Summary
Molecular mechanisms that concordantly regulate stress, lifespan and age-related physiological changes remain incompletely understood. Here, we demonstrate that in Drosophila, a p38 MAP Kinase (p38K)/Mef2/MnSOD pathway is a co-regulator of stress and lifespan in vivo. Hence, over-expression of p38K extends lifespan in a MnSOD-dependent manner, while inhibition of p38K causes early lethality and precipitates age-related motor dysfunction and stress sensitivity, that is rescued through muscle-restricted (but not neuronal) add-back of p38K. Additionally, mutations in p38K are associated with increased protein carbonylation and Nrf2-dependent transcription, while adversely affecting metabolic response to hypoxia. Mechanistically, p38K modulates expression of the mitochondrial MnSOD enzyme through the transcription factor Mef2, and predictably, perturbations in MnSOD modify p38K-dependent phenotypes. Thus, our results uncover a muscle-restricted p38K-Mef2-MnSOD signaling module that influences lifespan and stress, distinct from the Insulin/JNK/FOXO pathway. We propose that potentiating p38K might be instrumental in restoring the mitochondrial detoxification machinery and combating stress-induced aging.
Introduction
The p38 MAP Kinase (p38K) is a well-known Stress Activated Ser/Thr Protein Kinase (SAPK) that has been studied in as diverse paradigms as stress (Coulthard et al., 2009; Obata et al., 2000), cellular senescence and cancer (Loesch and Chen, 2008; Maruyama et al., 2009), immune response (Ashwell, 2006; Kurz and Tan, 2004), pain (Ji and Suter, 2007) and inflammation (Schieven, 2009). However, a potential role for p38K in lifespan regulation and its mechanistic basis are not widely established.
Among invertebrate models, C. elegans possesses three p38K genes, pmk-1, pmk-2 and pmk-3 that can phosphorylate the transcription factor Atf-2 (Berman et al., 2001; Sakaguchi et al., 2004) and are themselves activated by two kinases NSY-1 (MAPKKK) and SEK-1 (MAPKK) (Kim et al., 2002). C. elegans p38K participates in the oxidative stress response, regulates the phosphorylation and nuclear entry of the Nrf2 transcription factor SKN-1 and the fork-head transcription factor Daf-16 (Inoue et al., 2005; Kondo et al., 2005), and pmk-1 mutants are compromised in their immune response (Alper et al., 2010; Troemel et al., 2006). Although normal PMK-1 activity is required for lifespan extension seen in daf-2 (insulin signaling pathway) mutants (Troemel et al., 2006), PMK-1 and Daf-2/Daf-16 regulate independent subsets of genes suggesting that these two signaling pathways function independently. More recent studies have also suggested that the germline in C. elegans controls innate immunity and lifespan through non-overlapping signaling pathways involving p38K (Alper et al., 2010).
In Drosophila, the two p38K genes (Adachi-Yamada et al., 1999; Han et al., 1998a; Suzanne et al., 1999; Zhuang et al., 2006) have been studied in relation to stress (Craig et al., 2004; Cully et al.; Inoue et al., 2001; Sano et al., 2005) and the fly immune system (Davis et al., 2008; Ha et al., 2009; Han et al., 1998b; Shinzawa et al., 2009). While p38Ka regulates stress and the DUOX system in the midgut (Craig et al., 2004; Ha et al., 2009), p38Kb is involved in the immune response system in the gut, general infection tolerance (Shinzawa et al., 2009; Chen et al., 2010) and age-dependent stem cell proliferation and differentiation in the Drosophila intestine (Park et al., 2009). Thus, although p38K has been studied widely in the context of stress and immune system function, direct genetic demonstration of a role for the p38 MAP Kinases in lifespan regulation and physiologically relevant age-related phenotypes is currently lacking.
Here, we report that p38K in Drosophila regulates lifespan, sensitivity to oxidative stress and age-dependent alterations in motor performance. We find that these phenotypes require p38K function in muscle tissue and are mediated by p38K-dependent regulation of the mitochondrially localized Manganese Superoxide Dismutase (MnSOD or SOD2) through the transcription factor Mef2. In light of a neuronal Insulin/JNK/FOXO signaling pathway in lifespan regulation (Clancy et al., 2001; Evans et al., 2008; Holzenberger et al., 2003; Hwangbo et al., 2004; Libina et al., 2003; Lin et al., 2001; Murphy et al., 2003; Oh et al., 2005; Tatar et al., 2001; Wang et al., 2003, 2005; Wolkow et al., 2000), we propose that tissue-restricted signaling modules might regulate stress and longevity in metazoans.
Results
Generation of hypomorphic mutations in Drosophila p38b MAP kinase
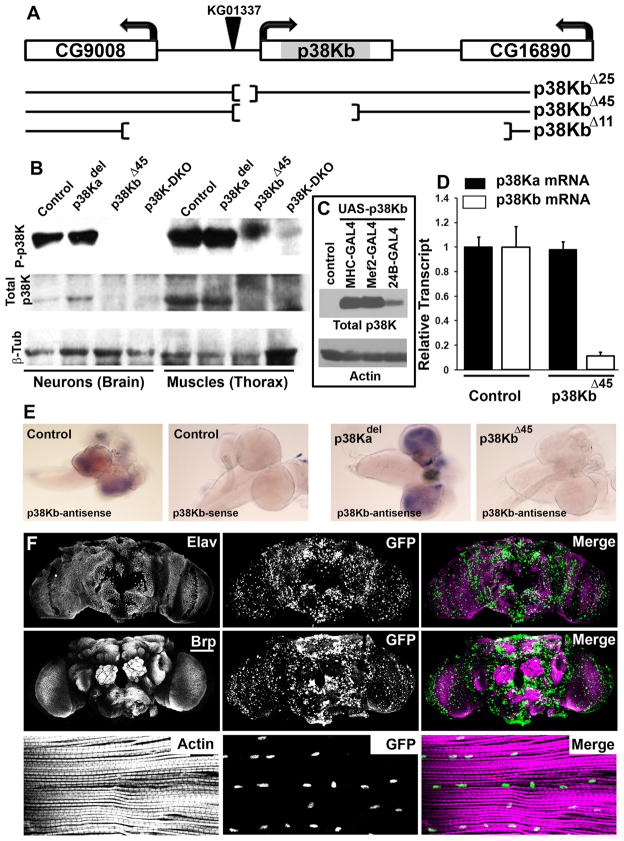
Drosophila has two closely homologous p38 Kinase genes, p38Ka (Mpk2, CG5475) and p38Kb (p38b, CG7393). We generated p38Kb mutations by employing standard transposon excision strategies resulting in three deletion mutants (Figure 1A) in addition to a precise excision, Ex 41. p38KbΔ25 removes 299 base pairs upstream of the transcription start, p38KbΔ45 removes most of the p38Kb coding region (1065 bp deletion) and p38KbΔ11 removes the entire p38Kb gene (3453 bp deletion) and portions of the two flanking genes (CG9008 and CG16890). All three excision alleles were viable when homozygous suggesting redundancy with p38Ka. On testing mRNA expression in p38Ka, p38Kb, and double mutant animals, we found that p38Kb mRNA was absent in p38KbΔ45 animals but detectable in p38Ka mutants, while p38Ka mRNA was undetectable in p38Ka null animals but present in p38KΔ45 animals (Figure S1). Similarly, RNA in situ hybridization showed absence of p38Kb transcript in p38KbΔ45 animals, while p38Kb transcript was present in both wild type controls and p38Ka null brains (Figure 1E). In addition, quantitative real-time PCR showed that levels of p38Ka transcript are not increased in p38KbΔ45 animals (Figure 1D), suggesting that p38Ka upregulation is not a mechanism for compensation.
Figure 1. p38Kb is expressed widely in adult Drosophila.
A) Schematic of the p38Kb genomic region depicting three transposon (KG01337) excision induced deletion mutations. B) Western analysis of head and thorax protein in p38Ka and p38Kb mutants probed with anti-phospho-p38K and anti-total p38K antibodies (β-tubulin is used as a loading control). C) Western blot with anti-total p38K antibodies shows muscle overexpression of p38Kb using MHC-GAL4, Mef2-GAL4 or 24B-GAL4 (control is below detection). D) Quantification of qRT-PCR experiments to show abundance of p38Ka and p38Kb mRNA in p38K mutants. E) RNA in situ experiments in the larval brain to detect p38Kb transcript in control and mutant animals. F) p38b-GAL4 expression in both adult brain and flight muscles visualized through the expression of a nuclear-GFP transgene (middle column and green in merged image). Brains are either counter-stained with an antibody to Elav (top row) or an antibody to the active zone protein Brp (middle row). Muscles are counter-stained with fluorescently conjugated Phalloidin to label actin bands. Scale bar for middle row is 50μm and for bottom row is 20μm. Error bars in all figures denotes SEM. See also Figures S1 and S2.
