Abstract
Induction of early pituitary progenitors is achieved through combined activities of signals from adjacent embryonic tissues. Previous studies have identified a requirement for oral ectoderm derived Sonic Hedgehog (Shh) in specification and/or proliferation of early pituitary progenitors, however how different Gli genes mediate Shh signaling to control pituitary progenitor development has not yet been determined. Here we show that Gli2, which encodes a major Gli activator, is required for proliferation of specific groups of pituitary progenitors but not for initial dorsoventral patterning. We further show that the action of Gli2 occurs prior to the closure of Rathke’ pouch. Lastly, we show that Shh/Gli2 signaling controls the diencephalic expression of Bone morphogenetic protein 4 (Bmp4) and Fibroblast growth factor 8 (Fgf8), two genes that are known to play critical roles in patterning and growth of Rathke’s pouch. Our results therefore suggest both cell-autonomous and non-cell autonomous requirements for Gli2 in regulation of pituitary progenitor specification, proliferation and differentiation.
Keywords: Gli1, Gli2, Gli3, Shh, mouse, pituitary, patterning, proliferation
INTRODUCTION
The pituitary gland is a master endocrine organ that produces a number of hormones regulating many essential physiological functions in the body. It is comprised of the adenohypophysis (anterior and intermediate lobes) and neurohypophysis (posterior lobe). The anterior lobe contains five different hormone-producing cell types that secrete adrenocorticotropic hormone (ACTH), growth hormone (GH), prolactin (PRL), thyroid-stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) while the posterior lobe contains nerve endings that secrete oxytocin and vasopressin (Reviewed in Camper et al., 2002; Zhu et al., 2007). Both hypopituitarism and hyperpituitarism have been identified in a number of human congenital pituitary disorders (Dattani et al., 1998; Pfaffle et al., 1992; Roessler et al., 2003; Thomas et al., 2001; Wu et al., 1998).
Development of the pituitary gland requires extensive interactions between different embryonic tissues. Between ~E8.5 and E9.0, a portion of the oral ectoderm thickens and invaginates to form a structure called Rathke’s pouch (RP). Several lines of evidence suggest that induction of RP requires signals derived from the diencephalon. First, mutation of T/ebp (Nkx2.1), which is expressed in the ventral diencephalon but not in RP, causes apoptosis and degeneration of nascent RP (Takuma et al., 1998). Second, mutation of Bmp4, which is expressed in the ventral diencephalon, causes a loss of early RP (Takuma et al., 1998; Winnier et al., 1995). Similar phenotypes were also observed in embryos over-expressing Noggin, a BMP antagonist, in the oral ectoderm (Treier et al., 1998). On the other hand, mutation of Noggin results in expansion of pituitary tissues (Davis and Camper, 2007). Lastly, analysis of embryos mutant for Fgfr2, which encodes a receptor for FGF1/3/7/10 and is expressed in the diencephalon, revealed a requirement for FGF signaling in the diencephalon (De Moerlooze et al., 2000). In both Fgfr2 and Fgf10 mutants, increased apoptosis causes severe defects in infundibulum and RP development (De Moerlooze et al., 2000; Ohuchi et al., 2000). By ~E11, RP closes off from the oral ectoderm and by ~E12, RP detaches from the oral ectoderm. Interestingly, the critical period for pituitary progenitor specification appears to be between ~E10 and ~E12/13. Nascent RP isolated from E10.5 mouse embryos will express early markers of pituitary pouch Isl1 but not Lhx3 (Lim1) when cultured in vitro. When combined with ventral diencephalic tissues, nascent RP can be induced to express Lhx3 and other pituitary markers (Ericson et al., 1998). The action of diencephalic tissues can be recapitulated by beads soaked with FGF8, suggesting at least that FGF8 secreted from the diencephalon is critical in specifying different pituitary cell types. However, by E11.5, the pituitary explants become refractory to FGF8 induction (Ericson et al., 1998). By E12/13 (E13/15 in rat embryos), isolated RP can differentiate into different pituitary cell types even when cultured alone, suggesting that pituitary progenitors have already been specified when RP detaches from the underlying oral ectoderm (Nemeskery et al., 1976).
In addition to dorsal diencephalic signals, Shh is expressed in the oral ectoderm immediately adjacent to RP (Treier et al., 1998). The requirement of Shh signaling in pituitary development is supported by the following experiments. Over-expression of Shh in the developing RP using an αGSU promoter, which drives ectopic expression of Shh in RP starting from ~E12.5, results in an expansion of ventral pituitary cell markers TSH and LH and causes pituitary hyperplasia (Treier et al., 2001). On the other hand, blocking Shh signaling through expression of a Shh antagonist, Hedgehog-interacting protein (Hhip), using the Pitx1 promoter causes pituitary hypoplasia (Treier et al., 2001). However, interpretation of some of these results is complicated by the fact that manipulations of secreted signals can affect multiple tissues. In addition to the oral ectoderm, Shh is also abundantly expressed in the ventral diencephalon, immediately above the early RP. As a result, RP may be exposed to Shh derived from both oral ectoderm and ventral diencephalon, and the above experiments might have affected oral ectoderm and diencephalon development in addition to development of RP. Preliminary analysis of the pituitary phenotype resulting from a loss of Gli2, which encodes the major Gli activator, provides some initial insight into the requirement of Gli genes. Mutations in Gli2 result in a variable loss of pituitary, demonstrating a requirement for Shh/Gli2 signaling in pituitary development (Park et al., 2000). However, whether different cell types are generated in the anterior pituitary or whether the development of posterior pituitary is affected has not yet been determined. To address how Gli genes mediate Shh signaling to control pituitary specification and proliferation, we analyzed pituitary phenotypes of embryos mutated for individual Gli genes, which are required for the transcription output of Shh signaling. We then conditionally disrupted Gli genes in RP and found a critical role for Shh signaling before RP detached from the oral ectoderm. Lastly, we found Shh signaling is also required in the diencephalon to control pituitary development through the regulation of two critical growth factors.
