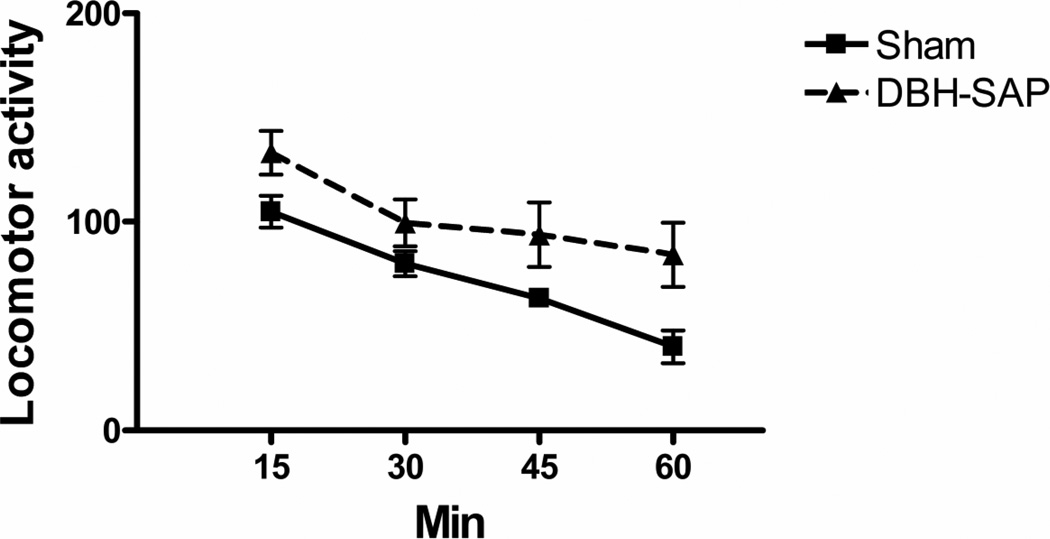
TST: Treatment with the ITX produced a significant decrease in immobility (t12 = 3.70, p = 0.005) (Fig 6). RFS: A significant decrease in immobility (t12 = 2.37, p < 0.05) and a significant increase in distance swum (t12 = 2.47, p < 0.05) were found in the ITX-lesioned animals (Fig. 7). Motor activity (open field): ITX lesioned mice showed a significant overall increase in locomotor activity (F1,12 = 6.89, p < 0.05) (Fig. 8).
Figure 6.
Effect of DBH-SAP ITX on immobility in tail suspension test. N = 6–8. p < 0.005 versus Control conjugate.
Figure 7.
Effect of DBH-SAP ITX on immobility (upper panel) and distance swum (lower panel) in subsequent repeated forced swim test. N as in Fig 7. * p < 0.05 versus Control conjugate.
Figure 8.
Effect of DBH-SAP ITX on 1 h locomotor activity in open field test. N’s as in Fig 7. The DBH-SAP group differed from the Control at p < 0.05 by ANOVA simple contrast.
4. Discussion
The present results show that ivt DSP4 and DBH-SAP ITX can cause marked and persistent changes in baseline and stress-induced depressive-like behaviors and locomotor activity in mice. Infusion of 75 µg of DSP4 into the 4th ventricle produced, at 2–4 weeks, significant reductions of immobility in the tail suspension (−35%) and RFS tests (−45.2%), a significant increase of distance swum in the latter by 203.1% and an increase in resistance to anhedonia caused by endotoxin administration. The neurotoxin also significantly increased open field locomotor activity by 148% which may partially explain its anti-immobility but not anti-anhedonic effect. The DBH-SAP ITX, which was employed to replicate the effects of ivt DSP4 with an independent lesioning technique, also produced chronic reductions of immobility in the tail suspension (−56%) and RFS tests (−53.5%) along with a significant increase of swimming distance in the latter (88.3%). The ITX also increased locomotor activity in the open field, although to a lesser degree than DSP4 (42.5%). Although there is controversy as to whether the tail suspension test is a model of stress-induced depression (Renard et al., 2003; Stone et al., 2011a), the repeated forced swim and endotoxin-anhedonia paradigms do represent such models (Stone et al., 2007) and the fact that all responded similarly to both lesioning methods supports the notion that the results apply to the stress-induced disorder. Since the depression tests in this study were conducted after placement of the lesions, it is not known at present whether the lesions only prevent the development of the behaviors or can also reverse them once established.
