Abstract
Background
Increased arterial stiffness has been shown to be associated with aging and cardiovascular risk factors. Speckle-tracking algorithms are being used to measure myocardial strain. We evaluated if speckle-tracking could be used to measure carotid arterial wall strain (CAS) reproducibly in healthy volunteers and then examined if CAS was lesser in individuals with diabetes.
Methods
Bilateral electrocardiography-gated ultrasound scans of the distal common carotid arteries [D-CCA] (3 cardiac cycles, 14 MHz linear probe, mean 78.7 [Standard deviation (SD) 8.9]) frames per second were performed twice (2–4 days apart) on 10 healthy volunteers to test repeatability. Differences in CAS between healthy (n=20) and diabetic subjects (n=21) were examined. Peak CAS was measured in each of 6 equal segments and averages of all segments (i.e., global average), of the 3 nearest the probe, and of the 3 farthest from the probe (i.e., far wall average) were obtained.
Results
Global CAS (intraclass correlation coefficient [ICC]=0.40) and far wall average (ICC=0.63) had the greatest test-retest reliability. The global and far wall averaged CAS were lower in diabetics (4.29% [Standard Error (SE) 0.27%]; 4.30% [SE 0.44%], respectively) than in controls (5.48% [SE 0.29%], p=0.001; 5.58% [SE 0.44%], p=0.003, respectively). This difference persisted after adjustment for age, gender, race, and hemodynamic parameters.
Conclusions
Speckle-tracking for measuring carotid arterial wall strain is feasible and modestly reliable. Diabetic subjects had a lower carotid arterial wall strain obtained with speckle-tracking when compared with healthy controls.
Introduction
Arterial stiffness, a mechanical property of the arterial wall, has been shown to be associated with cardiovascular risk factors and cardiovascular events. (1–7). Local arterial stiffness can be estimated using calculations based on changes in arterial diameter i.e. arterial distension during the cardiac cycle (4).
Echo-tracking devices have emerged as the most popular method for measuring vessel distension because of their higher resolutions (on the order of 0.001 mm) (8). However, echo-tracking is sensitive to tissue motion in directions other than that of the ultrasound insonation and to interference from other reflectors (e.g., speckles) within the arterial wall (9). Furthermore, the reproducibility of echo-tracking has been shown to be only similar to that of B-mode ultrasound derived changes in diameter (9).
Advances in echocardiography now permit quantification of myocardial stiffness via myocardial strain measures based on speckle-tracking (10). Strain is a percent or fractional measure of the spatial deformation of an object relative to its original size and has been validated in animal models as a surrogate measure of myocardial stiffness (10, 11). Speckle tracking is angle independent, has been shown to reduce error in myocardial strain measurements over tissue Doppler imaging, and hence may offer significant advantages (12, 13). We aimed to adapt the myocardial speckle-tracking technology and evaluate its feasibility for measuring carotid arterial strain.
We hypothesized that we could: a) measure carotid arterial strain reproducibly using the speckle-tracking technique; and b), that such a technique would detect differences associated with diabetes, a cardiovascular risk factor known to be associated with increased arterial stiffness. We chose to examine circumferential strain as it more closely approximates changes in luminal dimensions than radial strain, which represents change in wall thickness (14).
Methods
Study Population
Diabetic subjects (n=21) were recruited from individuals presenting to the non-invasive cardiac laboratory at the Ben Taub General Hospital (Houston, TX) for routine transthoracic echocardiography. A diagnosis of diabetes was confirmed using criteria from the American Diabetes Association 2010 Guidelines or active use of insulin and/or oral hypoglycemic therapies with a historical diagnosis (15). Healthy individuals without any previously known cardiovascular risk factors (n=20) were also recruited as a control comparison group (Table 1). All volunteers were recruited on presentation to the non-invasive cardiac laboratory, and thus were not asked specifically to refrain from food, caffeine, alcohol, tobacco, or vasoactive medications prior to the study. This scenario was considered to represent conditions encountered in a clinical setting.
Table 1.
Clinical characteristics of volunteers for the diabetic vs. control carotid strain comparison*
| Variable | Controls (n = 20) | Diabetics (n = 21) | p-value |
|---|---|---|---|
| Age (years) | 56.6 (1.9) | 57.0 (1.8) | 0.88 |
| Male (%) | 50.0 | 47.6 | 0.88 |
| Caucasian (%) | 30.0 | 28.6 | 0.92 |
| African-American (%) | 30.0 | 33.3 | 0.82 |
| Hispanic (%) | 25.0 | 28.6 | 0.80 |
| Asian (%) | 15.0 | 9.5 | 0.59 |
| Systolic BP (mm Hg) | 120.8 (2.7) | 125.7 (4.5) | 0.64 |
| Diastolic BP (mm Hg) | 73.8 (1.6) | 75.2 (2.6) | 0.86 |
| Pulse Pressure (mm Hg) | 47.0 (1.5) | 50.5 (2.9) | 0.68 |
| Heart Rate (bpm) | 63.3 (2.3) | 77.6 (2.3) | 0.0001 |
| Hypertensive (%) | 0 | 76.2 | <0.0001 |
| Antihypertensive Medication (%) | 0 | 85.7 | <0.0001 |
| Current Smoker (%) | 0 | 23.8 | 0.02 |
| Past Smoker (%) | 30.0 | 23.8 | 0.65 |
All values are means or proportions (SE). Proportions were compared between controls and diabetics using two-sided tests of proportions; continuous variables were compared between controls and diabetics using two-sided Student’s t-test or Wilcoxon rank sum test as appropriate.