Next, we carried out western blot analysis with anti-phospho-p38K antibodies and anti-total p38K antibodies that do not distinguish between p38Ka and p38Kb (the correct band is identified based on over-expression of wild type p38Kb; Figure 1C). Our results show that p38Kb is the more prominent entity since p38KbΔ45 mutants showed a stronger reduction in phospho-p38K signal than a p38Ka deletion mutant (Figure 1B). Expectedly, the strongest reduction was seen in animals that are double mutant for p38KbΔ25 and p38Kadel (called p38K-DKO henceforth for Double Knock Out). Since tissue staining with the anti-p38 Kinase antibody proved unsatisfactory, we used a P-element replacement technique (Sepp and Auld, 1999) to generate an enhancer trap line for the p38Kb, a p38Kb-GAL4 (inserted 83 base pairs upstream of the p38Kb gene). We found widespread GAL4 expression (seen with a UAS-GFP reporter) in both the brain and flight muscles (Figure 1F). Nuclear entry of p38Kb was also detected by transgenically expressed p38Kb::GAL4-DBD::VP16-TD chimeric protein (p38Kb fused to the GAL4 DNA binding domain and the VP16 transcription activating domain) that upon entry into the nucleus activates transcription from a UAS-GFP transgene (Figure S2) (Kumar et al., 2003). Similarly, a FLAG-tagged kinase-dead version of p38Kb constructed by mutating a Lysine residue at position 53 to an Arginine was present in both the cytoplasm and in the nucleus in muscle cells (Figure S2). Taken together, these data document expression of p38Kb in the neuro-muscular system, confirm the cellular localization of p38 Kinase, and through the generation of p38Kb deletion mutants, enable experiments to study phenotypic consequences of loss of p38 Kinase in Drosophila.
p38 Kinase regulates lifespan
Although p38Ka and p38Kb null mutants are viable, the loss of both p38Ka and p38Kb is strictly lethal (homozygous mutant combinations of p38KbΔ45 and p38Kadel), similar to observations made in previous studies (Craig, et al., 2004; Ha et al., 2009; Park et al., 2009; Shinzawa et al., 2009; Chen et al., 2010). A double mutant combination of the hypomorphic p38KbΔ25 allele and p38Kadel (p38K-DKO) however, produced viable adults. p38K-DKO adult flies appeared normal on eclosion but had a severely reduced lifespan (Figure 2A; Table S1). Single mutants of p38Ka (p38Kadel) or p38Kb (p38KΔ45) also had significantly reduced lifespan, with the loss of p38Kb resulting in a stronger phenotype (Figure 2A). Given that Ex 41 did not show any deficits in lifespan, the reduced lifespan phenotype in p38K mutants most likely maps to the p38K genes. Further, the short lifespan in p38K-DKO animals could be rescued significantly through the expression of wild type p38Kb in muscles (using the previously characterized endoderm-specific Mef2-GAL4 driver; Ranganayakulu et al., 1995; Demontis and Perrimon, 2010) but not in neurons (using the pan-neuronal elavC155-GAL4 line) (Figure 2B). These results raised the possibility that p38Kb activity in adult muscles is required for normal lifespan.
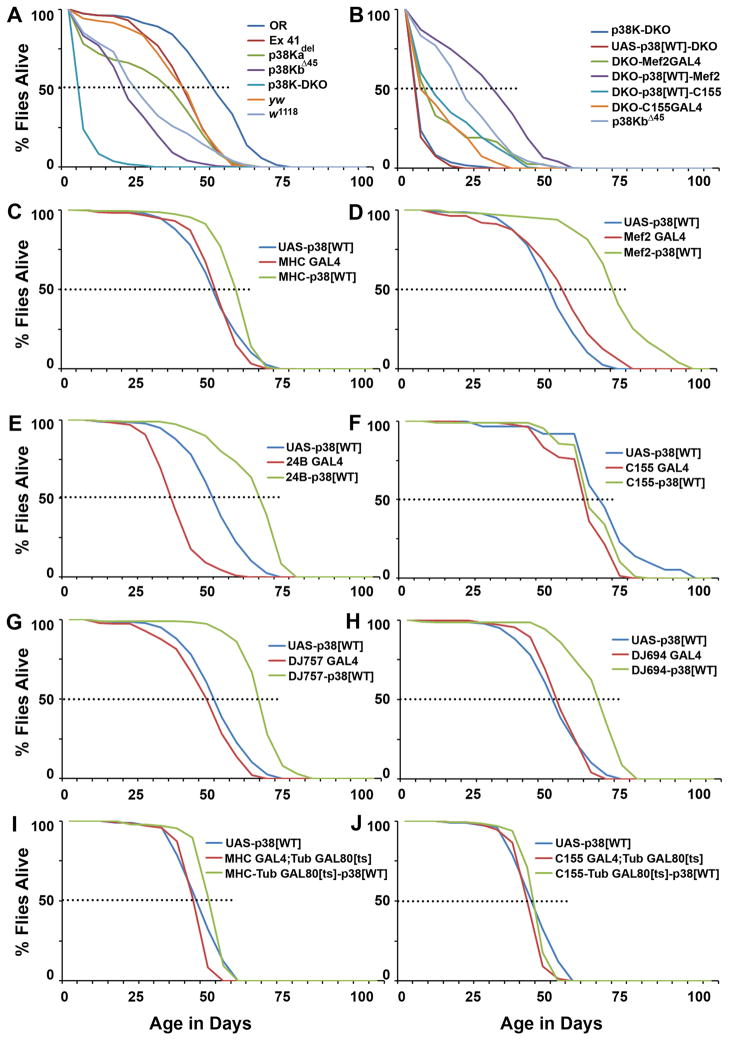
Figure 2. Muscle p38 MAP Kinase activity controls lifespan in Drosophila.
A) Lethality profiles of p38K mutants as compared to controls. Dotted lines represent 50% lethality. B) Reduced lifespan in the p38K-DKO animals is rescued through add back of p38Kb in muscles (Mef2-GAL4) but not in neurons (elavC155-GAL4). C, D and E) Lifelong expression of wild type p38Kb in muscles, using the MHC-GAL4, Mef2-GAL4 and 24B-GAL4 respectively, significantly extends lifespan in a control wild type genetic background. F) Pan-neuronal expression of p38Kb in a wild type genetic background fails to extend lifespan as compared to GAL4-only or UAS-only controls. G, H) Expression of p38K in adult muscle using the GAL4 lines DJ757 and DJ694 also extends lifespan. I) A similar phenotype is observed when p38K is expressed in adult muscles using the TARGET system to limit expression from the MHC-GAL4 line post-eclosion. J) Adult only expression of p38K in the nervous system does not impact lifespan. Males and females were tested independently with similar outcomes. (Mean lifespan in days: Oregon-R controls = 48; yw controls = 37; p38K-DKO = 5; p38Kadel = 29; p38KbΔ45 = 20; p38K-DKO-Mef2-GAL4 = 12; p38K-DKO-Mef2-GAL4-UAS-p38Kb[WT] = 30; p38K-DKO-elavC155-GAL4 = 10; p38K-DKO-elavC155-GAL4-UAS-p38Kb[WT] = 14; p<0.01 in each case, Log Rank test). Female only data shown. See also Table S1.
Although reduced longevity in p38K double mutant flies might be due to general debility, flies lived significantly longer than appropriate genetic controls when wild type p38Kb was expressed in muscle tissue with GAL4 lines that are reported to be muscle-specific. These include the MHC-GAL4 (Schuster et al., 1996; Sanyal et. al., 2002), Mef2-GAL4 (Ranganayakulu et al., 1995; Demontis and Perrimon, 2010), 24B-GAL4 (Sen et al., 2011; Sweeney et al., 1995), DJ694-GAL4 (Seroude et al., 2002) or DJ757-GAL4 (Seroude et al., 2002; Melicharek et al., 2010) ( (Figure 2C, D, E, G and H) (Table S1). Conversely, no effect on lifespan was observed when p38Kb was expressed pan-neuronally (Figure 2F) using an elavC155-GAL4 driver line (Sink et al., 2001). Significant, albeit somewhat modest, extension of lifespan was also observed when p38K was expressed in muscles post-eclosion using the temperature-sensitive TARGET system (McGuire et al., 2003) (Figure 2I). Again, no lifespan extension resulted when p38K was expressed in the adult nervous system post-eclosion (Figure 2J). Taken together, these results suggest that p38K activity in muscles regulates lifespan and that double mutant flies might be dying prematurely.