The present findings are therefore in agreement with the recent biochemical and pharmacological experiments discussed above showing evidence of heightened LC functional activity in depressives and an inverse relationship of LC activity with antidepressive effects. They also appear to be consistent with the results of a recent optogenetic study of mouse LC function in which strong phasic stimulation of the LC was found to produce an immediate arrest of behavioral activity although depressive behavior was not measured (Carter et al., 2010). In addition they also parallel a number of recent findings on the role of related A2 noradrenergic neurons in sickness behavior, a syndrome evolutionarily related to depression (Frenois et al., 2007; DellaGioia and Hannestad, 2010). Although A2 and LC noradrenergic neurons innervate different, though partially overlapping, structures, and do not have identical physiological or behavioral roles (Itoi and Sugimoto, 2010), the two cell groups appear to have a similar function in regard to the inhibition of behavioral activity during stress although probably in response to different precipitating conditions and effected through different efferent brain structures. Thus, both the A2 and LC neurons are strongly activated by stressors with A2 responding more to physiological perturbations and LC to behavioral and psychological ones (Dayas et al., 2001; Buller et al., 2001). Secondly, both are strongly activated by both endotoxin administration (Hare et al., 1995) and brainstem infusion of α1-adrenoceptor antagonists (Stone et al. submitted), two conditions that produce almost total behavioral inactivity (Stone et al., 1999; 2009; Gaykema and Goehler, 2009) as well as increased depressive behaviors (Yirmiya, 1996; Stone and Quartermain, 1999; DellaGioia and Hannestad, 2010). Third, local treatments that inhibit the activity of each cell group such as local anesthesia of the A2 and local infusion of agonists of inhibitory α1- and α2-receptors at the LC cause the disinhibition of active motivated behaviors and antidepressant effects after either endotoxin in the case of A2 (Marvel et al., 2004; Gaykema and Goehler, 2011) or stress, fear or novelty in the case of the LC (Simson et al., 1986; Weiss et al., 1986; Stone et al., 2009; 2011a). And fourthly, the effect of acute pharmacological inhibition of A2 cells on endotoxin-induced behavioral inactivity is in agreement with the effects of chronic ITX lesions of this nucleus (Gaykema and Goehler, 2011) while the effects of acute pharmacological inhibition of LC neurons with α1-agonists on depressive behaviors during stressful stimuli agree with the present effects of LC lesions (Stone et al., 2011a).
In contrast to the above concordance, the results of the present study are diametrically opposite to a recent study of mouse depressive behavior after a different ITX lesion of the LC (Itoi et al., 2011). The latter authors utilized local LC infusion of an ITX targeting IL-2Rα, under the control of the DBH promoter, to lesion the mouse LC and found that virtually complete elimination of the nucleus caused a marked increase in forced swim immobility and a reduction of locomotion in the open field, effects opposite to those of the present study. This group also found that swim immobility was not affected by systemic DSP4 administration but that the latter did significantly reduce motor activity in the open field.
Two significant differences between these studies that may have contributed to the different results are in the strains of mice used and the characteristics of the lesions. With respect to mice, the Itoi et al study utilized an inbred strain which is known to have one of the highest levels of immobility (untreated) in the forced swim test (C57BL/6N) while the present study used an outbred strain that is known to have one of the lowest (Swiss Webster) (López-Rubalcava and Lucki, 2000; Ripoll et al., 2003). It is possible, therefore, that the LC lesions simply exaggerated differential behavioral tendencies that were already present.
Alternately, they may have resulted from differences in the lesion characteristics since the ITX method used by Itoi et al achieved complete and apparently specific LC destruction whereas the two methods used in the present study produced only partial damage and involved several monoamine cell groups. Thus ivt DSP4 was found to reduce the LC cell count by only about 30% while the DBH-SAP ITX produced an approximate 60% loss. The 30% cell loss with DSP4 was not sufficient to alter resting levels of either NE or MHPG in the frontal cortex or hypothalamus although this reduction might not be a valid index of the actual level of impairment since resting levels of MHPG may not reflect the function of these neurons during stress or during the performance of behaviors in the depression tests. Also, lesions of this small size have generally not been found sufficient to trigger postsynaptic compensatory reactions, such as receptor sensitization, which may alter or obscure net behavioral changes following more complete lesions (Zigmond and Stricker, 1980; Haidkind et al., 2003). In support, there is some evidence that lesion size of the dorsal noradrenergic system in rats is an important determinant of behavioral effect since a low dose of DSP4 (10 mg/kg), which produces a 60% depletion of forebrain NE, has been found to increase forced swim immobility while a higher dose (30 mg/kg), causing 80–90% depletion, leads to the reverse effect (Harro et al., 1999). This pattern, however, is opposite to what was observed in the present study, i.e, reduced immobility after incomplete lesion. With respect to motor activity, however, both a partial depletion of NE in the rat ventral striatum (30%) produced by 6OHDA and partial lesions of the LC, DR and VTA in rats after prolonged darkness have been reported to produce persistent increases in motor activity (Schwarting and Carey, 1988; Gonzalez and Aston-Jones, 2008).