BP = blood pressure
Exclusion criteria included age <18 years, history of carotid stenting or carotid endarterectomy, history of radiation therapy to the neck, or a life expectancy less than 6 months. The study was approved by the institutional review board of the Baylor College of Medicine and the Harris County Hospital District. Written consent was obtained prior to study entry. All coronary heart disease risk factors as defined by the Adult Treatment Panel III guidelines (16) were noted for each subject.
Acquisition of Ultrasound Images
All subjects were interviewed and scanned by the same study investigator. The subjects were positioned supine with elevation of the head of the bed at a 30° incline. Electrocardiography (ECG)-gated B-mode bilateral carotid artery ultrasound scans were obtained (carotid artery pre-sets, Vivid 7 echocardiography machine; General Electric Healthcare, Waukesha, WI) over 3 cardiac cycles at 14 MHz with an M12L vascular transducer. Images were acquired at a mean 78.7 frames per second (fps) (SD 8.9 fps) and at a dynamic range of 72 dB. The gain was set to just below the presence of echoes in blood. The probe marker was always oriented to the left of the patient, regardless of the side imaged.
The probe was placed anteriorly over the carotid artery, and image sweeps from the proximal common carotid artery (CCA) to the carotid bifurcation were used to survey the CCA anatomy in transverse (cross-section). The transverse distal CCA, approximately one centimeter inferior to the carotid bulb, was used for our analysis. Subjects were instructed to perform a breath hold maneuver and to refrain from swallowing during the acquisition. The presence of significant motion artifact was noted on each scan, since motion would interfere with speckle-tracking and thus measured strain values. If motion artifacts occurred in a scan, image acquisition was repeated for that view when possible. Images were exported in Digital Imaging and Communications in Medicine (DICOM) format for offline analysis with an anonymization code unique to each subject.
Speckle-tracking Strain Analysis
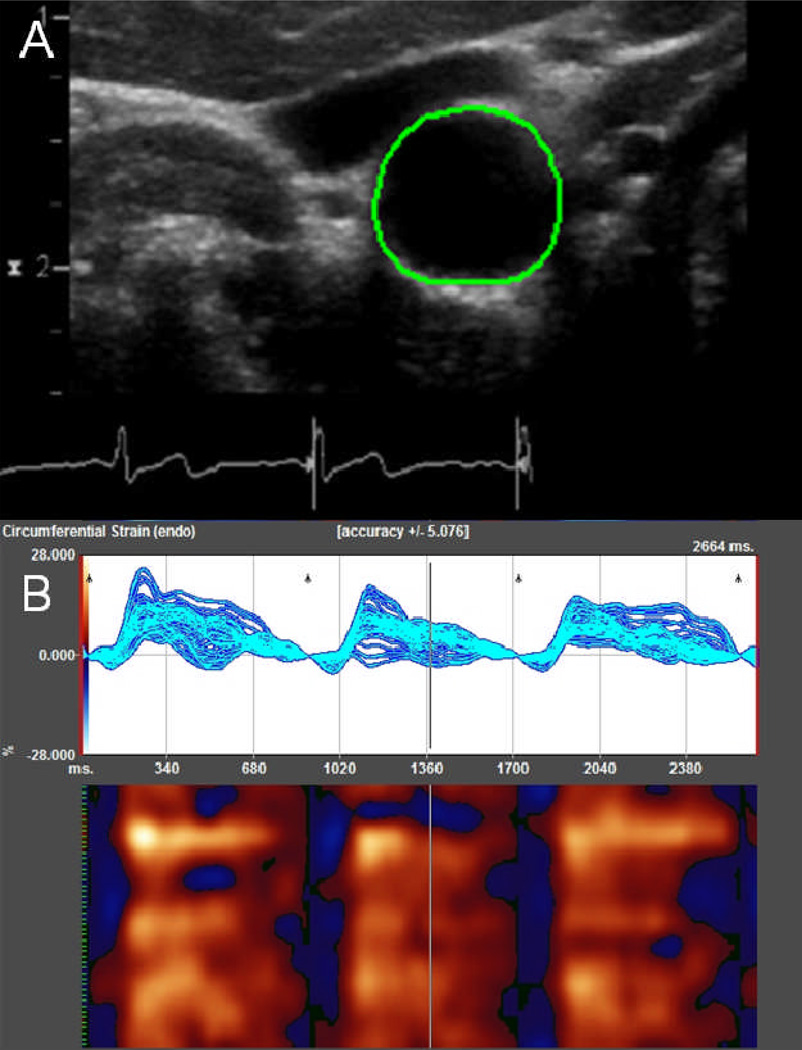
Ultrasound images were uploaded to a computer workstation with a software package designed to measure myocardial strain using speckle-tracking algorithms (2D Cardiac Performance Analysis, TomTec, Munich, Germany). TomTec provided the analysis software but otherwise had no input into the study design, data accrual, analysis, or manuscript preparation. On images of the transverse distal CCA, the image frame at the end of electrical diastole (start of QRS wave) was identified, and the vessel on this frame was manually marked with a contour at the lumen-intima boundary (Figure 1A). The analysis tool was then run to obtain strain data.
Figure 1. Measuring circumferential carotid arterial strain using speckle-tracking.
Contour generated after manual marking of intima-luminal border of the right distal common carotid artery (A). Strain values depicted by software system over 3 cardiac cycles (B). The top graph shows the change in circumferential strain over time at each of the 48 points along a closed loop contour. The graph below it illustrates a color map representation, with each of the 48 points represented on the y-axis; time, on the x-axis; red, positive strain values; and blue, negative values.