Loss of muscle p38K results in motor deficits that worsen with age
Progressive decline in motor function in flies has frequently been used as a biomarker of aging (Grotewiel, 2005; Demontis and Perrimon, 2010). Therefore, we tested p38K mutant animals for the presence of motor deficits and asked if they worsen with age. We selected age groups (1, 3 and 15 day old animals) at which control and wild type flies are not expected to show significant deterioration in any motor activity (Cook-Wiens and Grotewiel, 2002). We first observed mild but significant age-dependent impairment in flight behavior in p38K-DKO animals as compared to age-matched controls (Figure S3A). Negative geotaxis, however, was prominently impaired in p38Kb and p38K-DKO mutants and deteriorated more rapidly with age in p38K-DKO animals both when measured in a simple one-trial climbing assay (Figure 3A and movies S1 and S2) and in the counter-current apparatus that tests flies repeatedly for their ability to climb vertically (Figure S3B) (Benzer, 1967). Mef2 driven add-back of wild type p38Kb in muscles rescued these phenotypes (Figure 3A).
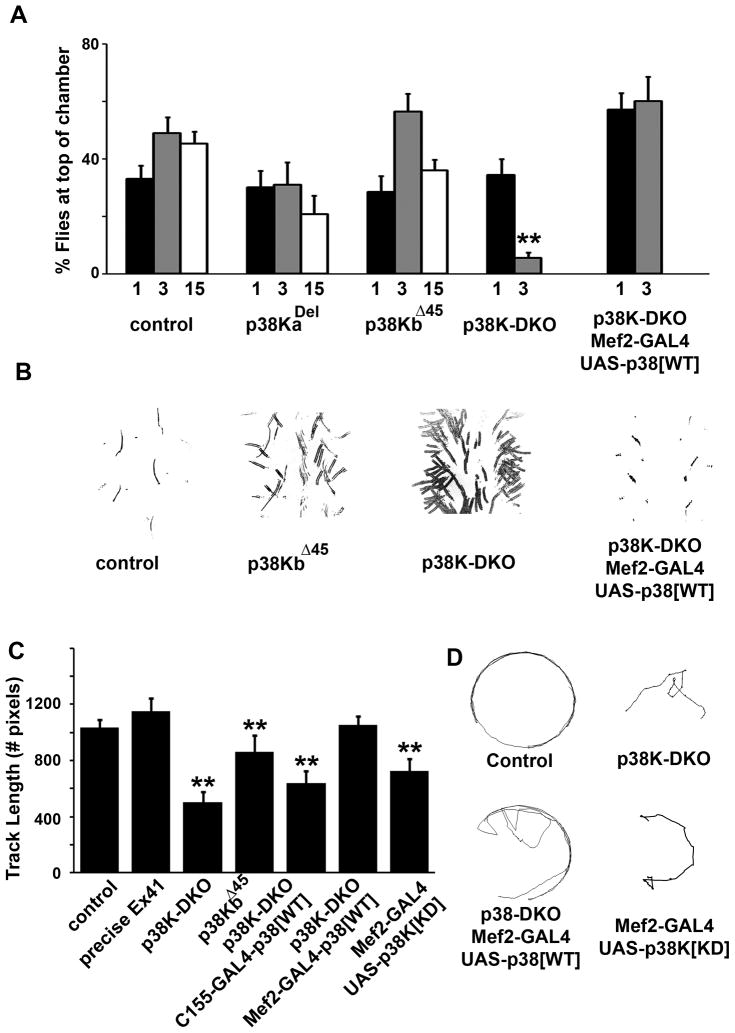
Figure 3. p38K protects against age-dependent motor deficits.
A) Graph plotting the percentage of flies that successfully complete a simple negative geotaxis test (climbing assay) and the variation in their performance with age (1, 3 and 15 day old flies). B) Altered gait and walking patterns in single and p38K-DKO mutants (traces depict “footprints” made by flies on carbon coated glass plates). C) Videographic analysis of exploratory walking in open field tests of individual flies from control and p38K mutant or transgenic animals. D) Representative tracks made by individual flies of particular genotypes. p<0.01 for all significant differences denoted by asterisks, one-way ANOVA. Only female data shown. See also Figures S3, and movies S1, S2, S3, and S4.
In 3 day old mutant flies, we also noticed aberrant walking behavior. Various distortions were noted, among them a tendency to drag the abdomen, dragging a leg, shuffling of the meta-thoracic legs and frequent slippage (Figure 3B). These phenotypes were also occasionally seen in single mutants, more obviously in p38KbΔ45, and were rescued through expression of wild type p38Kb protein in muscle. To quantify this defect, we videotaped walking in individual flies (open field exploratory test) and measured the distance and trajectory of walking for a period lasting one minute (Connolly, 1966). Figure 3C shows that walking speed in p38K-DKO flies was reduced as compared to wild type animals or Ex 41 (see movies S3 and S4). While walking in wild type animals improved with age, older DKO flies remained poor walkers. This defect was also rescued completely through muscle (but not neuronal) expression of wild type p38Kb, and was phenocopied in wild type animals through muscle-specific expression of a kinase-dead p38Kb transgene. Consistently, no age-dependent deterioration was observed in the function of the giant-fiber system in p38K-DKO mutant animals at any age (Figure S3) (Martinez et al., 2007). Taken together, these behavioral analyses highlight the importance of p38Kb in muscles for normal motor activity and further suggest that loss of p38K might result in age-related motor deficits.
p38 Kinase regulates sensitivity to oxidative stress in Drosophila
Previous measures of stress sensitivity in p38K mutant flies have been limited to p38Ka mutants (Craig et al., 2004). To measure the stress sensitivity of p38Kb mutants as well as in DKO animals, we subjected age matched control and mutant animals (1–2 days old) to two classical stressful stimuli: dry starvation and heat shock both of which lead to stress-induced lethality. When heat shocked at 37°C for 5 hours, nearly all p38K-DKO animals died within a subsequent 24 hour period (Figure S5A), while control animals were almost completely viable. Both single mutants also showed significantly increased lethality as compared to controls. Interestingly, heat-sensitivity in a wild type genetic background was also suppressed through muscle (but not neuronal) expression of wild type p38Kb. Similarly, when reared under conditions of dry starvation, 50% of p38K-DKO animals, but only 20% controls died within 15 hours. As before, muscle expression of wild type p38Kb significantly suppressed sensitivity to dry starvation in wild type animals (Figure S4B) while neuronal supply of p38Kb proved ineffective. These results confirm that loss of p38 Kinase significantly enhances sensitivity to stress in flies and also demonstrate that increasing p38K function in muscles provides resistance to such stress.
Genetic mutations in C. elegans have been used previously to test the role of p38K in the regulation of oxidative stress (Inoue et al., 2005). In flies, while p38Ka mutants show moderate effects of peroxide induced stress (Craig et al., 2004), the role of p38Kb in oxidative stress has not been investigated. Thus, we first tested p38Kb mutants and p38K double mutant flies for sensitivity to oxidative stress. Figure 4A shows that p38KbΔ25; p38Kadel (p38K-DKO) double mutants were significantly more sensitive to oxidative stress as compared to control genotypes and sucrose fed animals (Figures 4E and F). Single p38Kb mutants also displayed heightened sensitivity, and surprisingly in our hands, p38Ka mutants were marginally less sensitive than age-matched genetic controls (Figure 4A). Mef2-GAL4 driven expression of wild type p38Kb once again significantly rescued sensitivity to oxidative stress (Figure 4B). Consistent with a detoxification role for p38Kb, expression of normal p38Kb protein in wild type animals either in muscles (Mef2-GAL4) or in the p38Kb expression domain (p38Kb-GAL4) conferred additional resistance to hydrogen peroxide induced oxidative stress (Figure 4C, D). In sum, these results strongly suggest that muscle p38Kb MAP kinase activity is both necessary and sufficient for normal oxidative stress response in Drosophila.