With respect to lesion specificity, ivt DSP4 in the present study was found to produce a significant loss of neurons in the DR as well as in the LC whereas the DBH-SAP ITX partially lesioned the VTA and produced a small but nonsignificant cell loss in the DR as well. An effect of DSP4 on brain 5HT levels has been reported previously (Jonsson et al., 1981) while the action of the ITX on the VTA could be related to the known trophic action of the LC on dopaminergic neurons (Mavridis et al., 1991; Marien et al., 1993; Gesi et al., 2000; Cassano et al., 2009). Previous studies have revealed a high vulnerability of brainstem monoaminergic neurons to injury from various physiological insults (Kohlhauser et al., 1999) and the present findings could be a reflection of this sensitivity to some aspect of the ivt infusion. It was not possible to determine the separate contributions of LC and DR lesions in the present experiment with either fluoxetine or reboxetine pretreatment as both drugs were found to have nonspecific protective effects on the DSP4 lesions of these structures.
The combined effects of ivt DSP4 on the DR and LC could represent a significant factor in causing its marked antidepressant and hyperactive effects. The serotonergic raphe system has long been known to be involved in the inhibition of behavioral activity (Eagle et al., 2009) as well as in the regulation of depressive behavior. Although most current findings favor an antidepressant role for brain 5HT, there is increasing evidence that this system is involved in the production of anxious and depressive behaviors after stress (Maswood et al., 1998; Grahn et al., 2002; Takase et al., 2005) and is activated rather than inhibited in nontreated depressive patients (Barton et al., 2008). Furthermore, antidepressant drugs that inhibit reuptake in both the serotonergic and noradrenergic systems tend to be more efficacious than those acting on each system alone (Herrera-Guzman et al., 2009). It is possible therefore the noradrenergic and serotonergic systems, which represent the two neuronal systems most closely associated with depressive illness and antidepressant drug action, have partially redundant functions on depression and need to be both affected in order to produce significant and lasting effects on the disorder.
In addition to contradicting the antidepressant role of the noradrenergic system, the present results also argue against its proposed role as a stimulant of motor activity and also revisit the controversial relationship between motor activity and depressive behavior. First, however, it should be recognized that while the a motor activity role for NE is supported by a number of reports of behavioral hypoactivity after LC-noradrenergic lesions (Heybach et al., 1978; Owen et al., 1982; Ogren et al., 1983; Britton et al., 1984; Archer and Frediksson, 2003), there are an equal number showing little or no influence (Roberts et al., 1975; Porceddu et al., 1983; Sahakian et al., 1983; Britton et al., 1984; Archer et al., 1986; Sawynok et al., 1995; Neophytou et al., 2001; Srinivasan and Schmidt, 2004) and even some showing increases in baseline or stimulated activity in lesioned animals with high degrees of NE depletion (Ellison, 1975; Mason et al., 1978; 1979; Schwarting and Carey, 1988; Hatip-Al-Khatib and Bolukbasi, 1999; Murrough et al., 2000). These inconsistencies probably reflect inadequate experimental control of the complexities of the noradrenergic systems which possess the same receptors having opposite neural and behavioral functions depending on whether they are located at the LC or in terminal regions, and in which NE release can be affected either independently or concordantly with that of its peptide cotransmitter, galanin, by use of a selective NE-reuptake inhibitor or manipulation of nerve impulse rate, respectively (Stone et al., 2011b).
Building on the work of Weiss et al (2005), our group and others have presented a possible schema in which these factors might operate in both depression and motor activity and which might explain the relationship between the two (Gaykema and Goehler, 2011; Stone et al., 2011b). In this schema, it is assumed that both hyperactivity and antidepressant action result from a reduced neurotransmission in noradrenergic neurons of the LC and the lateral tegmental noradrenergic cell groups, A1 and A2, which leads to a reduced release of NE and galanin in cortical and subcortical regions involved in stress and active motivated behaviors. In stress areas, such as the paraventricular hypothalamus, amygdala and bed nucleus of the stria terminalis, whose neural activity is increased during behavioral inhibition and depression, NE is thought to have excitatory neurophysiological actions in conjunction with corticotrophin releasing hormone systems and to produce strongly aversive emotional responses (Heinrichs and Koob, 2004). In contrast, in areas associated with active motivated behavior such as the ventral tegmental area, nucleus accumbens, secondary motor, orbital and piriform cortex and lateral septal nucleus, whose neural activities are reduced during these conditions, NE and/or galanin are thought to have inhibitory actions on output neurons, causing a suppression of active motivated behaviors. The original noradrenergic hypothesis of motor activity was based on the findings of hypoactivity after drugs that depleted brain NE and of the restoration of active behavior with repletion of the amine. The new schema, however, permits a reinterpretation of these findings consistent with a behavioral inhibitory role for the NE systems if it is assumed that the key site(s) of transmitter depletion is the LC, A1 or A2 where the reduction in NE release deprives local inhibitory α-adrenergic receptors of stimulation and thus produces an intense activation of noradrenergic neurons with a concomitant release in forebrain structures of the non-depleted cotransmitter, galanin, producing an inhibition of behavioral activity (Weiss et al., 2005; Stone et al., 2011b). This view is supported by the finding of intense LC activation after reserpine treatment equivalent to that produced by either endotoxin or local blockade of α1-receptors (Stone et al., 2006) and also makes the prediction, as yet untested, that local infusion of an α-agonist in the either the LC or lateral tegmental nuclei should reverse reserpine-induced hypoactivity.