The speckle-tracking algorithm divided a manually marked contour into 48 equidistant points and tracked the vessel intimal wall using a cross-correlation velocity vector imaging algorithm, which was accelerated with a Fast Fourier transform function and optimized for myocardial strain analysis (17). In principle, the algorithm would enable tracking of the smallest strain corresponding to the motion of a fraction of the US wavelength (on the order of microns) (18). Strain was calculated with reference to the end of electrical diastole of each cardiac cycle (Figure 1B). The raw data were exported to Excel (Microsoft, Redmond, WA) spreadsheets for further processing.
Post-processing of Speckle-tracking Strain Analysis
Since a myocardial analysis tool was used, the six segment convention of the myocardial short-axis views was adopted for identification of vessel wall segments (19). Anterior regional segments were identified as near wall segments; inferior regional segments, as far wall segments. The septal segment was identified as medial for the right CCA and lateral for the left, since the probe marker always pointed to the left, and similarly the lateral segment was identified as lateral for the right CCA but medial for the left. The anterior segment and the inferior segment themselves were identified as mid segments (i.e., near wall, mid; far wall, mid, respectively).
For each arterial wall segment, the peak strain value (i.e., the most positive value) was determined for each of the 3 cardiac cycles, and the mean peak strain value was calculated. With this method, the circumferential strain values for the 6 segments corresponding to myocardial segments on short-axis views were obtained.
Superficial probe pressure may affect vessel wall segments near the probe. We observed medial-lateral echo dropout in our images (Supplemental Figure 1), and the reliability of strain measurements has been shown in phantom models to be lower in medial-lateral segments, likely due to heterogeneity in the density of ultrasonic lines/ echo dropout that can lead to unreliable strain measurements when the US beam is not aligned with direction of strain (20, 21). Thus, machine settings were optimized to achieve frame rates not too low to result in errors in lateral displacement and not too high to result in a decreased number of ultrasound scan lines. Further, we opted, a priori, to use the far wall as the primary segment for analysis. We also examined the global net average of all segments for comparison with traditional luminal measures. We later tested inter-visit repeatability and intra- and inter-visit reproducibility of the strain measures in each segment and of their averages.
Distensibility Measurement
For comparison of speckle-tracking derived strain with US based distensibility measurements, we measured the minimum and maximum of mean arterial diameters along 1 cm of the distal CCA on longitudinal views with an analysis system (Carotid Analyzer, Medical Imaging applications, LLC, Coralville, IA), which has been shown to have a mean difference of 0.02 pixel (95% confidence interval −2.98 to 3.02 pixels) between repeat measurements (22). Luminal strain was calculated by taking the difference between the two and dividing the minimum of mean arterial diameters. This method differs from previous methods that have used M-mode assessments of the carotid artery or aorta, by assessing diameters over a length of the CCA instead of a single beam axis.
Statistical Methods
Statistical analyses were performed using STATA 11 (STATA, College Station, TX). The Shapiro-Wilk test was used to determine non-normality of the data. Appropriate parametric and non-parametric tests (e.g., two-tailed two sample t-tests and Wilcoxon rank-sum tests) were employed to compare the characteristics and strain measures between the groups of diabetic and healthy controls. Values for each side were examined separately and as an average. Regression models were then used to adjust for covariates in the side-averaged strain comparisons (i.e., mean of left and right measures) with normalization of the dependent and predictor variables as needed. Two models were examined adjusting for participant characteristics and hemodynamic parameters since these may account for variations in carotid arterial strain, and both models included age, race, gender, heart rate, current smoking, and past history of smoking. The first model added systolic and diastolic blood pressures as covariates, and the second model added pulse pressure. These analyses were conducted with and without exclusion of image scans having motion artifacts.
Evaluation of Reproducibility
A separate set of healthy volunteers (n=10; mean age 34.1 [SE 3.2] years, 5 males, 5 females; 20 carotid arteries total) underwent US imaging as described in the above sections to allow testing for reproducibility. After a baseline scan, all the volunteers returned 2–4 days later for repeat imaging at approximately the same time of the day as the baseline scan. All carotid artery wall segments (e.g., lateral, mid, and medial segments of the near and far wall regions) were analyzed. One reader conducted the speckle-tracking strain analysis on the images acquired at both visits for inter-visit reproducibility analyses and repeated the analysis for the first visit for the intra-observer repeatability analyses. Another reader analyzed the same set of images for the inter-observer reproducibility analyses. Intraclass correlation coefficients (ICC[2,1]) and coefficient of variations were used to assess inter-visit repeatability and intra- and inter-reader reproducibility (23). Bland-Altman plots were also used to assess for bias (24).
Results
Reproducibility Analyses
Given the potential influence of heart rate and pulse pressure on strain values, we tested repeatability with and without consideration of these hemodynamic parameters. When segmental carotid arterial strain (CAS) values were examined for significant inter-visit differences, all wall segmental CAS and their averages showed no significant difference between visits (p >0.05). This trend persisted after accounting for heart rate and pulse pressure (Supplemental Table 1).