MATERIAL AND METHODS
Mouse breeding
Mouse breeding was carried out according to protocols approved by Case Western Reserve University (CWRU) Institutional Animal Care and Use Committee. The Rosa26-lacZ Reporter (R) mouse (Soriano, 1999) was used to monitor Cre-mediated recombination. To generate FoxG1Cre;Gli conditional mutant embryos, FoxG1Cre;Gli2zfd/+ male mice were bred to Gli2flox/flox;R/R or Gli2flox/flox;Gli3xt/+;R/R female mice. To generate embryos with activated Hh signaling in RP, Pitx2Cre male mice were bred to R26SmoM2 female mice. To generate Gli2 mosaic embryos, Gli2lacZ/+ mice were bred with Gli2zfd/+; Rosa26 (R26)-EGFP/R26-EGFP mice. The resulting morula stage embryos were co-cultured with CD1 embryos. Embryos that had aggregated after overnight culture were then transferred into pseudo-pregnant female for further development. The genotyping of Cre, Gli2flox, Gli2lacZ, Gli2zfd and Gli3xt mice has been described (Bai et al., 2002; Bai and Joyner, 2001; Corrales et al., 2004; Jeong et al., 2004; Maynard et al., 2002; Mo et al., 1997).
Histology, immunostaining and RNA in situ hybridization
Embryos were dissected in cold PBS and fixed in 4% paraformaldehyde (PFA) for 1-3 hrs at 4°C, washed with PBS, sunk in 30% sucrose and embedded in OCT (Tissue-Tek). Sagittal tissue sections were collected at 12 μm on a Leica Cryostat. 3-6 embryos were analyzed for each embryonic stage. Methods of X-gal staining, antibody staining and RNA in situ hybridization have been described (Bai et al., 2002; Bai and Joyner, 2001). The following antibodies were used: Pax6 (1:500, Covance), Isl1 (1:100, Abcam), Lhx3 (1:100, Developmental Studies Hybridoma Bank), BrdU (1:20, Becton-Dickinson), Ki67 (1:500, Abcam), phospho-Histone H3 (1:100, Upstate), cleaved Caspase-3 (1:200, Cell Signaling), ACTH (1:1000), GH (1:1000), LH (1:500), PRL (1:2500) and TSH (1:500, all from National Hormone & Peptide Program) and β-galactosidase (1:500, Biogenesis). Hoechst 33258 (Molecular Probes) was used to visualize nuclear staining. All sections were examined with a Leica DMLB epi-fluorescence microscope fitted with a SPOT camera in the CWRU Genetics Imaging Facility (supported by NIH-NCRR, RR-021228).
BrdU labeling, cell cycle, cell death analysis and quantification
Pregnant mice were injected intraperitoneally with BrdU (Sigma) at 100 μg/g body weight 1 hr prior to dissection. The percentage of BrdU+, Ki67+, pHH3+, Caspase3+ cells was calculated by dividing the number of the positive cells by the total number of nuclei (Hoechst) inside RP or other specified region. To calculate the fraction of S-phase cells within cell cycle, we divided the number of BrdU+ cells by the total number of Ki67+ cells and converted the number to a percentage. For each time point, we used 2 mid-sagittal sections from each embryo and 4-6 embryos from at least two different litters for analysis. As the number of Caspase3+ cells is low in both WT and Gli2 mutants, we counted 3-4 sections from each embryo (total 1-5 Caspase3+ cells per embryo, with n=3 for Gli2 mutants and n=6 for WT). The number in charts was displayed as mean ± S.E.M. Student’s t-test was used to calculate the P value and to determine whether the results were significantly different from each other (** indicates P<0.01; * indicates P<0.05).
Quantification of pituitary cell types at E17.5. To ensure consistent sampling of cell types among different embryos, we performed immunostaining analysis for each marker on two mid-sagittal sections from each embryo. A total of 6 WT embryos, 6 Gli2−/− embryos and 4 FoxG1Cre;Gli2−/− embryos from at least three litters were used in the analysis.
We used the following method to normalize the contribution of EGFP-labeled cells to RP of mosaic embryos. We first selected an area in the dorsal mid/hindbrain region, which does not have active Shh/Gli signaling, and calculated the percentage of EGFP+ cells over the total number of cells. This number was used as a baseline for the level of mosaicism in that embryo. We then calculated the percentage of EGFP+ cells within RP or in different regions of RP, and normalized the percentage against the baseline in that embryo. Results from three embryos were then used to determine whether there was a significant difference between the contribution of WT and Gli2 mutant cells.
RESULTS
Loss of Gli2 leads to a selective loss of specific pituitary cell types
Previous studies reported a variable loss of the pituitary (3/6) in Gli2 mutants when examined at E12.5 (Park et al., 2000). To determine how Gli2 mediates Shh signaling to influence pituitary development, we examined patterning of the pituitary gland and the production of different pituitary cell types in wild type (WT) and mutant embryos.
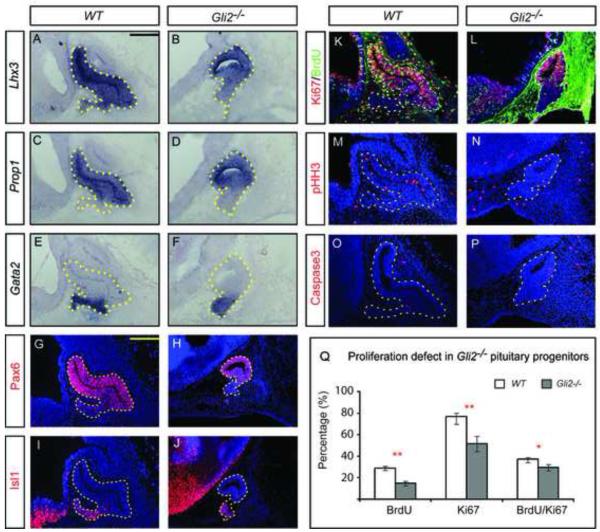
We first examined the expression of two markers that are normally expressed throughout the E12.5 pituitary pouch, Lhx3 and Prop1. Lhx3 encodes a LIM-homeodomain transcription factor that is required for specification of pituitary cell lineages (Sheng et al., 1996) and Prop1 encodes a paired-like homeodomain transcription factor required for initial determination of the Pit1 (Poulf1)-expressing lineage of the pituitary (Sornson et al., 1996). In Gli2 mutants, both Lhx3 and Prop1 were found throughout the pituitary gland, suggesting that early pituitary cells were specified normally (Fig. 1A-D). We then examined the expression of markers for different regions of the pituitary pouch. Gata2 encodes for a ventrally restricted transcription factor that is normally expressed in pituitary cells located at the anterior tip of RP, most of which have just exited cell cycle at E12.5 (Dasen et al., 1999; Treier et al., 1998) (Fig. 1E). In Gli2 mutants, Gata2+ cells were found in the anterior tip of the pouch, as was the case in WT embryos (Fig. 1F). We also examined the expression of two other pituitary markers, Pax6 and Isl1. Pax6 is normally expressed in a dorsal-to-ventral gradient and Isl1 is restricted to the pituitary tip at E12.5 (Fig. 1G,I). The expression pattern of Pax6 and Isl1 in Gli2 mutants is similar to the pattern seen in WT embryos (Fig.1H,J). This demonstrates that loss of Gli2 does not affect the overall patterning of the pituitary.