With respect to depression, NE reuptake inhibitors will have two effects in this model: they will reduce noradrenergic nerve impulse rate by stimulation of noradrenergic autoreceptors and will enhance signaling at postsynaptic adrenergic receptors in projection areas. The evidence suggests that during acute treatment, the enhanced postsynaptic receptor stimulation predominates because hypoactivity in the open field (Niesink and Van Ree, 1982; Hughes and Pither, 1987; Plaznik et al., 1993), with little or no antidepressant action in various behavioral screens is observed initially (Kitada et al., 1981; Lin et al., 2011). These effects most likely occur as a result of the increased stimulation of postsynaptic α2-adrenoceptors because these receptors are thought to be located extrasynaptically where they will be readily exposed to extracellular NE (Glass et al., 2002) and have been found to have inhibitory effects on active behavior (Herman et al., 1976; Spyraki and Fibiger, 1982; Nassif et al., 1983) as well as pro-depressive actions by several investigators (Garcia-Sevilla et al., 1999; Marcus et al., 2010). With repeated treatment, however, the postsynaptic signaling at α2-receptors declines possibly as a result of receptor desensitization (Niesink and Van Ree, 1982; Cuomo et al., 1983; Heal, 1984) while the reduced impulse flow does not and, in fact, becomes stronger, (West et al., 2009) and is temporally correlated with the recovery of behavioral activity as well as with the emergence of antidepressant activity.
So the schema of a behaviorally inhibitory role of noradrenergic systems appears consistent with a wide range of pharmacological findings on motor activity and antidepressant action and supports a close relation between the two. However, there are still several findings that do not agree with it. The first concerns the lack of antidepressant effect of stimulant drugs, such as amphetamine. Although depressed patients have reduced levels of daily activity which are restored by successful antidepressant treatment (Merrick, 1992; Willner, 1997; Demyttenaere et al., 2005; Schrijvers et al., 2009), stimulants, even though they markedly suppress LC neural activity (Akaoka et al., 1991), have generally not been found to be clinically effective antidepressants and produce false positives in animal screening tests since they reverse forced swim immobility (Porsolt et al., 1978). A possible reason for this is that they can also activate other central stress nuclei, such as the paraventricular hypothalamus (Swerdlow et al., 1993; Armario, 2010), which aggravate depression and may offset any positive effect of the behavioral stimulation. This may be why using a stimulant, such as modafinil, together with fluoxetine, an antidepressant that reduces stress (Musazzi et al., 2010), has been reported to increase the efficacy of antidepressant action (Abolfazli et al., 2011) and may warrant further studies of combining anti-stress agents with stimulants to achieve more effective antidepressants.
A second contradiction concerns the role of postsynaptic α2-receptors in depressive and antidepressive processes. Although there have been several reports of the hastening and potentiation of antidepressant effects by systemic α2-antagonists both preclinically and clinically and on both behavior and neural substrates (Sanacora et al., 2004; Yanpallewar et al., 2010), some investigators, including our group, have shown that these drugs can increase depressive behaviors and that postsynaptic α2-receptors may have antidepressant actions (Stone and Quartermain, 1999; Zhang et al., 2009). There is now evidence that different α2-receptor subtypes have different functions in depressive phenomena and differing anatomical localizations which may underlie these contradictory results (Schramm et al., 2001; Gyires et al., 2009).
Finally it should be noted that, in contrast to the delayed effect of antidepressants given systemically, infusions of α-agonists, such as clonidine and 6-fluoronorepinephrine directly into the brainstem, can produce immediate reductions of rodent depressive behaviors (Simson et al., 1986). In confirmation of earlier work (Petty et al., 1982), we have recently found that desmethylimipramine can also produce an immediate antidepressant response in the repeated forced swim model in mice if injected into the 4th ventricle and also appears to have a delayed brain uptake from the periphery when given systemically to stressed animals (Lin et al., 2011). This suggests that part of the delayed action of classical noradrenergic antidepressants may result from an impaired brain uptake and that these drugs, when present in the brainstem in sufficient concentrations, may also be capable of rapidly inhibiting central stress circuits.