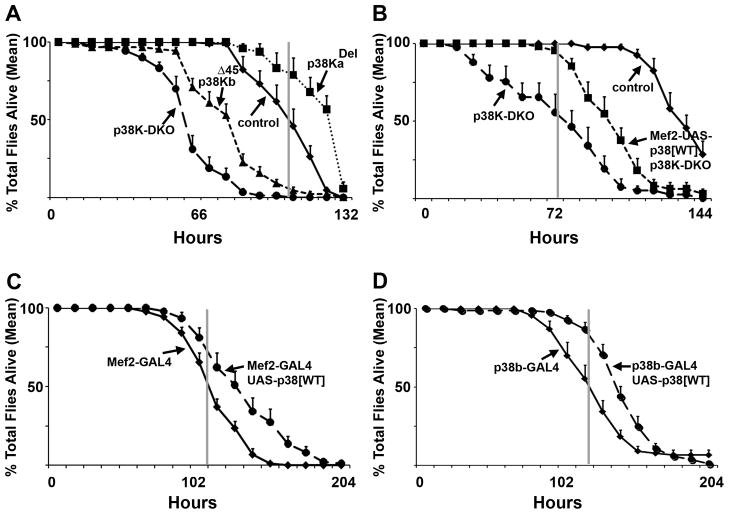
Figure 4. Muscle p38Kb is necessary and sufficient for resistance to oxidative stress.
A) p38KbΔ45 mutants and p38K-DKO animals are hyper-sensitive to Hydrogen Peroxide exposure through continuous feeding. B) Peroxide sensitivity in p38K-DKO animals can be rescued significantly by the expression of wild type p38Kb in muscles (using Mef2-GAL4). C) Expression of wild type p38Kb in wild type animals confers additional resistance to Peroxide. A similar effect is seen when p38Kb is expressed in a spatio-temporal domain specified by the p38Kb-GAL4 line (D). p values = control vs p38KbΔ45 <0.05 at 48 hrs and <0.01 for 64 hrs to 104 hrs; control vs p38K-DKO <0.05 at 32 hrs and 112 hrs and <0.01 40 hrs to 104 hrs; p38K-DKO vs Mef2 rescue <0.05 at 24 hrs to 40 hrs and <0.01 for 48 hrs to 72 hrs and 88 hrs and 96 hrs; p38K-DKO vs C155 rescue <0.05 at 32, 56, 64, 88, 104, and 112 hrs, <0.01 at 120 hrs; UAS-p38 wt/Mef2 vs OR/Mef2 <0.05 at 108, 120 and 156 hrs, <0.01 at 132 and 144 hrs; UAS-p38 wt/C155 vs OR/C155 <0.05 at 96 and 156 hrs, <0.01 from 108 to 144 hrs. See also Figure S4.
Cellular markers of oxidative stress are upregulated in p38K mutants
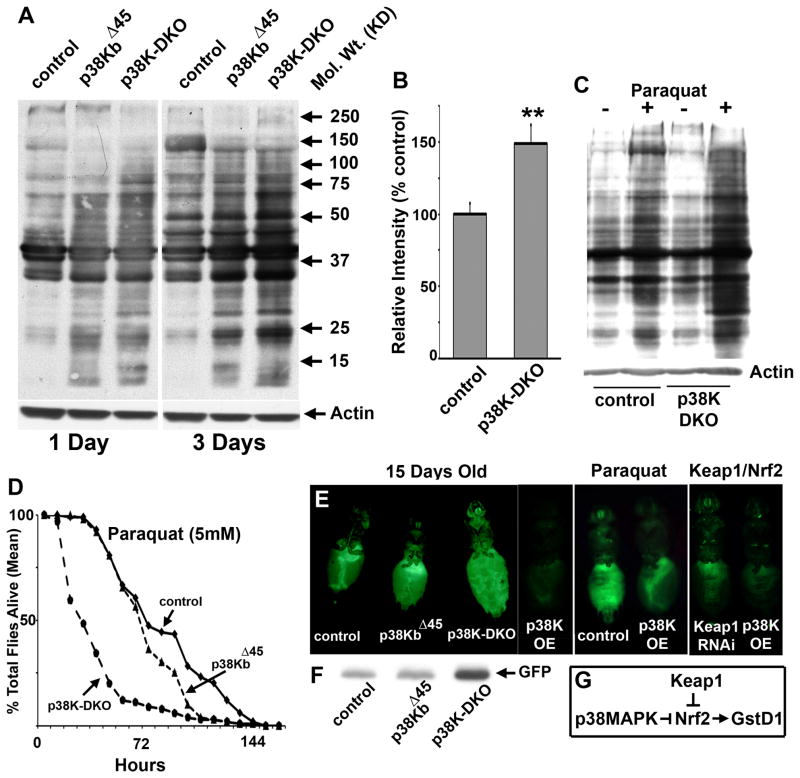
A common molecular outcome of oxidative stress, and a useful marker of age-related deterioration, is the irreversible addition of carbonyl groups to proteins in a site specific manner (at residues proline, lysine, arginine or threonine) (Levine, 2002). To measure the extent of protein oxidation in animals lacking p38 MAP Kinase, we performed “oxyblots” in which protein carbonylation is detected on a modified western blot through the chemical derivatization of carbonyl groups to 2,4-dinitrophenylhydrazone (DNP-hydrazone) followed by detection using antibodies to the DNP moiety. As shown in Figure 5A, increased overall protein carbonylation was detected in p38K mutant animals. Interestingly, protein carbonylation also increased more conspicuously in older p38K mutant animals than in age-matched controls (Figure 5B). Consistent with this idea, a significantly greater impact of the oxidizing herbicide Paraquat on protein carbonylation was seen in p38K-DKO animals than in age-matched genetic controls (Figure 5C) that is also compatible with the deleterious effect of Paraquat on the lifespan of p38K mutant and control flies (Figure 5D).
Figure 5. Cellular markers of oxidative stress are upregulated in p38K mutants.
A) Oxyblot analysis measuring total protein carbonylation in neuronal and muscle tissue (head and thorax) of age-matched control, p38KbΔ45, and p38K-DKO animals. B) Quantitative comparison of total protein carbonylation between 3 day old control and p38K-DKO animals. (Actin is used as a loading control). C) Paraquat feeding increases protein carbonylation in control animals and to a greater extent, in p38K-DKO animals (“+” denotes Paraquat feeding for a 4 hour period). (D, E, F) Quantitation of GFP expression from an in vivo ARE (anti-oxidant response element) dependent reporter of GST-D1 transcription in the whole fly supports a model in which p38K normally functions to inhibit Nrf2 activity (G). See also Figure S5.
A key signaling pathway associated with protective responses to oxidative stress is the Nrf2-Keap1 cassette, which controls the expression of a number of detoxifying enzymes (such as GST) and antioxidant proteins from the cis-regulatory Anti-oxidant Response Element or ARE (Kobayashi et al., 2004; Nguyen et al., 2009). Regulation of Nrf2, a CNC family transcription factor, by p38K is currently unclear (Andreadi et al., 2006; Naidu et al., 2009), but experiments in C. elegans support the idea that p38K activates Nrf2 (Inoue et al., 2005). In order to determine the relationship between p38 MAPK signaling and Nrf2 function in Drosophila, we adopted a transgenic reporter of GST-D1 transcription that has been used to assay stress responses in an Nrf2/Keap1 model of stress (Sykiotis and Bohmann, 2008). We estimated GFP reporter expression as an in vivo readout of oxidative stress and tested p38 Kinase mutants for an altered GST transcriptional response. As shown in Figure 5E and F, strongly increased GFP expression was observed in the p38K double mutant animals, confirming elevated stress response in these animals. Since reporter expression is regulated by the Nrf2 transcription factor, these results also suggest that absence of p38 Kinase signaling, directly or indirectly, potentiates GST transcription, perhaps through Nrf2. Consistent with this idea, expression of wild type p38Kb inhibited basal and Paraquat stimulated reporter expression as well as increased reporter expression seen in Keap1 RNAi knockdown animals (Figure 5E). Together, these results suggest that in Drosophila, p38K is a negative regulator of Nrf2 activity (Figure 5G). However, it is formally possible that increased stress in the absence of p38K stimulates Nrf2 activity through p38K-independent mechanisms.