Figure 1.
Gli2 mutant pituitaries have normal patterning but show defects in proliferation. (A-F) RNA in situ hybridization of wild type (WT) and Gli2 mutants using probes of Lhx3, Prop1 and Gata2. (G-J) Immunostaining of Pax6 and Isl1. (K-P) Analysis of proliferation and cell death by immunostaining of Ki67, BrdU, pHH3 and Caspase3 in Rathke’s pouch. Note: The strong background staining in (L) is likely caused by leaky vasculature and elevated levels of mouse endogenous IgG in Gli2 mutants. (Q) Quantification BrdU incorporation, the expression of Ki67 and Caspase3. The percentage of BrdU+, Ki67+, pHH3+, Caspase3+ cells was calculated by dividing the number of the positive cells by the total number of nuclei (Hoechst). All embryos (n=6) were at E12.5. * indicates P<0.05, ** indicates P<0.01. Scale bars: 100 μm.
Although patterning of the Gli2 mutant pituitary is largely normal, most Gli2 mutant pituitaries were found to be smaller than WT pituitaries (Fig.1), suggesting defects in cell proliferation and/or excessive cell death in mutant pituitary tissues. To distinguish between these two possibilities, we examined expression of Ki67, a marker for proliferating cells, BrdU incorporation, a marker for cells in S-phase, and phospho-Histone H3 (pHH3), a marker for cells in M-phase. At E12.5, we detected reductions in the percentage of cells expression Ki67 in Gli2 mutant pituitaries (Fig. 1K,L,Q, P=0.009), BrdU+ cells (Fig. 1K,L,Q, P=0.0006) and cells in M-phase based on pHH3 levels (Fig. 1M,N, P=0.0123). To test if excessive cell death contributed, we examined the expression of cleaved-Caspase3 and found no significant difference between mutant and WT pouches (Fig. 1O,P). Together, these data suggest that loss of Gli2 affects proliferation of pituitary progenitor cells at E12.5.
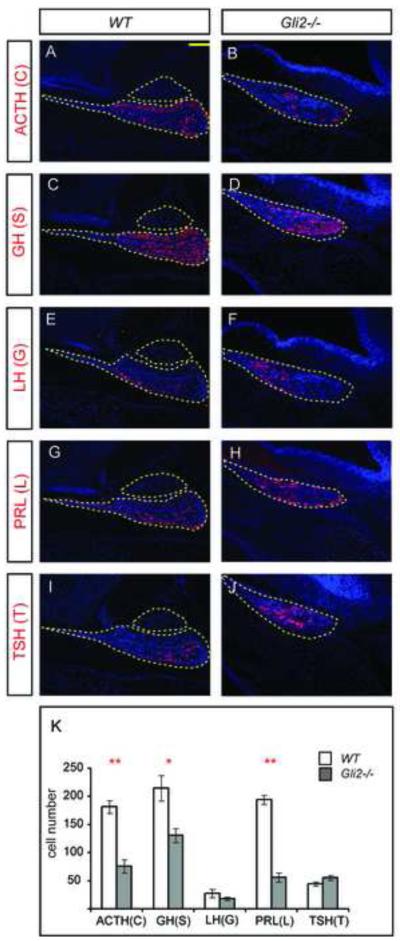
To determine whether loss of Gli2 affects the production of different pituitary cell types, we analyzed the expression of cell-type specific markers at E17.5, a stage when all mature cell types are normally present (Fig. 2A-J). Five hormone-secreting cell types we examined include corticotropes (C), which secrete ACTH; thyrotropes (T), which secrete TSH; gonadotropes (G), which secrete LH; somatotropes (S), which secrete GH; and lactotropes (L), which secrete PRL. We found that all five markers were detected in Gli2 mutant pituitaries (Fig. 2 A-J). However, when the number of different cell types was counted in Gli2 mutants, we found a reduction in the number of C, S and L cells (Fig. 2K), suggesting a requirement for Gli2 for normal generation of these three cell types.
Figure 2.
The number of corticotropes, somatotropes and lactotropes is reduced in Gli2 mutants at E17.5. (A-J) Immunostaining of different pituitary cell type markers ACTH, GH, LH, PRL and TSH at in WT and Gli2 mutant pituitaries. Outlined areas in WT represent anterior and posterior pituitaries. In Gli2 mutants, only anterior pituitary is present. (K) Quantification of each pituitary cell type in WT and Gli2 mutants (* indicates P<0.05, ** indicates P<0.01, n=6 embryos). Two mid-sagittal sections from each embryo were used to quantify the number of different cell type.
Progenitor proliferation in the nascent pituitary requires cell-autonomous expression of Gli2
Several possibilities could account for the proliferation defects in Gli2 mutant pituitaries. First, Gli2 is required cell-autonomously in RP to respond to Shh signal derived from the oral ectoderm, as suggested by previous studies (Treier et al., 1998). Second, Gli2 could be required for cells in RP to respond to diencephalic derived Shh. Third, Gli2 may be required in the diencephalon for the expression of other growth signals, as diencephalic defects were found in Gli2 mutants in our previous study (Park et al., 2000). To distinguish these possibilities, we generated Gli2 mosaic embryos and analyzed mutant cells in an otherwise WT background.