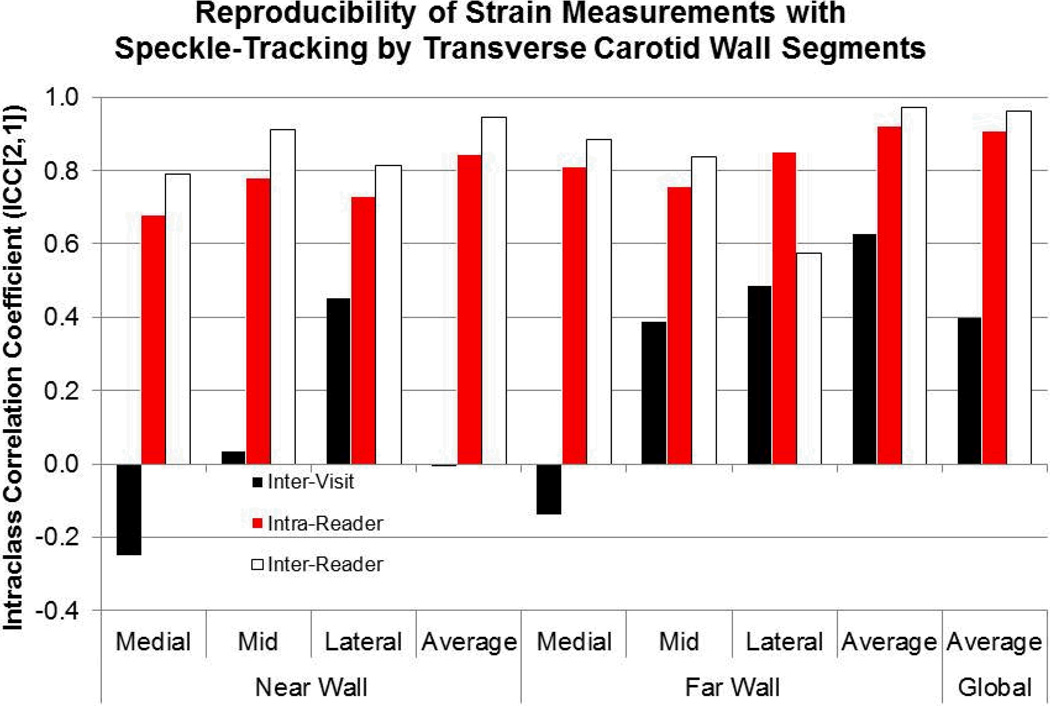
For the primary repeatability study, test-retest analysis showed the greatest correlation for the far wall segments (ICC=0.63) and the global net averaged strain of all segments (intraclass correlation coefficient [ICC]=0.40) (Figure 2). The inter-visit coefficients of variation (CV) for the averages of the far wall segments and of all segments were 27% and 26%, respectively. When the strain to heart rate-pulse pressure product (i.e. heart rate times pulse-pressure) ratio was used, test-retest analysis showed the highest correlation again for far wall average (ICC=0.59) (Supplemental Table 2). These observations supported our a priori decision to examine the average strain of the 3 far wall segments for our primary analysis.
Figure 2. The reproducibility of carotid arterial strain measures using speckle-tracking.
Test-retest of image acquisition, intra-reader repeatability, and inter-reader reproducibility by intraclass correlation coefficients (ICC) type (2,1) of carotid arterial strain measures by arterial wall segments on a separate set of volunteers (n=10, 20 carotid scans).
Intra- and inter-reader reproducibility was assessed for each segment, with agreement given as intraclass correlation coefficients (ICC’s). The highest agreements for within-reader (intra-reader) and between-reader (inter-reader) correlations were again observed for the far wall average and the net average of all arterial wall segments (Figure 2). The intra-reader CV for these averages were 15% and 12%, respectively; and the inter-reader CV, 14% and 10%, respectively. Bland-Altman plots showed no systematic bias for all reproducibility measures and reasonable intra-reader and inter-reader limits of agreements (Supplemental Figure 2, Supplemental Table 3).
Comparison of Diabetics and Healthy Controls
The mean age of all individuals (diabetics and healthy volunteers) was 56.8 years. As a whole, the group consisted of 49% males and 29% Caucasians; had mean systolic, diastolic, and pulse pressures of 123.3 (SE 2.7) mm Hg, 74.5 (SE 1.6) mm Hg, and 48.8 (SE 1.7) mm Hg, respectively; and had a mean heart rate of 70.6 (SE 1.9) bpm (Table 1). No significant differences between the two groups were noted in baseline characteristics, except that diabetics were more likely to be hypertensive, on antihypertensive medications, current smokers and to have higher heart rates than controls (Table 1). Of the 21 diabetics, 9 were on beta-blockers, 2 on non-dihydropyridine calcium channel blockers, and 1 on amiodarone. No controls were on medications that affect heart rate. Despite repeated acquisitions, motion artifact was still present in scans of the right carotid artery in two diabetic volunteers. When the two individuals with motion artifacts were excluded, the baseline differences between the two groups remained similar.
Some participants (diabetics n=7, controls n=2) had atherosclerotic plaques at or above the level of the carotid artery bulb, but none had plaques in the carotid artery segments analyzed. No subject had a history of aortic valve disease, which may influence arterial hemodynamic measurements and potentially strain measurements, or any irregular heart rhythm such as atrial fibrillation.