5. Conclusion
In summary, the present results indicate that marked and persistent reductions in baseline or stress-induced depressive-like behaviors and in behavioral activation can be produced in mice by lesions of brainstem monoaminergic neurons that are partial and/or involve multiple neuronal systems. The contrast with previous studies involving more complete and specific noradrenergic lesions may help reveal new information on the operation and affective functions of these brain systems.
Highlights.
The role of central noradrenergic neurons in depression was reexamined in mice
Immobility and anhedonia and were chronically reduced but motor activity increased after lesions with central DSP4 or a noradrenergic immunotoxin
Both lesion procedures produced partial noradrenergic lesions and also affected other monoamine systems
The result support a behavioral depressive and inhibitory role of one or more central monoaminergic systems
ACKNOWLEDGMENTS
Supported in part by NIMH/5RO1MH045265 (EAS) and MH50947 (AJD). The authors thank Vivien Low and Constance Moussouris for their technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
STATEMENT OF INTEREST
None
References
- Abolfazli R, Hosseini M, Ghanizadeh A, Ghaleiha A, Tabrizi M, Raznahan M, et al. Double-blind randomized parallel-group clinical trial of efficacy of the combination fluoxetine plus modafinil versus fluoxetine plus placebo in the treatment of major depression. Depress Anxiety. 2011;28:297–302. doi: 10.1002/da.20801. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Roussel B, Lin J-S, Chouvet G, Jouvet M. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett. 1991;123:20–22. doi: 10.1016/0304-3940(91)90148-m. [DOI] [PubMed] [Google Scholar]
- Archer T, Fredriksson A, Jonsson G, Lewander T, Mohammed A, Ross S, et al. Central noradrenaline depletion antagonizes aspects of d-amphetamine-induced hyperactivity in the rat. Psychopharmacology. 1986;88:141–146. doi: 10.1007/BF00652230. [DOI] [PubMed] [Google Scholar]
- Archer T, Frediksson A. An antihypokinesic action α2-adrenoceptors upon MPTP-induced behaviour deficits in mice. J Neural Transm. 2003;110:183–200. doi: 10.1007/s00702-002-0777-5. [DOI] [PubMed] [Google Scholar]
- Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, et al. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Britton DR, Ksir C, Britton KT, Young D, Koob GF. Brain norepinephrine depleting lesions selectively enhance behavioral responsiveness to novelty. Physiol Behav. 1984;33:473–478. doi: 10.1016/0031-9384(84)90171-9. [DOI] [PubMed] [Google Scholar]
- Buller K, Xu Y, Dayas C, Day T. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1beta-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinology. 2001;73:129–138. doi: 10.1159/000054629. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano T, Gaetani S, Morgese MG, Macheda T, Laconca L, Dipasquale P, et al. Monoaminergic changes in locus coeruleus and dorsal raphe nucleus following noradrenaline depletion. Neurochem Res. 2009;34:1417–1426. doi: 10.1007/s11064-009-9928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010;34:130–143. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Lehmann O, Theobald DE, Pena Y, Zakaria R, Ghosh R, et al. Serotonin depletion impairs waiting but not stop-signal reaction time in rats: implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology. 2009;34:1311–1321. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- Ellison GD. Behavior and the balance between norepinephrine and serotonin. Acta Neurobiol Exp. 1975;35:499–515. [PubMed] [Google Scholar]
- Esposito E, Ossowska G, Samanin R. Further evidence that noradrenaline is not involved in the anti-immobility activity of chronic desipramine in the rat. Eur J Pharmacol. 1987;136:429–432. doi: 10.1016/0014-2999(87)90319-0. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, OConnor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Escriba PV, Ozaita A, La Harpe R, Walzer C, Eytan A, et al. Up-regulation of immunolabeled alpha2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J Neurochem. 1999;72:282–291. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain Behav Immun. 2009;23:926–930. doi: 10.1016/j.bbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: Brain substrates for fatigue? Brain Behav Immun. 2011;25:443–460. doi: 10.1016/j.bbi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Revs. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Colago EE, Pickel VM. Alpha-2A-adrenergic receptors are present on neurons in the central nucleus of the amygdala that project to the dorsal vagal complex in the rat. Synapse. 2002;46:258–268. doi: 10.1002/syn.10136. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Gold P, Chrousos G. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiat. 2002;7:254–262. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci USA. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O’Connor KA, Deak T, Sparks PD, et al. Blockade of alpha1 adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behav Brain Res. 2002;134:387–392. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Gyires K, Zadori ZS, Torok T, Matyus P. Alpha-2-adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int. 2009;55:447–453. doi: 10.1016/j.neuint.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Haidkind R, Eller M, Harro M, Kask A, Rinken A, Oreland L, et al. Effects of partial locus coeruleus denervation and chronic mild stress on behaviour and monoamine neurochemistry in the rat. Eur Neuropsychopharmacol. 2003;13:19–28. doi: 10.1016/s0924-977x(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Hare AS, Clarke G, Tolchard S. Bacterial lipopolysacharide-induced changes in fos protein expression in the rat brain: correlation with thermoregulatory changes and plasma corticosterone. J Neuroendocrinol. 1995;7:791–799. doi: 10.1111/j.1365-2826.1995.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Harro J, Pähkla R, Modiri AR, Harro M, Kask A, Oreland L. Dose-dependent effects of noradrenergic denervation by DSP-4 treatment on forced swimming and β-adrenoceptor binding in the rat. J Neural Transm. 1999;106:619–629. doi: 10.1007/s007020050184. [DOI] [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Bolukbasi F. Destruction of the noradrenergic system with DSP4 potentiates the behavioral effects of MK-801 in rats. Pharmacol Biochem Behav. 1999;62:233–237. doi: 10.1016/s0091-3057(98)00169-5. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Herman ZS, Brus R, Drybanski A, Szkilnik R, Slominska-Zurek J. Influence of 6-hydroxydopamine on the behavioral effects induced by apomorphine or clonidine in rats. Psychopharmacology. 1976;50:73–80. doi: 10.1007/BF00634158. [DOI] [PubMed] [Google Scholar]
- Herrera-Guzman I, Gudayol-Ferre E, Herrera-Guzman D, Guardia-Olmos J, Hinojosa-Calvo E, Herrera-Abarca JE. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J Psychiatr Res. 2009;43:855–863. doi: 10.1016/j.jpsychires.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Heybach JP, Coover GD, Lints CE. Behavioral effects of neurotoxic lesions of the ascending monoamine pathways in the rat brain. J Comp Physiol Psychol. 1978;92:58–70. doi: 10.1037/h0077434. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Pither JM. Chronic imipramine effects on exploratory behavior in rats. Pharmacol Biochem Behav. 1987;27:359–362. doi: 10.1016/0091-3057(87)90581-8. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety, and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N, Suzuki S, Sawada K, Das G, Uchida K, et al. Targeting of locus ceruleus noradrenergic neurons expressing human interleukin-2 receptor alpha-subunit in transgenic mice by a recombinant immunotoxin anti-Tac(Fv)-PE38: a study for exploring noradrenergic influence upon anxiety-like and depression-like behaviors. J Neurosci. 2011;31:6132–6139. doi: 10.1523/JNEUROSCI.5188-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Ponzoni A, Ross S. DSP4 (N-(2-dhloroethyl)-N-ethyl-2-bromobenzylamine) - a useful denervation tool for central and peripheral noradrenaline neurons. Eur J Pharmacol. 1981a;72:173–186. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Johnson L, Szebeni K, Stockmeier CA, Ordway GA. Glutamate signaling proteins and tyrosine hydroxylase in the locus coeruleus of alcoholics. J Psychiat Res. 2008;42:348–355. doi: 10.1016/j.jpsychires.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada Y, Miyauchi T, Satoh A, Satoh S. Effects of antidepressants in the rat forced swimming test. Eur J Pharmacol. 1981;72:145–152. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- Kitayama IT, Otani M, Murase S. Degeneration of the locus ceruleus noradrenergic neurons in the stress-induced depression of rats. Ann NY Acad Sci. 2008;1148:95–98. doi: 10.1196/annals.1410.059. [DOI] [PubMed] [Google Scholar]
- Kohlhauser C, Kaehler S, Mosgoeller W, Singewald N, Kouvelas D, Prast H, et al. Histological changes and neurotransmitter levels three months following perinatal asphyxia in the rat. Life Sci. 1999;64:2109–2124. doi: 10.1016/s0024-3205(99)00160-5. [DOI] [PubMed] [Google Scholar]
- Lin Y, Suckow RF, Sarfraz Y, Stone EA. Further evidence for an immediate antidepressant action of intracerebral drug administration in a model of chronic depression. Int J Neuropsychopharmacol. 2011;14:691–696. doi: 10.1017/S1461145710001161. [DOI] [PubMed] [Google Scholar]
- López-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Wiker C, Franberg O, Konradsson-Geuken A, Langlois X, Jardemark K, et al. Adjunctive alpha2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int J Neuropsychopharmacol. 2010;13:891–903. doi: 10.1017/S1461145709990794. [DOI] [PubMed] [Google Scholar]
- Marien M, Briley M, Colpaert F. Noradrenaline depletion exacerbates MPTP-induced striatal dopamine loss in mice. Eur J Pharmacol. 1993;236:487–489. doi: 10.1016/0014-2999(93)90489-5. [DOI] [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Imm. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mason ST, Corcoran ME, Fibiger HC. Noradrenergic processes involved in the locomotor effects of ethanol. Eur J Pharmacol. 1979;54:383–387. doi: 10.1016/0014-2999(79)90068-2. [DOI] [PubMed] [Google Scholar]