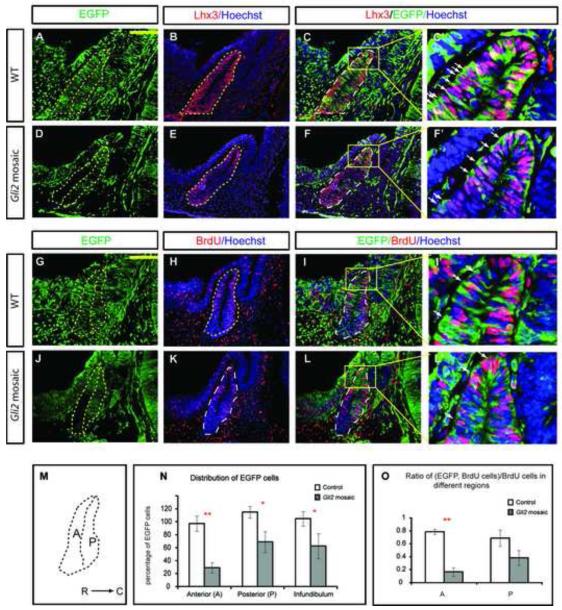
Two different Gli2 alleles, Gli2zfd and Gli2lacZki (Bai and Joyner, 2001; Mo et al., 1997), were used to generate Gli2 mutant embryos. EGFP+ embryos were derived by crossing Gli2lacZki/+ and Gli2zfd/+; EGFP/EGFP mice. The EGFP-labeled embryos were then aggregated with morula stage, un-labeled CD1 embryos in vitro. After overnight incubation, those embryos that had successfully aggregated were transferred into pseudo-pregnant females and allowed to develop. Embryos were then dissected and genotyped with primers for the two Gli2 alleles. Those that contained Gli2zfd, Gli2laczki and WT Gli2 alleles were mosaic embryos containing WT and Gli2 mutant cells while those that contained only Gli2lacZki and WT Gli2 alleles were used as controls. Both control and mosaic embryos appeared to have morphologically normal RP (Fig. 3A-F). To determine whether Gli2 mutant cells can compete with WT cells and adopt a pituitary cell fate, we stained mosaic pituitary with Lhx3 antibody (Fig. 3A,D). We found that all EGFP+ Gli2 mutant cells expressed Lhx3 in mosaic pituitary examined (Fig. 3B,E, n=3), suggesting that Gli2 mutant cells can contribute to pituitary and adopt pituitary cell fate in mosaic embryos (Fig. 3C,C’,F,F’). However, there were fewer EGFP positive cells in mosaic Gli2 pouches (Fig. 3E,K,N) than in controls (Fig. 3B,H). To determine whether GFP+ mutant cells are less likely to be integrated into mosaic pituitaries, we divided RP into two regions, anterior wall (A) and posterior wall (P), along the pouch lumen (Fig. 3M). When we calculated the percentage of GFP+ cells in each division and normalized this number to the level of mosaicism in each embryo (see Materials and Methods), we found mutant cells contributed significantly less to the anterior wall of the pituitary (Fig. 3N, PA= 0.002). Interestingly, mutant cells contributed less to the posterior wall as well, although the reduction was not as dramatic (Fig. 3N, PP=0.031). Lastly, mutant cells were also underrepresented in the infundibulum (Fig. 3N, PI=0.011), suggesting a requirement of Shh/Gli2 signaling in the development of posterior pituitary.
Figure 3.
In mosaics, Gli2 mutant cells do not contribute as well in the anterior edge of the pituitary due to reduced proliferation there. (A-F) Immunostaining of Lhx3 in WT and Gli2 mosaic embryos. (C’,F’,I’,L’) are higher magnification images of corresponding boxes indicated. Note: EGFP is expressed in cytoplasm while Lhx3 and BrdU are present in nuclei. (G-L) Immunostaining of BrdU and EGFP in WT and Gli2 mosaic embryos. EGFP+ cells in WT embryos (A-C, G-I) were Gli2+/+ while EGFP+ cells in Gli2 mosaic (D-F, J-L) were Gli2zfd/lacki. (M) Schematic representation of the subdivisions of the pituitary. R, rostral; C, caudal. (N) Distribution of EGFP+ cells in anterior wall (A) and posterior wall (P) of the anterior pituitary, and in infundibulum. (O) Distribution of BrdU+;EGFP+/BrdU+ cells in different regions of the anterior pituitary (PA<0.01; PP=0.105). See Material and Methods for normalization of the number of EGFP+ cells in each embryo. The anterior pituitary was outlined by dotted lines. White arrowheads indicate cells double-labeled with both green and red. Scale bars: 100 μm. * represents P<0.05, ** represents P<0.01.
One potential reason why there are fewer mutant cells in the anterior wall of the pouch is that mutant cells do not proliferate as well as WT cells. To determine whether this was the case, we counted the number of EGFP+ BrdU+ mutant cells and the total number of unlabeled, BrdU+ S-phase cells (Fig. 3G-L), and calculated the percentage of mutant cells over the total number of S-phase cells in each region (Fig. 3O). Indeed, we found EGFP+ cells were proliferating less in the anterior wall of the pouch (Fig. 3O). There were also fewer proliferating EGFP+ cells in the posterior wall, although the reduction was not as drastic as in the anterior wall of the pouch, suggesting that the reason there were fewer Gli2 mutant cells inside RP is that these is a cell-autonomous requirement of Gli2 for proliferation.
Removal of Gli2 function before the closure of RP causes proliferation defects in some pituitary progenitors
Previous studies have shown that pituitary development is controlled by Shh expressed from the oral ectoderm (Treier et al., 1998; Treier et al., 2001). To determine the critical period of Shh/Gli signaling for pituitary proliferation, we conditionally removed Gli2 function in the pituitary by using a conditional Gli2 allele and two Cre lines, FoxG1Cre (Hebert and McConnell, 2000) and Pitx2Cre (Liu et al., 2003).