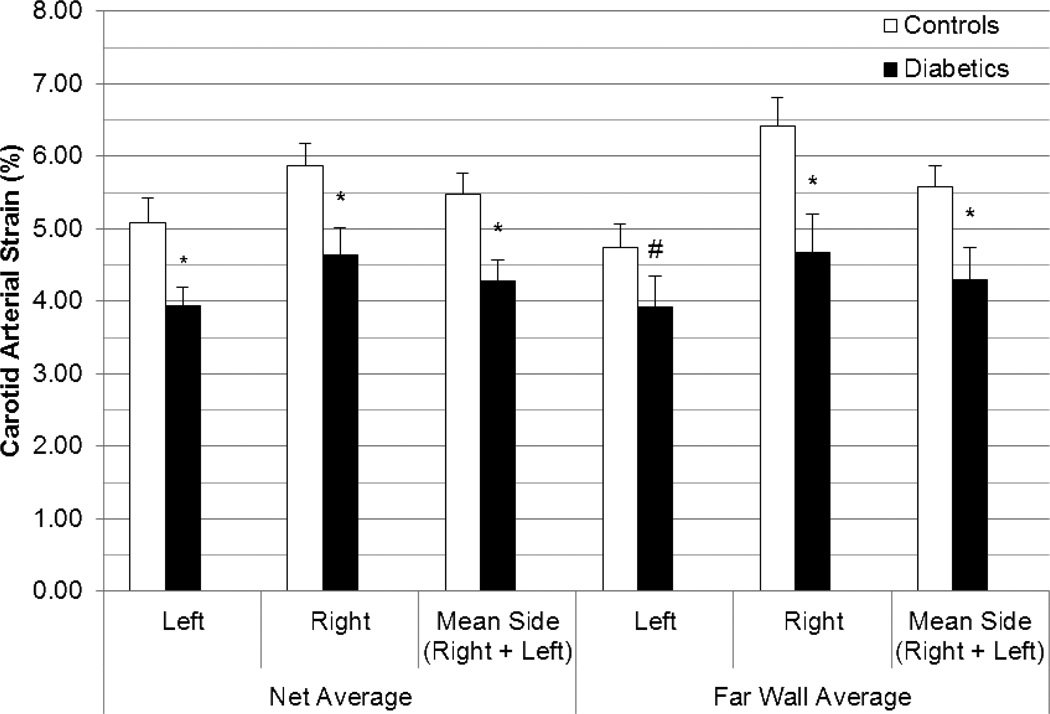
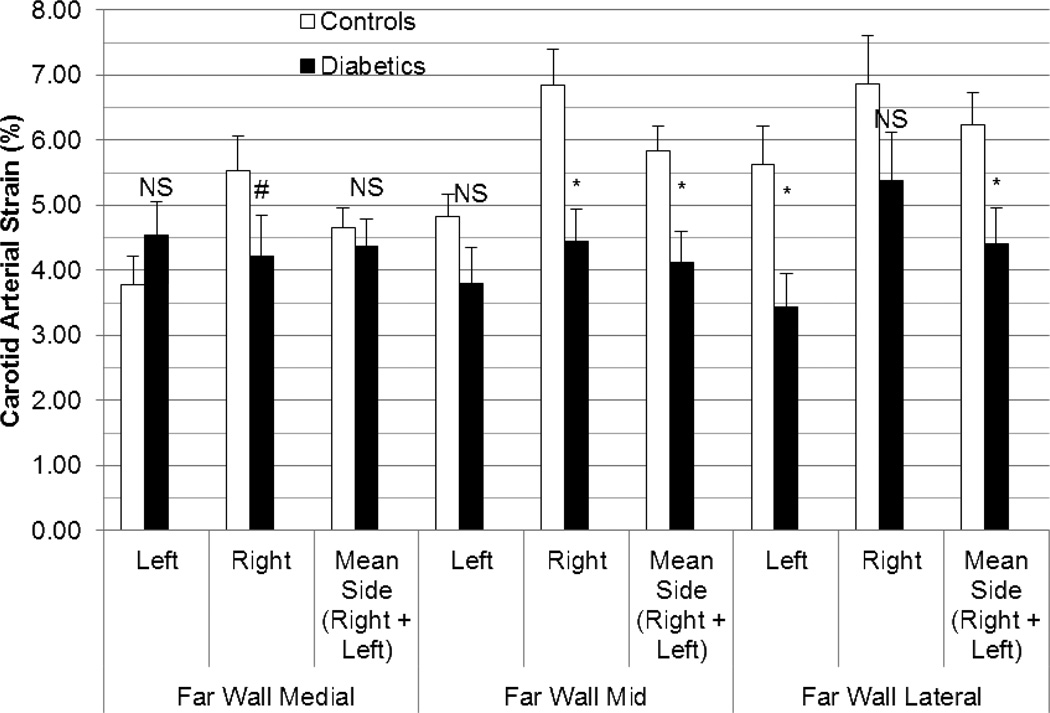
Overall, side-averaged CAS values (i.e., individual mean of left and right measures together) were normally distributed among healthy controls (p >0.05) but positively skewed among diabetics (p <0.01) for all segments and their averages. Therefore, all comparison tests were non-parametric. CAS values were lower in diabetics than in controls for the far wall average (4.30% [SE 0.44%] v. 5.58% [SE 0.29%], p=0.003) and global net average (4.29% [SE 0.29%] v. 5.48% [SE 0.30%], p=0.001) (Figure 3). When each far wall segment was examined, CAS values continued to be significantly lower in diabetics than in controls for the far wall lateral (p=0.004) and mid segments (p=0.0008) but not for the far wall medial segment (p=0.18) (Figure 4). When scans with motion artifact were excluded, side-averaged CAS values continued to remain lower in diabetics (n=19) than in controls (n=20) for the global net average (3.95% [SE 0.18%] v. 5.48% [SE 0.30%], p=0.0001) and for the far wall average (3.78% [SE 0.25%] v. 5.58% [SE 0.29%], p=0.0002).
Figure 3. Comparison of peak circumferential carotid arterial strain in controls vs. diabetics by net average and far wall average values.
Strain values listed are means with standard error bars. Tests of comparison were performed using the Wilcoxon rank sum test. (L=Left, R=Right, # p < 0.05, * p <0.001)
Figure 4. Comparison of peak carotid arterial strain in controls vs. diabetics by medial, mid, and lateral far wall segments.
Strain values listed are means with standard error bars. Tests of comparison were performed using the Wilcoxon rank sum test. (L=Left, R=Right, NS=non-significant, # p < 0.05, * p <0.001)
For the entire group (diabetics and healthy controls), the carotid arterial strain (CAS) values on the left were much lower than on the right for the global net average (p =0.003), far wall average (p=0.0007), and the far wall segments except for the far wall medial segment, which had no difference (p<0.0001 for far wall mid, p=0.005 for the far wall lateral, p=0.33 for the far wall medial) (Figure 3, Supplemental Table 4). After exclusion for the two individuals with motion artifacts, the lower left-sided strain values persisted.