p38 MAP Kinase regulates expression of the mitochondrial antioxidant enzyme MnSOD
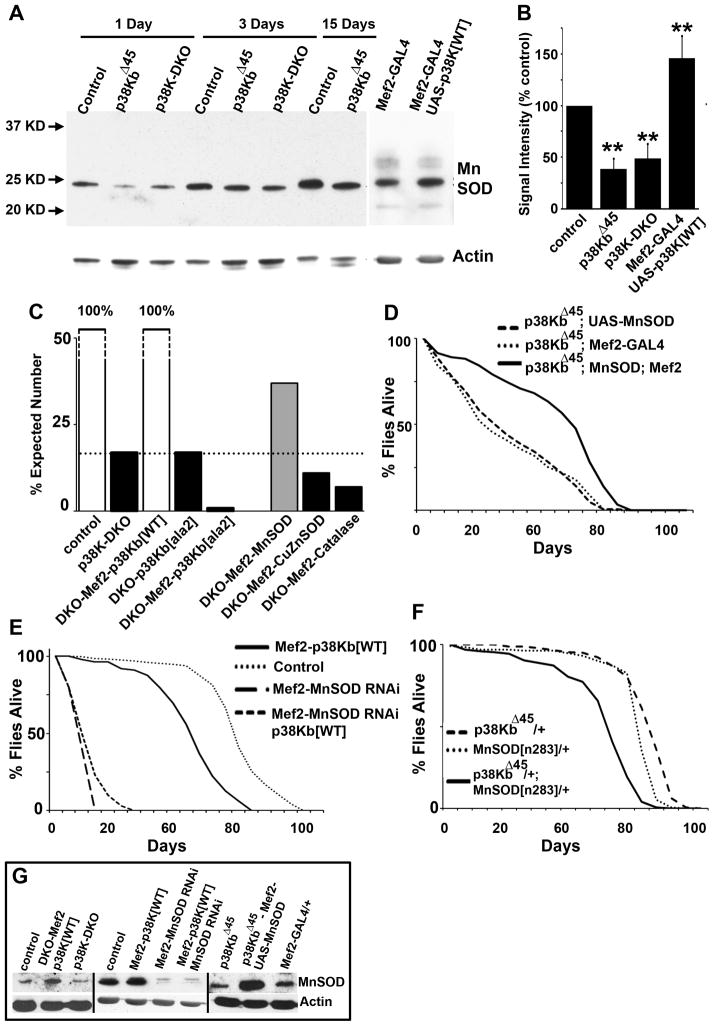
We noticed that both p38K and MnSOD mutant phenotypes are localized in muscle tissue and involve reduced lifespan, increased sensitivity to oxidative stress and motor defects (Duttaroy et al., 2003; Godenschwege et al., 2009; Kirby et al., 2002; Piazza et al., 2009). To test the hypothesis that p38K-dependent regulation of MnSOD leads to the convergence of these phenotypes, we estimated MnSOD protein levels in muscle tissue in p38K mutant animals. As shown in Figure 6A, MnSOD levels are significantly reduced in the absence of p38Kb (p38KbΔ45) or in p38K-DKO animals. This reduction persists through age and a roughly 50% reduction in MnSOD is observed in p38K mutant animals that are either 1, 3 or 15 days old as compared to age-matched controls (Figure 6B). Furthermore, in wild type animals MnSOD expression was increased through expression of wild type p38Kb in muscles (Figure 6A and 6B). Together, these results suggest that p38 MAP Kinase activity is both necessary and sufficient for MnSOD expression.
Figure 6. p38K controls MnSOD expression in muscles to regulate lifespan.
A) Western blot to measure MnSOD expression in different genotypes. B) Quantification of western blots in A. C) Quantification of viability in p38K mutants and the effect of manipulating MnSOD, CuZnSOD and Catalase in a p38K mutant background. D) Lifespan measurements in p38K mutants and the effect of supplementing MnSOD. E) Lifespan profile of animals overexpressing p38Kb with simultaneous knockdown of MnSOD in muscle. F) Dominant genetic interaction between p38KbΔ45 and MnSODn283 mutant alleles in lifespan regulation. G) Western analysis of MnSOD protein levels following manipulations of MnSOD and p38Kb.
Next, we tested physiological consequences of p38K-dependent MnSOD regulation. Only 17% of p38K-DKO flies make it to adulthood (Figure 6C). This reduced viability could be completely rescued through muscle-specific add-back of wild type p38Kb, but not by a mutant p38Kb (p38Kb-ala2) in which the Tyrosine and Threonine residues that are the targets of phosphorylation by an upstream kinase have been altered to Alanine (Figure 6C). Significantly, supplementing MnSOD, but not CuZnSOD or Catalase, rescued the viability of p38K-DKO animals two-fold (Figure 6C). This result is consistent with the idea that reduced viability in p38K mutants is, at least in part, due to reduction in MnSOD mediated detoxification of oxidative radicals. Importantly, addition of either wild type p38Kb or MnSOD in a p38K-DKO background increased MnSOD protein levels in adult Drosophila muscle tissue (Figure 6G).
To test if reduced lifespan in p38K mutants is due to loss of MnSOD, we assessed the effect of adding MnSOD to homozygous p38KbΔ45 mutants. Analysis of MnSOD in this sensitized mutant background is more likely to reveal MnSOD-centric roles for p38K as compared to the more drastic p38K reduction in DKO. As shown in Figure 6D and G, increased MnSOD expression significantly rescued the abbreviated lifespan phenotype in p38KbΔ45 mutants. Thus, 90% percent of flies with elevated MnSOD were alive when 50% p38KbΔ45 mutants were dead. Additionally, RNAi mediated knock-down of MnSOD completely abolished p38Kb-dependent lifespan extension (Figure 6D and G). Since these effects of p38 Kinase and MnSOD could potentially occur through independent pathways, we also tested for dominant interaction between null alleles of p38K and MnSOD. As shown in Figure 6F, both p38KΔ45 and MnSODn283 alleles heterozygous over a wild type allele had normal lifespans (Table S1). However, a transheterozygous combination of these two alleles had a significantly reduced lifespan. In sum, these observations suggest interaction between p38K and MnSOD in lifespan regulation.
p38 Kinase regulates MnSOD expression through the transcription factor Mef2
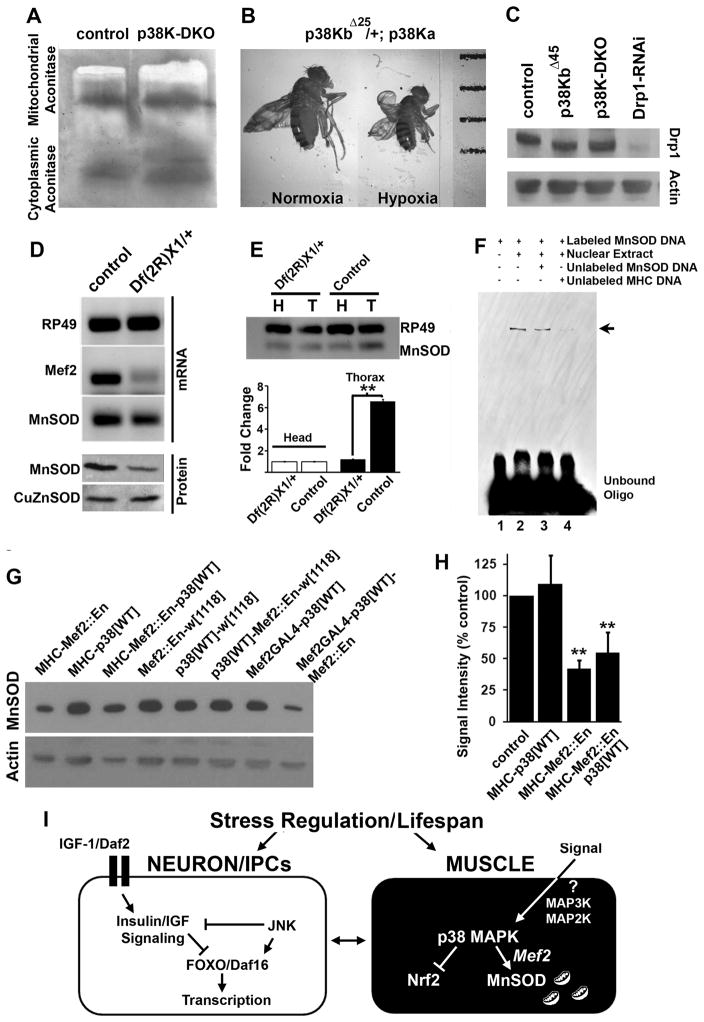
What might be the mechanism by which p38 Kinase regulates MnSOD? One possibility is that p38 Kinase somehow affects overall mitochondrial physiology. To explore this idea, we measured the enzymatic activity of cis-aconitase, a citric acid cycle enzyme that is predominantly localized to the mitochondrial matrix and is highly sensitive to oxidative stress (Gardner and Fridovich, 1991). Figure 7A shows that mitochondrial aconitase activity was unchanged in p38K-DKO mutant animals as compared to controls. This is not surprising considering the abundance of MnSOD in muscles and the prior observation that 50% reduction in MnSOD2 in MnSODn283/+ heterozygous animals does not lead to an appreciable change in mitochondrial aconitase activity (Paul et al., 2007). To further investigate the implication of reduced MnSOD expression in p38K mutant flies, we tested the response of p38K mutants to hypoxia (5% O2). Insects are able to maintain normal physiological functions in hypoxia and hypoxic conditions can significantly rescue shortened lifespan in both CuZnSOD and MnSOD mutants (Haddad, 2006; Wicks et al., 2009). When grown under chronic hypoxic conditions, however, p38K-DKO animals failed to eclose (Figure S5B). We next examined animals that are heterozygous for the p38KbΔ25 mutation in combination with homozygous p38Kadel (p38KbΔ25/+; p38Kadel/p38Kadel) and found that these animals had smaller pupae and adults under hypoxic conditions as compared to the same genotype grown in normoxia (Figure 7B and Figure S5A). Finally, p38K-DKO adults had a similarly abbreviated lifespan in hypoxia as they did under normoxic conditions (Figure S5C). These somewhat unexpected results suggest that reducing Oxygen supply is not sufficient to suppress lifespan phenotypes of p38K mutants. This is probably because hypoxia can rescue lifespan only under conditions of extreme lack of SOD activity as well as the fact that p38K controls multiple cellular proteins and processes, in addition to MnSOD. Finally, we found that levels of another mitochondrial protein, Drp1, remain unaffected by perturbations in p38K (Figure 7C). These results indicate that p38K does not influence overall parameters of mitochondrial physiology, and the effect on MnSOD is likely to be specific.