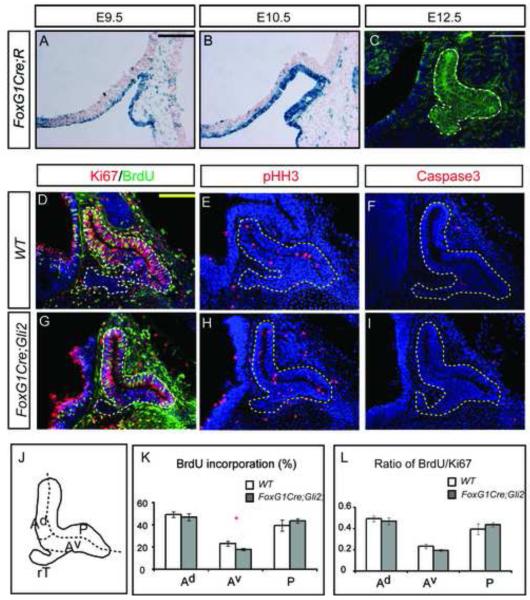
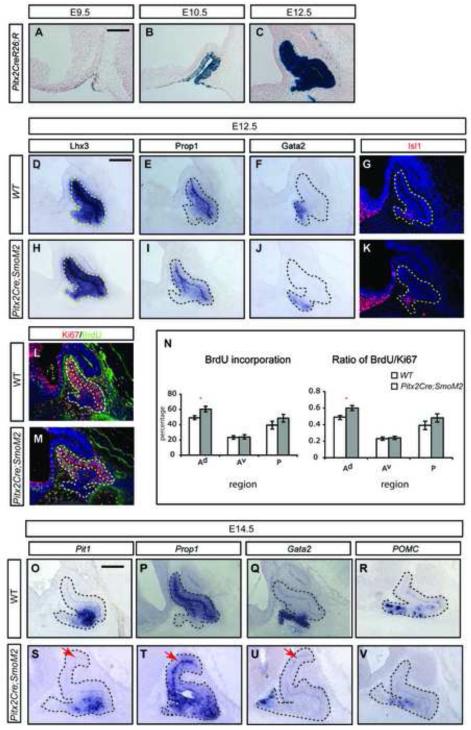
FoxG1 (Forkhead box G1) is initially expressed in the anterior neural ridge and in the early telencephalon (Shimamura and Rubenstein, 1997). To determine whether FoxG1Cre can be used to remove Gli2 function before RP closes, we used FoxG1Cre to recombine a Rosa26-lacZ reporter (R) and monitored the activity of •-galactosidase expressed from the Rosa26-lacZ reporter. We found that at E9.5, all cells encompassing the invaginating RP were positive for •-galactosidase (Fig. 4A). The staining of •-galactosidase within all RP cells was confirmed at E10.5 and E12.5 (Fig. 4B,C), suggesting that FoxG1Cre can be used to disrupt Gli2 function before the closure of RP. As FoxG1 is not expressed in the diencephalon, the manipulation should not affect the normal role of the diencephalon in regulating the growth and patterning of the pituitary.
Figure 4.
Early loss of Gli2 function results in proliferation defects in progenitors in the anterior-ventral pituitary. (A-C) FoxG1Cre activity can be detected before the closure of RP, as detected by X-gal staining at E9.5 (A) and E10.5 (B), and by immunostaining at E12.5 (D-I). Analysis of proliferation and cell death in E12.5 WT and FoxG1Cre;Gli2 conditional mutants. (J) Schematics of sub-divisions of the pituitary. (K,L) Quantification of BrdU incorporation and the ratio of BrdU/Ki67 in different regions of the pituitary. Scale bars: 100 μm.
At E12.5, the pituitaries of conditional Gli2 mutants (FoxG1Cre; Gli2flox/zfd) appeared morphologically normal. Analysis of early pituitary markers, Lhx3, Porp1, Gata2, Pax6 and Isl1, also revealed a pattern of expression resembling that of the WT embryos (Fig. S1A-K), consistent with our results that Gli2 does not control pituitary patterning (Fig. 1). Nevertheless, we reasoned that if Gli2 is required to mediate Shh signaling to control progenitor proliferation before the closure of RP, then we would expect proliferation defects in the FoxG1Cre; Gli2flox/zfd conditional mutants. To this end, we examined the proliferation of conditional mutant cells at E12.5 by analyzing BrdU incorporation and expression of Ki67 and pHH3. Compared with WT embryos, we found no significant differences in the percentage of cells incorporating of BrdU, or cells expressing Ki67 and pHH3 in conditional Gli2 mutants (Fig. 4D,G). Furthermore, removal of Gli2 did not change the pattern of cell death, as there was no significant change in the number of cells expressing Caspase3 (Fig. 4F,I). To determine whether there are regional differences in cell proliferation, we divided the pouch into four regions: anterior (dorsal and ventral, Ad and Av respectively), rostral tip (rT) and posterior (P) (Fig. 4J). At this stage, most of the rT cells had already exited the cell cycle while the rest of the cells in RP were still proliferating. Interestingly we found a decrease in the percentage of BrdU incorporation in the Av region of the FoxG1Cre;Gli2 mutants (Fig. 4K). The reduction in BrdU incorporation appeared to reflect a reduction in proliferation, rather than changes in cell cycle length, as the number of proliferating cells (Ki67+) was also decreased and the ratio of BrdU/Ki67 remained largely unchanged (Fig. 4L).
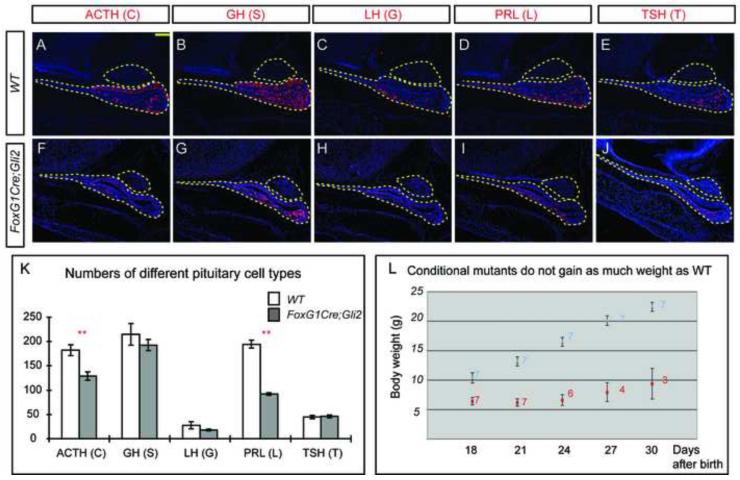
If proliferation of progenitors in the Av region was affected, then we would expect to see a reduction in the number of mature pituitary cells generated from this region. We therefore quantified the number of each mature pituitary cell type in FoxG1Cre;Gli2 mutants at E17.5 (Fig. 5A-J). Consistent with this expectation, we found a smaller number of corticotropes (C) and lactotropes (L) (Fig. 5K), suggesting a requirement of Gli2 in the proliferation and differentiation of their precursors.
Figure 5.