There was no significant difference for CAS values derived from measures of luminal dimensions between diabetics (mean CAS of left and right sides 8.00% [SE 0.55%]) and healthy controls (mean CAS of left and right sides 7.14% [SE 0.51%]; p=0.32), even after exclusion of the 2 participants with motion artifact (diabetic CAS 8.25% [SE 0.58%] v. control CAS 7.14% [SE 0.51%], p=0.16).
Regression analyses were performed on log-transformed values of CAS to normalize the variables. On univariate analysis, diabetic status, current smoking status, heart rate were significant predictors of lower far wall average and global net average strain values; age and blood pressure measurements, notably pulse pressures (Supplemental Figure 3), were not (Supplemental Table 5). After exclusion of scans with motion artifact, this relationship between CAS and predictors persisted (Supplemental Table 5). When the comparisons were adjusted for covariates (Model 1 including age, gender, race, heart rate, current and past smoking, plus systolic and diastolic blood pressures; Model 2 including age, gender, race, heart rate, current and past smoking, plus pulse pressure), diabetic status was no longer significantly associated with speckle-tracking derived CAS values for both models. However, when the two participants with motion artifact were excluded, diabetic status became significantly associated with the global net average strain (p=0.01 for both models) and the far wall average strain (p=0.01 for the first model, p=0.02 for the second model) (Table 2). Overall, these results suggest that diabetes significantly and adversely impacts circumferential carotid arterial strain even after adjustments for covariates.
Table 2.
Linear regression of carotid arterial strain measurement for each wall segment, normalized by log transformation, regressed against diabetic status, adjusted for covariates.* Blood pressures were inverted to normalize the variables.
| Log-transformed CAS measurements | ||||||
|---|---|---|---|---|---|---|
| Beta-coefficient (95% CI) p-value |
Net Avg. | Far Wall Avg. | Lateral Far Wall | Mid Far Wall | Medial Far Wall | |
| Model 1† | Presence of diabetes (without image exclusions) | −0.146 (−0.332, 0.041) p=0.12 |
−0.176 (−0.426, 0.073) p=0.16 |
−0.316 (−0.692, 0.060) p=0.10 |
−0.158 (−0.478, 0.162) p=0.32 |
−0.071 (−0.332, 0.190) p=0.58 |
| Presence of diabetes (with image exclusion) |
−0.226 (−0.405, −0.047) p=0.02 |
−0.284 (−0.521, −0.048) p=0.02 |
−0.470 (−0.840, −0.101) p=0.01 |
−0.182 (−0.508, 0.144) p=0.26 |
−0.190 (−0.440, 0.059) p=0.13 |
|
| Model 2‡ | Presence of diabetes (without image exclusions) | −0.132 (−0.321, 0.057) p=0.17 |
−0.151 (−0.407, 0.106) p=0.24 |
−0.281 (−0.660, 0.099) p=0.14 |
−0.143 (−0.462, 0.176) p=0.37 |
−0.041 (−0.319, 0.238) p=0.77 |
| Presence of diabetes (with image exclusion) | −0.228 (−0.407, −0.050) p=0.01 |
−0.283 (−0.522, −0.044) p=0.02 |
−0.463 (−0.832, −0.095) p=0.02 |
−0.189 (−0.511, 0.133) p=0.24 |
−0.188 (−0.451, 0.075) p=0.16 |
|
Covariates common to both models included age, race, gender, heart rate, current smoking, and past history of smoking.
Model 1 covariates included the common covariates plus systolic and diastolic blood pressures.
Model 2 covariates included the common covariates plus pulse pressure.
Discussion
In summary, we showed that measuring carotid arterial strain (CAS) was feasible and modestly reliable using a novel speckle-tracking based technique and that CAS values were lower in diabetics compared with non-diabetics. Furthermore, we found inter-visit, intra-reader, and inter-reader agreements were consistently higher for the mean CAS of all segments and the far wall segments (Figure 2). The inter-visit ICC suggested moderate reliability based on criteria set forth by Fleiss (0.40 to <0.60 for moderate ICC) whereas the intra-/inter-reader ICC’s were excellent (ICC ≥0.75) (25). CAS values were lower in diabetics versus non-diabetics, and this difference persisted even after adjustments for covariates and appropriate exclusions (Table 2).
Measurements of arterial wall characteristics have become increasingly recognized as possible cardiovascular risk markers in large epidemiologic studies. The elastic property of large arterial vessels depend greatly on the elastin content (organized as elastic lamina) of the arterial media, and to a lesser extent on the collagen content of the vessel (26–28). Both components in turn are produced by the smooth muscle cell population of the same layer (i.e., arterial media) (26–28).
As large arteries age, smooth muscle cells degenerate and decrease in number through apoptosis, and the elastin lamina also degenerate and become fragmented (28). In contrast, the more rigid collagen component has been shown to proportionally increase (28). These processes lead to increased arterial stiffness, a process best labeled with the term arteriosclerosis (27, 28). These observations have been borne out in clinical studies, which have found increased pulse pressures, a marker of arterial stiffness, to increase with age (27, 29). Certain pathologic states (e.g., hypertension through medial hypertrophy; atherosclerosis through foam cell infiltration and plaque deposition) have also been recognized to contribute and potentially to accelerate arterial stiffening (2–5, 27, 28, 30, 31). Similarly, non-insulin dependent diabetes has been postulated to increase proliferation of smooth muscle cells through elevated insulin levels and to lead to advanced glycosylation end-products (AGEs) involving the collagen and elastin of the medial layer through hyperglycemia (32). Therefore, several of the cardiovascular risk factors seem to be associated with arterial stiffness.