Figure 7. p38 Kinase regulates muscle MnSOD through the transcription factor Mef2.
A) Biochemical assay for mitochondrial and cytoplasmic aconitase in age-matched control and p38K-DKO animals. B) Partial reduction of p38K activity renders flies highly sensitive to hypoxia stress and results in smaller adult body size (hypoxia is 5% oxygen). C) Quantification of a mitochondrial protein Drp1 in p38K mutant animals. D) Semi-quantitative RT-PCR and western blotting to measure Mef-2 mRNA and protein expression and SOD protein expression in DfMef-2X1/+ animals. E) Comparison of MnSOD mRNA levels between thorax and head in control and DfMef-2X1/+ animals. F) Competitive EMSA (Electrophoretic mobility shift assay) to test the binding potential of one of the five Mef2 binding sites identified by in silico scans using Drosophila nuclear extracts. G) Western blots to measure MnSOD levels following inhibition of Mef2-dependent transcription in muscle tissue using either the MHC-GAL4 or Mef2-GAL4 driver line. These results are quantified in (H). I) Comparison of signaling pathways that regulate stress and lifespan. See also Figure S6.
To determine mechanisms by which p38K might regulate MnSOD, we compared Transcription Factor binding sites in the MnSOD and CuZnSOD genes in Drosophila (Frith et al., 2001). This analysis (Figure S6A) revealed the presence of strong binding clusters for the transcription factor Mef2 upstream of the MnSOD gene but not the CuZnSOD gene (Figure S6C). Similar Mef2 binding clusters are tightly conserved across several Drosophila species (Figure S6B, D). Interestingly Mef2 binding sites are also present in the mouse and human MnSOD genes (Figure S6E). These results raised the possibility that MnSOD is regulated by Mef2, a transcription factor well recognized as a downstream target of p38 Kinase signaling in mammals (Cox et al., 2003; Han et al., 1997; Zhao et al., 1999).
Potential regulation of MnSOD expression by Mef2 makes several predictions: a) changes in Mef2 activity should alter MnSOD transcript levels, b) the putative MnSOD genomic region identified in silico should have Mef2 binding activity, c) Mef2 perturbation should alter MnSOD protein expression, and d) p38 Kinase-dependent increase in MnSOD should be inhibited by blocking Mef2. Since most loss-of-function and null mutations in Mef2 display early lethality, we used adults heterozygous for a genomic deletion shown previously to delete portions of the Mef2 gene resulting in the amorphic allele Mef2X1. Figure 7D shows that in this deletion, Df(2R)X1, levels of not only Mef2 and MnSOD transcript but also MnSOD protein were reduced (while CuZnSOD remained unchanged) (Ranganayakulu et al., 1995; Lilly et al., 1995; Bour et al., 1995). More strikingly, MnSOD transcripts were preferentially and strongly reduced in muscle (thorax) as compared to neurons (head) in a Mef2 deletion background (Figure 7E). Electro-mobility shift analysis revealed that a radioactively labeled oligonucleotide representing the genomic region upstream of MnSOD bound to and was shifted by Drosophila nuclear extracts (Figure 7F). Since this shift was competed out by both unlabeled MnSOD derived oligonucleotide and an oligonucleotide derived from the MHC gene that is known to bind Mef2 (Sandman et al., 2006), these data suggest that the MnSOD upstream gene region is bound by a nuclear factor that could most likely be Mef2.
MnSOD protein, but not CuZnSOD was reduced in a genomic deficiency for Mef2 (Figure 7D, bottom panel). Similarly, when expression of Mef2-regulated genes was inhibited through muscle-specific expression of a transgenic construct that represses transcription from Mef2-binding cis-elements (UAS-Mef2::Engrailed) (Blanchard et al., 2010), MnSOD protein levels were reduced by ~ 50% (Figure 7G and H). Finally, expression of Mef2::En abolished increased MnSOD that results from over-expression of p38K in muscle tissue. Given that Mef2 is a well characterized target of p38 Kinase across species (and that p38K activation increases expression from a Mef2 transcriptional reporter in flies; Figure S6F, G), these results support the model (Figure 7I) that in Drosophila muscle tissue, p38K regulates MnSOD expression through the transcription factor Mef2, to regulate oxidative stress response and lifespan.
Discussion
The free radical theory of aging postulates that accumulating physiological damage as a result of oxidative stress contributes to aging during the normal lifespan of an animal (Harman, 1956; Kirkwood, 2005). However, recent data have prompted a re-evaluation of the precise connection between oxidative stress, particularly mitochondrial oxidative stress, and aging (Buffenstein et al., 2008; Howes, 2006; Lapointe et al., 2009). For instance, genetic experiments in which mutants in MnSOD are evaluated for their effect on oxidative stress and aging have been paradoxical. While homozygous Drosophila MnSOD mutants are clearly short-lived and display increased mitochondrial oxidative stress (Duttaroy et al., 2003; Kirby et al., 2002; Paul et al., 2007; Piazza et al., 2009), genetic ablation of MnSOD in C. elegans leads to lifespan extension while simultaneously elevating oxidative stress (Van Raamsdonk and Hekimi, 2009). In mice, MnSod+/− heterozygous animals show increased oxidative damage and age-related decline of mitochondrial function but no physiological signs of accelerated aging (Guachalla et al., 2009; Kokoszka et al., 2001; Zhang et al., 2009). Conversely, over-expression of MnSOD in normal long-lived backgrounds is reported to extend lifespan in one study in Drosophila (Sun and Tower, 1999) but does not extend lifespan in rodents (Jang et al., 2009). While our results do suggest physiological roles for MnSOD in the regulation of lifespan, they need to be interpreted with caution since our MnSOD manipulations are carried out in a sensitized p38K-manipulated background. Indeed, previous work shows that a 50% reduction in MnSOD by itself does not lead to lifespan deficits although it does significantly increase sensitivity to Paraquat induced oxidative stress (Duttaroy et al., 2003). In p38K mutants, however, we find increased stress sensitivity and a dramatic reduction in lifespan, suggesting the presence of other, as yet undetermined molecular pathways that are affected by the loss of p38K. Consistent with this idea, p38K heterozygous mutants are hyper-sensitive to hypoxia and result in substantially reduced pupal and adult body size, perhaps due to altered mitochondrial function and energy metabolism that precludes normal adaptive responses to hypoxia (Feala et al., 2009; Zhou et al., 2008). Given the large number of potential p38K targets in a cell, it is also likely that developmental consequences resulting from a loss of p38K contribute towards behavioral and lifespan phenotypes observed in these mutants. Overall, although regulation of aging may not be the sole function of p38K signaling, our results suggest that it influences stress and lifespan in vivo.