- Mason ST, Roberts DC, Fibiger HC. Noradrenergic influences on catalepsy. Psychopharmacology. 1978;60:53–57. doi: 10.1007/BF00429179. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Mavridis M, Degryse A-D, Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of parkinson's disease. Neuroscience. 1991;41:507–523. doi: 10.1016/0306-4522(91)90345-o. [DOI] [PubMed] [Google Scholar]
- Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117:51–57. doi: 10.1016/j.pain.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Merrick W. The Experience of Psychopathology: Investigating Mental Disorders in their Natural Settings. In: Vries Md., editor. Dysphoric moods in depressed and non-depressed adolescents. Cambridge: Cambridge Univ Press; 1992. pp. 148–156. [Google Scholar]
- Murrough J, Boss-Williams K, Emery M, Bonsall R, Weiss J. Depletion of brain norepinephrine does not reduce spontaneous ambulatory activity of rats in the home cage. Brain Res. 2000;883:125–130. doi: 10.1016/s0006-8993(00)02850-x. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif S, Kempf E, Cardo B, Velley L. Neurochemical lesion of the locus coeruleus of the rat does not suppress the sedative effect of clonidine. Eur J Pharmacol. 1983;91:69–76. doi: 10.1016/0014-2999(83)90363-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. Recent advances in the neurobiology of depression. Psychopharmacology Bulletin. 2002;36 Suppl 2:6–23. [PubMed] [Google Scholar]
- Neophytou SI, Aspley S, Butler S, Beckett S, Marsden CA. Effects of lesioning noradrenergic neurones in the locus coeruleus on conditioned and unconditioned aversive behaviour in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1307–1321. doi: 10.1016/s0278-5846(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM. Antidepressant drugs normalize the increased social behaviour of pairs of male rats induced by short term isolation. Neuropharmacology. 1982;21:1343–1348. doi: 10.1016/0028-3908(82)90144-7. 1982;21:1343-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M. Is immobility of rats in the forced swim test "behavioral despair?". Physiol Behav. 1988;42:93–95. doi: 10.1016/0031-9384(88)90266-1. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Archer T, Johansson C. Evidence for a selective brain noradrenergic involvement in the locomotor stimulant effects of amphetamine in the rat. Neurosci Lett. 1983;43:327–331. doi: 10.1016/0304-3940(83)90209-4. [DOI] [PubMed] [Google Scholar]
- Owen S, Boarder MR, Gray JA, Fillenz M. Acquisition and extinction of continuously and partially reinforced running in rats with lesions of the dorsal noradrenergic bundle. Behav Brain Res. 1982;5:11–41. doi: 10.1016/0166-4328(82)90088-2. [DOI] [PubMed] [Google Scholar]
- Petty F, Sacquitne JL, Sherman AD. Tricyclic antidepressant drug action correlates with its tissue levels in anterior neocortex. Neuropharmacology. 1982;21:475–477. doi: 10.1016/0028-3908(82)90034-x. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Palejko W, Stefanski R, Kostowski W. Open field behavior of rats reared in different social conditions: the effects of stress and imipramine. Pol J Pharmacol. 1993;45:243–252. [PubMed] [Google Scholar]
- Plaznik A, Tamborska E, Hauptmann M, Bidzinski A, Kostowski W. Brain neurotransmitter systems mediating behavioral deficits produced by inescapable shock treatment in rats. Brain Res. 1988;447:122–132. doi: 10.1016/0006-8993(88)90972-9. [DOI] [PubMed] [Google Scholar]
- Porceddu MIA, Melis M, DiChiara G. Role of ventral mesencephalic reticular formation and related noradrenergic and serotonergic bundles in turning behavior as investigated by means of kainate, 6-hydroxydopamine and 5,7-dihydroxytryptamine lesions. Brain Res. 1983;262:187–200. doi: 10.1016/0006-8993(83)91008-9. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–381. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Renard CE, Dailly E, David DJ, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17:449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJP, Dailly E, Hascoët M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Zis AP, Fibiger HC. Ascending catecholamine pathways and amphetamine-induced locomotor activity: importance of dopamine and apparent non-involvement of norepinephrine. Brain Res. 1975;93:441–454. doi: 10.1016/0006-8993(75)90182-1. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Winn P, Robbins TW, Deeley RJ, Everitt BJ, Dunn LT, et al. Changes in body weight and food-related behaviour induced by destruction of the ventral or dorsal noradrenergic bundle in the rat. Neuroscience. 1983;10:1405–1420. doi: 10.1016/0306-4522(83)90122-7. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Berman RM, Cappiello A, Oren DA, Kugaya A, Liu N, et al. Addition of the alpha2-antagonist yohimbine to fluoxetine: effects on rate of antidepressant response. Neuropsychopharmacology. 2004;29:1166–1171. doi: 10.1038/sj.npp.1300418. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A, Doak G. Caffeine antinociception in the rat hot-plate and formalin tests and locomotor stimulation: involvement of noradrenergic mechanisms. Pain. 1995;61:203–213. doi: 10.1016/0304-3959(94)00169-F. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The α2A-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers D, Maas YJ, Pier MP, Madani Y, Hulstijn W, Sabbe BG. Psychomotor changes in major depressive disorder during sertraline treatment. Neuropsychobiology. 2009;59:34–42. doi: 10.1159/000205516. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Carey R. Differential behavioural effects after subtotal depletions of dopamine or noradrenaline in the ventral striatum. Funct Neurol. 1988;3:29–36. [PubMed] [Google Scholar]