Regional stiffness measured using pulse wave velocity (tonometry) has been shown to be associated with incident coronary heart disease (7). Although local carotid artery stiffness measures have shown strong associations with cardiovascular risk factors, their associations with incident cardiovascular events have been weaker/ non-significant (33, 34). Recent results from the Atherosclerosis Risk in Communities Study support a significant association between ultrasound-based measures of arterial stiffness and incident stroke events, independent of established atherosclerotic risk factors (35). Thus, local measures of arterial stiffness may also hold potential as cardiovascular risk markers.
Local arterial stiffness can be estimated non-invasively through the measurement of arterial distension using imaging (e.g., magnetic resonance imaging, ultrasound imaging) (4). Imaging-based distension measurements rely on the detection of changes in arterial dimensions over the cardiac cycle, which can be obtained from lumen-wall border tracking (i.e., lumen size) or from direct wall tracking (4, 36, 37). For ultrasound, direct vessel wall tracking has been accomplished with echo-tracking and more recently with tissue Doppler imaging (TDI) and with speckle-tracking (8, 38–40).
Echo-tracking has been well studied and validated for measuring arterial distension but has its limitations (4, 8). Although Hoeks et al, found the echo-tracking resolution to be less than 0.001 mm in vitro, they also noted the standard deviation of distension measured in a human test subject to be 0.043 mm (8). They attributed this finding to arterial motion and physiological variations, which may be evidence of a threshold beyond which higher resolutions do not improve distension measurements (8). Furthermore, the technology is fundamentally based on M-mode, or single-beam axes, limiting its ability to assess motion assessment in other axial directions (8). Recently, Kawaski et al, used TDI to measure strain rates on longitudinal views of the common carotid artery in patients with coronary heart disease (38). However, the TDI method is limited by angle dependence (38).
We sought to evaluate the application of speckle-tracking for direct assessment of carotid arterial wall strain and are among the first groups to do so (40, 41). Speckle-tracking is a 2-dimensional angle-independent method of tissue-tracking that is used for assessing myocardial stiffness (10, 14, 42). Speckle-tracking tracks the arterial wall, through “acoustic fingerprints,” or persistent speckles specific to a local tissue region and therefore gets direct arterial wall information rather than depending on luminal changes.
Bjallmark et al, employed speckle-tracking analysis of carotid strain in a population of younger (n=10) and older (n=10) individuals and showed increasing age to be associated with lower strain values (39), which our models support. However, Bjallmark et al, acquired images for only the right common carotid artery, did not report on the inter-visit repeatability of their measurements, and examined a smaller population sample. Our work differs by demonstrating the reliability and repeatability of speckle-tracking for measuring CAS in arterial wall segments and furthermore examines CAS in a population known to have stiffer arteries, i.e. diabetics.
Furthermore, we examined CAS measures using a lumen based assessment of distension and found no significant differences between the two groups suggesting that speckle tracking may be more sensitive. Although our lumen-based distension measure was not obtained with echo-tracking, studies by others have shown the reliability of distension measured using echo-tracking and of that using B-mode ultrasound imaging to be comparable (9, 37). Furthermore, our lumen based distensibility measurement evaluates a larger segment of the artery compared with echo tracking.
Finally, we found arterial strain measurements to be significantly higher in the right than in the left carotid artery for the two of the three far wall segments, the average of all three far wall segments, and the net average of all arterial wall segments. Differences in right versus left measurements have been reported in common carotid intima-media thickness measurements, with the right having lesser values than the left (43–45). In fact, in one study of participants with and without migraine headaches, arterial distension was also found to be significantly greater in the right than in the left for both groups of individuals. These findings are consistent with the findings in our study. These observations suggest that there may be differences in blood pressure, shear forces, and vascular anatomy specific in the same individual between the right and the left carotid arteries and merits further investigation.
Clinical perspective
Ultrasound imaging offers a non-invasive and safe method of evaluating the arterial wall. Carotid artery measures of atherosclerosis (namely carotid intima-media thickness and plaque presence) has already been shown to be useful in coronary heart disease risk prediction and as a surrogate marker to test efficacy of anti-atherosclerotic therapy (46, 47). Ultrasound based stiffness measures provide potentially yet another measure to improve our assessment of vascular health. Pulse pressure is often considered a good marker of arterial stiffness (48); and in general, when the arterial stiffness increases, in order to maintain a similar arterial distensibility (strain), the pulse pressure should also proportionally increase.
However, as noted in our analysis, there is a very poor correlation between pulse pressure and CAS as measured by speckle-tracking. The poor correlation between these two measures of stiffness may be explained by the fact that CAS measures only local carotid stiffness while pulse pressure reflects the stiffness of the entire vascular tree. Furthermore, given that the association between CAS and diabetes persisted after adjustment for pulse pressure, one could hypothesize that a simple pulse pressure measurement may not be an adequate surrogate for CAS, and perhaps both provide complementary information on vascular health. Whether local arterial strain reflects the advanced sequelae of pathologic processes or remodeling due to various medical interventions remains a potential area for further clinical investigation. Clearly, much further work will be required before this technology will have any use in routine clinical practice.