A well described paradigm in the field of aging research is that of dietary restriction (DR) (Fontana et al., 2010; Narasimhan et al., 2009). A large number of studies, including several in flies, worms and mice, have confirmed that DR extends lifespan by engaging the insulin signaling pathway through the transcription factor FOXO (Barbieri et al., 2003; Hwangbo et al., 2004; Kimura et al., 1997; Tatar et al., 2001). Experiments in Drosophila have further shown that the JNK pathway normally antagonizes insulin signaling in neuroendocrine cells by promoting nuclear localization of FOXO (Wang et al., 2005). Our results are especially interesting in light of this model, since the requirement for p38K signaling in this context seems to be limited to muscle tissue as is the necessity for MnSOD. Based on this, we propose a model in which an Insulin/JNK pathway is operational in neurons whereas a p38K/Mef2/MnSOD pathway is functional in muscles. Currently, we do not know how a muscle-restricted p38K pathway interacts with the neuronally resident insulin/JNK pathway to fine-tune stress responses leading to changes in longevity. However, a recent study in flies has shown how FOXO activity in muscle tissue is capable of regulating aging and the age-related accumulation of protein aggregates (Demontis and Perrimon, 2010). This study also suggests cross-talk between such events in muscle and the regulation of dietary intake. It will be interesting to see in future experiments how the p38K pathway interacts with or influences DR and the insulin pathway.
Experimental Procedures
Generation of p38Kb mutants and transgenic strains
See supplementary methods for general fly strains and genetics. The P{SUPor-P}p38bKG01337 P-element 83 base pairs upstream of the transcriptional start of the p38Kb locus was excised and 50 independent excision lines were screened by PCR using primers flanking the P-element insertion site. Three deletions that removed portions of the p38Kb locus were identified and sequenced. The p38Kb-GAL4 line was generated by exchanging the P{SUPor-P}p38bKG01337 P-element with a P{GawB} containing P-element using a strategy outlined previously (Sepp and Auld, 1999). The UAS-p38Kb-Kinase dead transgene was made by substituting a Lys residue at 53 with an Arg (Wu et al., 1991) using site-directed mutagenesis. The transgene was cloned into pTWF using Gateway cloning (Invitrogen Inc.) to create a UAS-p38Kb-Kinase dead with a C-terminal FLAG epitope tag. The Alanine substituted p38K transgene was created through site-directed mutagenesis that replaced Thr 183 and Tyr 185 with Ala. This mutated transgene was then cloned into pUAST and used to generate transgenic Drosophila (Bestgene Inc.). For the p38K::GAL4DBD::VP16 fusion construct, the GAL4DBD::VP16 region was subcloned using PCR from a rl::GAL4DBD::VP16 transgenic described previously (Kumar et al., 2003). This was then cloned in frame downstream of a p38K gene that lacked the stop codon. The entire transgene was then transferred to pP{Hsp70-CaSper} followed by generation of transgenic flies. In these transgenics, the fusion protein is expressed ubiquitously using heat shock regimens from the hsp-70 promoter.
Hydrogen Peroxide feeding
9 sets of 10 animals per genotype per sex were aged one day and then starved for 6 hrs. Each sex was tested independently. Animals were fed either 1.3% agarose, 1% sucrose or 1.3% agarose, 1% sucrose, 1% Hydrogen Peroxide (Fisher). Animals were then assayed for survival every 8 hrs. p values are: control vs p38KbΔ45, p<0.05 (48 hours) and <0.01 (64 hours to 104 hours); control vs p38K-DKO, p <0.05 (32 hours, 112 hours) and <0.01 (40 hours to 104 hours); p38K-DKO vs p38K-DKO-Mef2-GAL4-UAS-p38Kb[wt], p <0.05 (24 hours to 40 hours) and <0.01 (48 hours to 72 hours, 88 hours, 96 hours); p38K-DKO vs p38K-DKO-elavC155-UAS-p38Kb[wt], p<0.05 (32 hours, 56 hours, 64 hours, 88 hours, 104 hours, and 112 hours), <0.01 (120 hours); UAS-p38Kb[wt]-Mef2-GAL4 vs OR-Mef2, p<0.05 (108 hours, 120 hours, 156 hours) and <0.01 (132 hours, 144 hours); UAS-p38Kb[wt]-elavC155 vs OR-elavC155 <0.05 (96 hours, 156 hours) and <0.01 (108 hours to 144 hours).
Walking assays
Single female animals aged 1, 3, or 15 days were anesthetized and their wings removed 24 hrs before testing. Individual animals were placed in a circular glass chamber and videotaped for 1 min. Total track length was measured using Image J and the SpotTracker II plugin. A total of 9 animals were tested for each genotype. Footprint analysis was performed on single female animals 3 days old. Glass slides were covered with a thin layer of candle soot. Animals were allowed to walk across the slides for three independent sets of tracks.
Measurement of viability and lifespan
Viability was determined as the percentage of animals expected to eclose. For analysis of lifespan, mutants were first backcrossed multiple times to control strains and closely matched genetic controls were used in each case. 10–20 female or male flies were housed in vials containing standard Drosophila culture medium and transferred to fresh vials every 2–3 days as needed. The number of dead animals was determined daily. Log rank and Wilcoxon tests were used for statistical analysis using the program JMP. For all significant differences p<0.01.
Antibodies, immunohistochemistry and western blotting
See supplementary methods for antibodies and working dilutions. Adult brains and thoraxes were processed as described previously (Sanyal, 2009). Western blots were performed according to standard protocols. 3 thoraxes or heads were homogenized in 30μl of 1X Laemmli buffer plus protease inhibitors (EDTA complete tablets). Oxyblots were performed per manufacturer’s instructions (Milipore). Lysates were made from 3 heads and thoraxes in 1X Laemmli buffer. Lysates were then derivatized to add DNPH moieties to protein carbonyls. DNPH moieteies were then detected with rabbit anti-DNPH (Milipore 1:150) and rabbit anti-HRP (Milipore 1:300). Aconitase assays were done as described previously (Paul et al., 2007).
Electro-mobility shift assays
Biotin labeled MnSOD oligonucleotides (GAAATTAAA aactatttttaaTTGAAACAT, Mef2 binding site shown in bold lower case) were mixed with the Drosophila Nuclear extract (Genetex Inc.). Gel mobility shifts were carried out following standard protocols. To eliminate the possibility that the shift resulted from non-specific binding we challenged this complex with cold MnSOD oligonucleotides (300X). Cold MnSOD oligonucleotides competed with the labeled oligonucleotides causing reduction in the band intensity, which suggests that the bound nuclear factor is specific for MnSOD sequence. Furthermore, we challenged this complex with cold Myosin Heavy Chain (Mhc) oligonucleotides (GAATATGTtttaaaaataaccAAAGACATT, Mef2 binding site in bold lower case), a well-known target for Mef2 (Sandman et al., 2006). Mhc oligonucleotides were found to compete with MnSOD oligonucleotides for the same nuclear factor.
Semi-quantitative PCR
Total RNA was obtained from the head and thorax of DfMEF-2X1/+ and the wild type control flies using Trizol. Multiplex PCR was used to simultaneously amplify MnSOD and RP49 mRNAs in the same PCR reaction.
Supplementary Material
Highlights.