Early loss of Gli2 function results in the reduction of corticotropes and lactotropes at E17.5. (A-J) Immunostaining of different pituitary cell type markers. (K) Quantification of different pituitary cell types in WT and FoxG1Cre;Gli2 mutants (* denotes P<0.05, ** denotes P<0.01, n=6 embryos). (L) FoxG1Cre;Gli2 mutants did not grow as well as WT pups after birth. Seven pups from four litters were tracked for each genotype. The number on each dot indicates surviving pups at that time. Scale bars: 100 μm.
The reduction in the number of pituitary cells could impede normal growth during the early postnatal period. To test whether this is the case, we monitored the growth of FoxG1Cre;Gli2 mutant pups. Compared with WT pups, mutant pups had a lower body weight (Fig. 5L), and even though mutant pups were born alive, most died within 30 days after birth. The growth defects in FoxG1Cre;Gli2 mutants likely reflect disruption of pituitary function, although the possibility that it might also result from loss of Gli2 function in endodermal tissues was not examined.
We also used Pitx2Cre, which is active in RP (Liu et al., 2003) slightly later than FoxG1Cre to disrupt Gli2 function. Pitx2Cre-mediated recombination of Rosa26-lacZ can be detected in a few cells at E9.5 and more cells at E10.5 and E12.5 (Fig. 6A-C). However, when Pitx2Cre was used to remove Gli2 function, we found no obvious defects in patterning or proliferation of mutant pituitaries at E12.5 (data not shown), suggesting that Gli2 is required before Pitx2Cre is active in RP.
Figure 6.
Activation of Hh signaling after the closure of RP resulted in increased proliferation in the anterior-dorsal pituitary. (A-C) X-gal staining revealed the activity of Pitx2Cre from E9.5 to E12.5. (D-I) Analysis of patterning markers in E12.5 WT embryos (D,F,H) and in embryos with activated Hh signaling (Pitx2Cre;SmoM2) using RNA in situ hybridization (D-F,H-J) and immunostaining (G, K). (L, M) Immunostaining of BrdU incorporation and the expression of Ki67. (N) Quantification of BrdU incorporation and Ki67 expression. (* indicates P<0.05, n=6 embryos). (O-V) Activation of Hh signaling did not affect pituitary patterning, as revealed by RNA in situ hybridization of markers at E14.5. Scale bars: 100 μm.
Overlapping roles of Gli genes in pituitary development
Previous studies have shown that the three Gli genes share overlapping and redundant functions (Bai et al., 2004). To address whether Gli2 is the primarily Gli transcription factor to mediate Shh signaling during pituitary development, we analyzed the pituitary phenotypes of Gli1 and Gli3 mutants at E12.5. Both mutants had normal pituitary patterning and proliferation at E12.5 (data not shown), consistent with the notion that Gli2 is the primary Gli transcription factor in the pituitary. We next examined pituitary development in Gli2/Gli3 double mutants, which do not express Gli1 and therefore lose all Gli function (Bai et al., 2004), and found no pituitary in all double mutants examined (n=4). One potential reason for the complete loss of pituitary in Gli2/Gli3 double mutants is that Gli mediated Shh signaling is required in the diencephalon for the initial formation of RP. To address this issue, we generated conditional mutant embryos that lost all Gli function specifically in the pituitary using FoxG1Cre (FoxG1Cre; Gli2flox/zfd;Gli3xt/xt). At E12.5, the pituitary gland in these conditional mutant embryos was smaller. However, when pituitary specific cell markers were used to examine the initial patterning, we found that Lhx3 is expressed normally throughout the pituitary gland (data not shown). In addition, Isl1 showed typical staining in the rostral tip and Pax6 appeared to express in a dorsal-to-ventral pattern, similar to WT embryos at this stage (Fig. S1K,L). These results confirm that early pituitary patterning is not affected by loss of Gli function, and that excessive production of Gli repressor in pitx1-Hhip transgenic embryos, as in Shh mutants, may be responsible for the severe pituitary phenotypes.
Activation of Hh signaling in RP increases pituitary proliferation without affecting patterning
If disruption of Gli function affects pituitary cell proliferation, then we expect that activation of Shh signaling increase pituitary proliferation. To test this, we activated Hedgehog (Hh) signaling using Pitx2Cre and a conditional allele encoding an active form of Smo inserted in the Rosa26 locus (R26SmoM2) (Jeong et al., 2004). At E12.5, Pitx2Cre; R26SmoM2 mutant pouch appeared similar to wild type pouches, as Lhx3, Prop1, Gata2 and Isl1 were expressed normally (Fig. 6D-K). The expression pattern of these markers remained unchanged even when examined at E14.5 (Fig. 6O-V), suggesting that activation of Hh signaling using Pitx2Cre does not alter the general patterning of the pituitary.
We next examined markers for cell proliferation in mutant pituitary pouches. Although there were no significant differences in the number of Ki67+ and pHH3+ cells in the whole pituitary pouch (data not shown), the number of proliferating cells (BrdU+ and BrdU/Ki67) was significantly increased in the Ad region of the pouch (Fig. 6L,M,N). The observation that cells in the dorsal anterior region over proliferate in response to Shh signaling suggests that these cells normally do not receive saturating amounts of Shh. The phenotype became more prominent at E14.5, as Pitx2Cre;SmoM2 mutant pituitaries were much bigger in size than WT controls (Fig. 6O-V). Together, these data support our observation that the primary role of Shh/Gli2 signaling is to control the proliferation, rather than patterning, of pituitary progenitors.