- Semba J, Takahashi R. Effect of monoamine precursors on the forced-swimming test in mice. Psychopharmacology. 1988;95:222–225. doi: 10.1007/BF00174513. [DOI] [PubMed] [Google Scholar]
- Simson PE, Weiss J. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- Simson PG, Weiss J, Hoffman LJ, Ambrose MJ. Reversal of behavioral depression by infusion of an alpha-2 adrenergic agonist into the locus coeruleus. Neuropharmacology. 1986;25:385–389. doi: 10.1016/0028-3908(86)90232-7. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC. Clonidine-induced sedation in rats: evidence for mediation by postsynaptic alpha 2-adrenoreceptors. J Neural Transm. 1982;54:153–163. doi: 10.1007/BF01254925. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Behavioral and neurochemical effects of noradrenergic depletions with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine in 6-hydroxydopamine-induced rat model of Parkinson's disease. Behav Brain Res. 2004;151:191–199. doi: 10.1016/j.bbr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Stone EA, Cotecchia S, Lin Y, Quartermain D. Role of brain α1B-adrenoceptors in modafinil-induced behavioral activity. Synapse. 2002;46:269–270. doi: 10.1002/syn.10127. [DOI] [PubMed] [Google Scholar]
- Stone EA, Grunewald G, Lin Y, Ahsan R, Rosengarten H, Kramer K, et al. Role of epinephrine stimulation of CNS α1-adrenoceptors in motor activity in mice. Synapse. 2003a;49:67–76. doi: 10.1002/syn.10212. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biol Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of depression. Prog Neuropsychopharmacol Biol Psychiat. 2007;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y. Activation of central stress systems by blockade of brainstem α1-adrenoceptors. Submitted. [Google Scholar]
- Stone EA, Lin Y, Ahsan R, Quartermain D. Role of locus coeruleus α1-adrenoceptors in motor activity in rats. Synapse. 2004;54:164–172. doi: 10.1002/syn.20074. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Quartermain D. Immobility from administration of the α1-adrenergic antagonist, terazosin, in the IVth ventricle in rats. Neurosci Lett. 2003b;353:231–233. doi: 10.1016/j.neulet.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. Marked behavioral activation from inhibitory stimulation of locus coeruleus α1-adrenoceptors by a full agonist. Brain Res. 2009;1291:21–31. doi: 10.1016/j.brainres.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. Antidepressant-like action of intracerebral 6-fluoronorepinephrine, a selective full alpha-adrenoceptor agonist. Int J Neuropsychopharmacol. 2011a;14:319–331. doi: 10.1017/S1461145710000507. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011b;67:193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Alpha 1-noradrenergic neurotransmission, corticosterone and behavioral depression. Biol Psychiatry. 1999;46:1287–1300. doi: 10.1016/s0006-3223(99)00234-6. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D. Brain α1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience. 1999;94:1245–1252. doi: 10.1016/s0306-4522(99)00394-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–637. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry. 2001;50:644. doi: 10.1016/s0006-3223(01)01260-4. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, et al. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Boss-Williams KA, Moore JP, Demetrikopoulos MK, Ritchie JC, West CHK. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:281–287. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG, Hoffman LJ, Ambrose MJ, Cooper S, Webster A. Infusion of adrenergic receptor agonists and antagonists into the locus coeruleus and ventricular system of the brain. Effects on swim-motivated and spontaneous motor activity. Neuropharmacology. 1986;25:367–384. doi: 10.1016/0028-3908(86)90231-5. [DOI] [PubMed] [Google Scholar]
- West CH, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12:627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-M, Gorman AL, Dunn AJ. The involvement of central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal in rats. J Pharmacol exp Ther. 1990;255:1064–1070. [PubMed] [Google Scholar]
- Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, et al. Alpha-2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O'Donnell JM. Postsynaptic alpha-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Stricker EM. Supersensitivity after intraventricular 6-hydroxydopamine: relation to dopamine depletion. Experientia. 1980;36:436–437. doi: 10.1007/BF01975133. [DOI] [PubMed] [Google Scholar]