Muscle p38K controls lifespan and oxidative-stress response in Drosophila
Loss of p38K in muscles leads to age-related motor deficits
p38K controls MnSOD expression in muscles through the transcription factor Mef2
Acknowledgments
This work was supported by grants from the URC, Emory University to SS, NSF postdoctoral fellowship and a PD-CERC T32 fellowship to AVM, and grant U54 NS039407-06A1 from NINDA to AD. The anti-Brp and anti-Elav (Gerald Rubin) antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S, McElwee MK, Apfeld J, Lackford B, Freedman JH, Schwartz DA. The Caenorhabditis elegans germ line regulates distinct signaling pathways to control lifespan and innate immunity. J Biol Chem. 2010;285:1822–1828. doi: 10.1074/jbc.M109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci U S A. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman K, McKay J, Avery L, Cobb M. Isolation and characterization of pmk-(1–3): three p38 homologs in Caenorhabditis elegans. Mol Cell Biol Res Commun. 2001;4:337–344. doi: 10.1006/mcbr.2001.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard FJ, Collins B, Cyran SA, Hancock DH, Taylor MV, Blau J. The transcription factor Mef2 is required for normal circadian behavior in Drosophila. J Neurosci. 2010;30:5855–5865. doi: 10.1523/JNEUROSCI.2688-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour BA, O’Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmayr SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordr) 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xie C, Tian L, Hong L, Wu X, Han J. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc Natl Acad Sci U S A. 2010;107:20774–20779. doi: 10.1073/pnas.1009223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Connolly KJ. Locomotor activity in Drosophila as a function of food deprivation. Nature. 1966;209:224. doi: 10.1038/209224a0. [DOI] [PubMed] [Google Scholar]
- Cook-Wiens E, Grotewiel MS. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp Gerontol. 2002;37:1347–1357. doi: 10.1016/s0531-5565(02)00096-7. [DOI] [PubMed] [Google Scholar]
- Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DM, Du M, Marback M, Yang EC, Chan J, Siu KW, McDermott JC. Phosphorylation motifs regulating the stability and function of myocyte enhancer factor 2A. J Biol Chem. 2003;278:15297–15303. doi: 10.1074/jbc.M211312200. [DOI] [PubMed] [Google Scholar]
- Craig CR, Fink JL, Yagi Y, Ip YT, Cagan RL. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 2004;5:1058–1063. doi: 10.1038/sj.embor.7400282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M, Genevet A, Warne P, Treins C, Liu T, Bastien J, Baum B, Tapon N, Leevers SJ, Downward J. A role for p38 stress-activated protein kinase in regulation of cell growth via TORC1. Mol Cell Biol. 30:481–495. doi: 10.1128/MCB.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Primrose DA, Hodgetts RB. A member of the p38 mitogen-activated protein kinase family is responsible for transcriptional induction of Dopa decarboxylase in the epidermis of Drosophila melanogaster during the innate immune response. Mol Cell Biol. 2008;28:4883–4895. doi: 10.1128/MCB.02074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics. 2003;165:2295–2299. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Chen WC, Tan MW. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7:879–893. doi: 10.1111/j.1474-9726.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feala JD, Coquin L, Zhou D, Haddad GG, Paternostro G, McCulloch AD. Metabolism as means for hypoxia adaptation: metabolic profiling and flux balance analysis. BMC Syst Biol. 2009;3:91. doi: 10.1186/1752-0509-3-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith MC, Hansen U, Weng Z. Detection of cis-element clusters in higher eukaryotic DNA. Bioinformatics. 2001;17:878–889. doi: 10.1093/bioinformatics/17.10.878. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- Godenschwege T, Forde R, Davis CP, Paul A, Beckwith K, Duttaroy A. Mitochondrial superoxide radicals differentially affect muscle activity and neural function. Genetics. 2009;183:175–184. doi: 10.1534/genetics.109.103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Guachalla LM, Ju Z, Koziel R, von Figura G, Song Z, Fusser M, Epe B, Jansen-Durr P, Rudolph KL. Sod2 haploinsufficiency does not accelerate aging of telomere dysfunctional mice. Aging (Albany NY) 2009;1:303–315. doi: 10.18632/aging.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Haddad GG. Tolerance to low O2: lessons from invertebrate genetic models. Exp Physiol. 2006;91:277–282. doi: 10.1113/expphysiol.2005.030767. [DOI] [PubMed] [Google Scholar]
- Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- Han SJ, Choi KY, Brey PT, Lee WJ. Molecular cloning and characterization of a Drosophila p38 mitogen-activated protein kinase. J Biol Chem. 1998a;273:369–374. doi: 10.1074/jbc.273.1.369. [DOI] [PubMed] [Google Scholar]
- Han ZS, Enslen H, Hu X, Meng X, Wu IH, Barrett T, Davis RJ, Ip YT. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol. 1998b;18:3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Howes RM. The free radical fantasy: a panoply of paradoxes. Ann N Y Acad Sci. 2006;1067:22–26. doi: 10.1196/annals.1354.004. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Tateno M, Fujimura-Kamada K, Takaesu G, Adachi-Yamada T, Ninomiya-Tsuji J, Irie K, Nishida Y, Matsumoto K. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. Embo J. 2001;20:5421–5430. doi: 10.1093/emboj/20.19.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, et al. Over-expression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, et al. A conserved p38 MAP Kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP, Hsiung F, Powers MA, Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development. 2003;130:3703–3714. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz CL, Tan MW. Regulation of aging and innate immunity in C. elegans. Aging Cell. 2004;3:185–193. doi: 10.1111/j.1474-9728.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Lapointe J, Stepanyan Z, Bigras E, Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/− mice. J Biol Chem. 2009;284:20364–20374. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci. 2008;13:3581–3593. doi: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2007;67:778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Naguro I, Takeda K, Ichijo H. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem. 2009;16:1229–1235. doi: 10.2174/092986709787846613. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Melicharek DJ, Ramirez LC, Singh S, Thompson R, Marenda DR. Kismet/CHD7 regulates axon morphology, memory and locomotion in a Drosophila model of CHARGE syndrome. Hum Mol Genet. 2010;19:4253–4264. doi: 10.1093/hmg/ddq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Naidu S, Vijayan V, Santoso S, Kietzmann T, Immenschuh S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol. 2009;182:7048–7057. doi: 10.4049/jimmunol.0900006. [DOI] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–666. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med. 2000;28:N67–77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim YS, Yoo MA. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging (Albany NY) 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza N, Hayes M, Martin I, Duttaroy A, Grotewiel M, Wessells R. Multiple measures of functionality exhibit progressive decline in a parallel, stochastic fashion in Drosophila Sod2 null mutants. Biogerontology. 2009;10:637–648. doi: 10.1007/s10522-008-9210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganayakulu G, Zhao B, Dokidis A, Molkentin JD, Olson EN, Schulz RA. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol. 1995;171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A, Matsumoto K, Hisamoto N. Roles of MAP kinase cascades in Caenorhabditis elegans. J Biochem. 2004;136:7–11. doi: 10.1093/jb/mvh097. [DOI] [PubMed] [Google Scholar]
- Sandmann T, Jensen LJ, Jakobsen JS, Karzynski MM, Eichenlaub MP, Bork P, Furlong EE. A temporal map of transcription factor activity: mef2 directly regulates target genes at all stages of muscle development. Dev Cell. 2006;10:797–807. doi: 10.1016/j.devcel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Sano Y, Akimaru H, Okamura T, Nagao T, Okada M, Ishii S. Drosophila activating transcription factor-2 is involved in stress response via activation by p38, but not c-Jun NH(2)-terminal kinase. Mol Biol Cell. 2005;16:2934–2946. doi: 10.1091/mbc.E04-11-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr Patterns. 2009;9:371–380. doi: 10.1016/j.gep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature. 2002;25:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- Schieven GL. The p38alpha kinase plays a central role in inflammation. Curr Top Med Chem. 2009;9:1038–1048. doi: 10.2174/156802609789630974. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Sen A, Yokokura T, Kankel MW, Dimlich DN, Manent J, Sanyal S, Artavanis-Tsakonas S. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J Cell Biol. 2011;192:481–495. doi: 10.1083/jcb.201004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics. 1999;151:1093–1101. doi: 10.1093/genetics/151.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroude L, Brummel T, Kapahi P, Benzer S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell. 2002;1:47–56. doi: 10.1046/j.1474-9728.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- Shinzawa N, Nelson B, Aonuma H, Okado K, Fukumoto S, Miura M, Kanuka H. p38 MAPK-dependent phagocytic encapsulation confers infection tolerance in Drosophila. Cell Host Microbe. 2009;6:244–252. doi: 10.1016/j.chom.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Sink H, Rehm EJ, Richstone L, Bulls YM, Goodman CS. sidestep encodes a target-derived attractant essential for motor axon guidance in Drosophila. Cell. 2001;105:57–67. doi: 10.1016/s0092-8674(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzanne M, Irie K, Glise B, Agnes F, Mori E, Matsumoto K, Noselli S. The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev. 1999;13:1464–1474. doi: 10.1101/gad.13.11.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wicks S, Bain N, Duttaroy A, Hilliker AJ, Phillips JP. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic Biol Med. 2009;46:176–181. doi: 10.1016/j.freeradbiomed.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans lifespan by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Wu J, Rossomando AJ, Her JH, Del Vecchio R, Weber MJ, Sturgill TW. Autophosphorylation in vitro of recombinant 42-kilodalton mitogen-activated protein kinase on tyrosine. Proc Natl Acad Sci U S A. 1991;88:9508–9512. doi: 10.1073/pnas.88.21.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4:e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZH, Zhou Y, Yu MC, Silverman N, Ge BX. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell Signal. 2006;18:441–448. doi: 10.1016/j.cellsig.2005.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.