Patterning of pituitary requires diencephalic function of Gli2
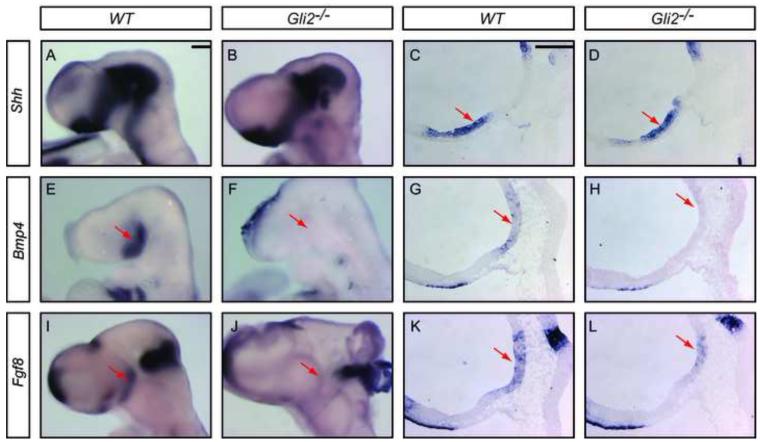
In addition to the partial loss of anterior pituitary as reported previously (Park et al., 2000), we found that all Gli2 mutants had no posterior pituitary (n=43). The complete loss of the posterior pituitary suggests a requirement of Gli2 in ventral diencephalon. As diencephalon-derived signals such as BMP4 and FGF8 are required for induction and proliferation of the anterior pituitary (Ericson et al., 1998; Takuma et al., 1998; Treier et al., 1998), we were curious whether the expression of these two signals was perturbed in Gli2 mutants. We therefore examined the expression of Shh, Bmp4 and Fgf8 in Gli2 mutants using in situ hybridization on E9.5 embryos and on embryo sections (Fig. 7). We found a normal expression of Shh in the ventral diencephalon of Gli2 mutants (Fig. 7A,B). However, while Bmp4 was detected strongly in the medial ventral diencephalon in WT embryos (Fig. 7E), no Bmp4 expression was detected in the ventral diencephalon of Gli2 mutants (Fig. 7F, n=3 embryos). Similarly, while Fgf8 is strongly expressed in the diencephalon of WT embryos (Fig. 7I), the expression in Gli2 mutants was greatly reduced (Fig. 7J, n=3 embryos). Similar results were obtained when in situ hybridization was performed on E9.5 embryo sections (Fig. 7C,D,G,H,K,L, n=7 embryos). Together, these results suggest that Gli2 is required for diencephalic expression of Bmp4 and Fgf8 and that the loss of expression of these two genes in diencephalon may be responsible for the variable pituitary phenotypes in Gli2 mutants.
Figure 7.
Gli2 is required for the expression of Bmp4 and Fgf8 in the ventral diencephalon. (A-L) RNA in situ hybridization of Shh, Bmp4 and Fgf8 in E9.5 embryos (A,B,E,F,I,J) and embryo sections (C,D,G,H,K,L). Red arrows indicate diencephalic expression. Scale bars: 100 μm.
DISCUSSION
Shh signaling is primarily required for proliferation, rather than specification, of early pituitary progenitors
Shh signaling has been shown to be involved in controlling cell fate specification and proliferation in different tissues. In the spinal cord, Shh can induce different types of spinal cord neurons based on the concentration of Shh or the length of action (Dessaud et al., 2008). In the cerebellum, however, the major function of Shh appears to control proliferation of granular precursors (Kenney and Rowitch, 2000). Analysis of Gli mutants and gain-of-function embryos provided further support that Gli transcription factors, in particular Gli2, mediate different functions of the Shh signaling in different tissues. For example, Gli2 mutants do not generate floor plate cells and have only residual V3 interneurons in the spinal cord (Ding et al., 1998; Matise et al., 1998). Similarly, Gli2 mutants have defects in the generation of ventral telencephalic neurons (Yu et al., 2009). On the other hand, Gli2 mutants show reduced proliferation of granular progenitors but no loss of cell types in the cerebellum (Corrales et al., 2004).
Because Shh mutants have severe patterning defects during early embryonic development, the role of Shh signaling in pituitary development has previously only been examined in gain- or loss-of-function transgenic embryos (Treier et al., 2001). Blocking Shh signaling by expressing Shh antagonist Hhip in the oral ectoderm caused loss of anterior pituitary markers Gata2 and brn4 (Pou3f4), and a smaller pituitary. On the other hand, ectopic expression of Shh in the anterior pituitary resulted in the expansion of anterior marker Gata2, increased production of pituitary cells and a bigger pituitary. In both situations, changes in the expression of anterior markers were accompanied by a corresponding change in cell proliferation. Our current study provides several lines of evidence that Shh/Gli signaling functions primarily to promote pituitary cell proliferation. First, the patterning of Gli2 mutant pituitaries is largely normal but the pituitaries have a clear proliferation defect. Second, Gli2 mutant cells are less likely to contribute to the pituitary, in particular to anterior pituitary, because these mutant cells do not proliferate as well as WT cells in mosaic embryos. Lastly, patterning of early pituitary is normal in conditional Gli2 mutants when Gli2 function is disrupted by using FoxG1Cre or Pitx2Cre. Even when both Gli2 and Gli3 are disrupted in the pituitary, cells in RP still express appropriate pituitary markers at E12.5.
Our analysis also revealed a critical window of Shh/Gli signaling in controlling proliferation and differentiation of pituitary progenitors. Removal of Gli2 using FoxG1Cre affects proliferation and differentiation of several pituitary progenitors. These effects are direct results of loss of Gli2 function in RP, as diencephalic expression of Bmp4 and Fgf8 is not altered in these embryos (Supplementary Figure S2). Additional removal of all Gli function in the pituitary have a more drastic effect, as FoxG1Cre;Gli2;Gli3 mutant pituitary glands are much smaller than FoxG1Cre;Gli2 pituitary glands. However, removal of Gli2 function using Pitx2Cre, which becomes active slightly later that FoxG1Cre, does not appear to have obvious effect on patterning or proliferation of the pituitary progenitors. Nevertheless, ectopic activation of Shh signaling using Pitx2Cre causes massive over-proliferation of all pituitary progenitors of the pituitary, suggesting that although Shh/Gli signaling is not required at this stage, pituitary progenitors are still responsive to ectopic Shh signaling. It further suggests that Shh signaling must be tightly regulated even at this later phase.
Although Hedgehog signaling is largely conserved across species, clear differences exist as to how Hh/Gli signaling regulates pituitary development in mouse and fish. In mouse, Gli2 functions as the major activator and is required for pituitary development (Park et al., 2000), while Gli1 and Gli3 are dispensable, although Gli1 is redundant in function with Gli2. Furthermore, conditional removal of Gli2 or all Gli function in pituitary reveals that Hh/Gli signaling controls proliferation, rather than patterning, or early pituitary progenitors. Our analysis also reveals that Hh/Gli signaling controls diencephalic expression of Bmp4 and Fgf8, which have been shown to control early pituitary patterning and proliferation. On the other hand, zebrafish dtr/gli1 is required for normal development as dtr/gli1 null mutants have a pituitary phenotype including a reduced expression of pituitary markers nk2.2 and Lim3, and reduction in the number of somatotropes, lactotropes and anterior corticotropes (Devine et al., 2009; Karlstrom et al., 2003). Blocking the function of yot/gli2 results in minor pituitary phenotypes as the number of corticotropes is reduced. However, blocking the function of both dtr/gli1 and yot/gli2ab function results in a significant reduction in almost all anterior pituitary cell types (Devine et al., 2009), a phenotype that is more severe than any single or compound mouse Gli mutants. It is possible that the differences observed are species specific, as the morphogenetic process of pituitary formation in zebrafish is distinct from that of the mouse or chick in at least two aspects. First, the zebrafish pituitary primordium is formed as a solid structure at the anterior edge of the head, which then moves caudally into the head in a process that is different from that in chick or mouse. Second, the formation of zebrafish pituitary gland does not involve a structure equivalent to Rathke’s pouch and the invagination of oral/placodal ectoderm (Glasgow et al., 1997; Herzog et al., 2003). In addition, there are differences in the transcriptional regulation of Gli genes in mouse and fish, as mouse Gli2 is not regulated by Hh signaling (Bai et al., 2002) while the zebrafish dtr/gli1 appears to be regulated by Hh signaling (Devine et al., 2009). Alternatively, the more severe pituitary phenotype in zebrafish could also result from knockdown of gene function in both pituitary and diencephalon.
Shh/Gli2 mediated diencephalic signal is required for pituitary development
Our analysis of pituitary-specific Gli mutants revealed that blocking of Shh signaling within pituitary progenitors reduces proliferation and differentiation of pituitary progenitors but does not significantly affect patterning of the pituitary gland. But if that is the case, why would half of the Gli2 mutants lack a pituitary? The answer may be in the ventral diencephalon. Starting from E8.0, Shh is strongly expressed in the ventral diencephalon, immediately above the forming RP. Our previous analysis identified loss of ventral diencephalic tissues and reduced expression of Nkx2.1 in some Gli2 mutants. As ventral diencephalon is an important tissue in the induction of RP, we examined the expression of diencephalic marker genes between E8.5 and E9.5. We found that Gli2 mutants had reduced expression of Bmp4 and Fgf8, which are growth factors shown to be critical for the development of a definitive pouch (Takuma et al., 1998; Treier et al., 1998). Our study therefore identified two requirements for Shh/Gli signaling that are critical for pituitary progenitor development: one within RP to promote proliferation of pituitary progenitors and a second role to control expression of Bmp4/Fgf8 in the ventral diencephalon to control early patterning of RP. Future work will be needed to determine whether the Gli2 transcription factor can bind directly to Bmp4/Fgf8 regulatory elements to control their expression in the ventral diencephalon.
Supplementary Material
Acknowledgements
We thank Drs. M. Rosenfeld and S. Camper for providing in situ probes, W. Jiang of CWRU transgenic facility for performing the embryo aggregation experiments, Dr. P. Conrad for microscope assistance and Drs. R. Atit, K. Molyneaux and R. Conlon for comments and suggestions. This study was supported in part by a CWRU fund (CBB) and an NIH R01 HL093484 (JFM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–72. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–15. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Camper SA, Suh H, Raetzman L, Douglas K, Cushman L, Nasonkin I, Burrows H, Gage P, Martin D. Pituitary gland development. In: Rossant J, Tam PPL, editors. Mouse Development, Patterning, Morphogenesis, and Organogenesis. Academic Press; San Diego: 2002. [Google Scholar]
- Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development. 2004;131:5581–90. doi: 10.1242/dev.01438. [DOI] [PubMed] [Google Scholar]
- Dasen JS, O’Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–98. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, Krauss S, Beddington RS, Robinson IC. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–33. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–60. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- Devine CA, Sbrogna JL, Guner B, Osgood M, Shen MC, Karlstrom RO. A dynamic Gli code interprets Hh signals to regulate induction, patterning, and endocrine cell specification in the zebrafish pituitary. Dev Biol. 2009;326:143–54. doi: 10.1016/j.ydbio.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–43. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Karavanov AA, Dawid IB. Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol. 1997;192:405–19. doi: 10.1006/dbio.1997.8761. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–51. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, Sasaki H, Schier AF. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130:1549–64. doi: 10.1242/dev.00364. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–67. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–85. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–70. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Jain MD, Balmer CW, LaMantia AS. High-resolution mapping of the Gli3 mutation extra-toes reveals a 51.5-kb deletion. Mamm Genome. 2002;13:58–61. doi: 10.1007/s00335-001-2115-x. [DOI] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–23. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Nemeskery A, Nemeth A, Setalo G, Vigh S, Halasz B. Cell differentiation of the fetal rat anterior pituitary in vitro. Cell Tissue Res. 1976;170:263–73. doi: 10.1007/BF00224303. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Pfaffle RW, DiMattia GE, Parks JS, Brown MR, Wit JM, Jansen M, Van der Nat H, Van den Brande JL, Rosenfeld MG, Ingraham HA. Mutation of the POU-specific domain of Pit-1 and hypopituitarism without pituitary hypoplasia. Science. 1992;257:1118–21. doi: 10.1126/science.257.5073.1118. [DOI] [PubMed] [Google Scholar]
- Roessler E, Du YZ, Mullor JL, Casas E, Allen WP, Gillessen-Kaesbach G, Roeder ER, Ming JE, Ruiz i Altaba A, Muenke M. Loss-of-function mutations in the human GLI2 gene are associated with pituitary anomalies and holoprosencephaly-like features. Proc Natl Acad Sci U S A. 2003;100:13424–9. doi: 10.1073/pnas.2235734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Jr., Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–7. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–18. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–33. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Dattani MT, Brickman JM, McNay D, Warne G, Zacharin M, Cameron F, Hurst J, Woods K, Dunger D, Stanhope R, Forrest S, Robinson IC, Beddington RS. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet. 2001;10:39–45. doi: 10.1093/hmg/10.1.39. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–86. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfaffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, 3rd, Rosenfeld MG. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet. 1998;18:147–9. doi: 10.1038/ng0298-147. [DOI] [PubMed] [Google Scholar]
- Yu W, Wang Y, McDonnell K, Stephen D, Bai CB. Patterning of ventral telencephalon requires positive function of Gli transcription factors. Dev Biol. 2009;334:264–75. doi: 10.1016/j.ydbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87:933–63. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.