Limitations
Our study had limitations. Brachial blood pressures were used instead of local carotid pressures, both of which have been shown to differ from each other and to differ in local remodeling effects within an individual (49, 50). However, it must be noted that in individuals with cardiovascular risk factors such as diabetes, the peripheral pulse pressure tends to be similar to the central pulse pressure while in healthy individuals the central pulse pressure tends to be lower (51). Despite this (i.e. likely greater central pulse pressures which in turn could increase arterial distension/ strain) diabetics in our study had lower arterial strain, suggesting that the arterial strain we measured by speckle tracking also reflects a stiffer arterial wall. The inter-visit intraclass correlation coefficients were modest and may have been due to variations in time from last meal, use of vasoactive substances (e.g., caffeine or alcohol), and diurnal variations. Additionally we used a system intended for myocardial analysis and used DICOM images instead of raw US data. Dedicated strain platforms for arterial wall imaging may further improve the repeatability and reproducibility of arterial strain measurements. We also acquired at higher frame rates than those used for myocardial analyses; however, the effects of higher frame rates on reproducibility (by potentially affecting spatial resolution) of strain measurements are unclear. Strain measurements were not compared with phantom or animal models using sonomicrometry crystals. Still, we tested the technique with a common clinical disease entity known to lead to increased arterial strain to lay the groundwork for the potential clinical vascular applications of the speckle-tracking technique.
The diabetic sample size was small, and we did not examine the association of strain values with the severity or duration of diabetes. We were also unable to adjust for glomerular filtration rates, as serum creatinine levels were not available on all subjects. Acoustic shadowing of the medial-lateral wall segments was observed and may have limited the reliability of strain measures in these segments. We analyzed strain measurements in only the distal common carotid artery, and differences in these measures may be present along the different carotid artery segments. We examined the carotid artery for only circumferential strain; other strain measures including longitudinal and radial strain should also be examined. Lastly, we adapted an ultrasound system and analysis package originally optimized for cardiac use to vascular applications.
Future Directions
The results of this study point to several potential avenues of investigation. Different scanning approaches need to be investigated to see if probe pressure does indeed influence strain measurements. Differences in strain values at different levels of the carotid vasculature should also be examined. Additional data on differences between left versus right sides for not only distensibility measures but also carotid intima-media thickness measures are still needed. Optimization of speckle-tracking systems for use with vascular applications will also be required.
Conclusion
Measurement of carotid arterial wall strain using speckle-tracking is feasible and reliable in the wall segments farthest from the ultrasound probe. These strain measures were significantly lower in diabetics even after adjustment for age, gender, race, and hemodynamic parameters, suggesting that this technique may be more sensitive to changes from early atherosclerosis than previous techniques based on luminal measurements.
Supplementary Material
Acknowledgments
We thank Berthold Klas and TomTec, Munich, Germany, for supplying the speckle-tracking strain analysis software and associated documentation. We thank the participants of the study for their contribution and Joanna Brooks, B.A., for editorial assistance. Dr. Yang was supported by a National Institutes of Health (NIH) / National Heart, Lung, and Blood Institute (NHLBI) T32 HL007812 training grant and an American Heart Association South Central Affiliate Postdoctoral Fellowship grant at different times during this study. Dr. Virani is supported by a Veterans Affairs Career Development Award. Dr. Nambi is supported by a NIH/NHLBI K23 HL096893 grant.
References
- 1.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994 Oct 15;140(8):669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 2.Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease) Circulation. 1999 Aug 31;100(9):951–957. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 3.Urbina EM, Srinivasan SR, Kieltyka RL, Tang R, Bond MG, Chen W, et al. Correlates of carotid artery stiffness in young adults: The Bogalusa Heart Study. Atherosclerosis. 2004 Sep;176(1):157–164. doi: 10.1016/j.atherosclerosis.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006 Nov;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 5.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2008 Aug 15;102(4):491–496. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskinen J, Magnussen CG, Taittonen L, Rasanen L, Mikkila V, Laitinen T, et al. Arterial structure and function after recovery from the metabolic syndrome: the cardiovascular risk in Young Finns Study. Circulation. 2010 Jan 26;121(3):392–400. doi: 10.1161/CIRCULATIONAHA.109.894584. [DOI] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010 Mar 30;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 8.Hoeks AP, Brands PJ, Smeets FA, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16(2):121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 9.Stadler RW, Taylor JA, Lees RS. Comparison of B-mode, M-mode and echo-tracking methods for measurement of the arterial distension waveform. Ultrasound Med Biol. 1997;23(6):879–887. doi: 10.1016/s0301-5629(97)00074-4. [DOI] [PubMed] [Google Scholar]
- 10.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007 Mar;20(3):234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Pislaru C, Bruce CJ, Anagnostopoulos PC, Allen JL, Seward JB, Pellikka PA, et al. Ultrasound strain imaging of altered myocardial stiffness: stunned versus infarcted reperfused myocardium. Circulation. 2004 Jun 15;109(23):2905–2910. doi: 10.1161/01.CIR.0000129311.73402.EF. [DOI] [PubMed] [Google Scholar]
- 12.Sivesgaard K, Christensen SD, Nygaard H, Hasenkam JM, Sloth E. Speckle tracking ultrasound is independent of insonation angle and gain: an in vitro investigation of agreement with sonomicrometry. J Am Soc Echocardiogr. 2009 Jul;22(7):852–858. doi: 10.1016/j.echo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Cho GY, Chan J, Leano R, Strudwick M, Marwick TH. Comparison of two-dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol. 2006 Jun 1;97(11):1661–1666. doi: 10.1016/j.amjcard.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 14.Artis NJ, Oxborough DL, Williams G, Pepper CB, Tan LB. Two-dimensional strain imaging: a new echocardiographic advance with research and clinical applications. Int J Cardiol. 2008 Jan 24;123(3):240–248. doi: 10.1016/j.ijcard.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33 Suppl 1:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–3421. [PubMed] [Google Scholar]
- 17.Vannan MA, Pedrizzetti G, Li P, Gurudevan S, Houle H, Main J, et al. Effect of cardiac resynchronization therapy on longitudinal and circumferential left ventricular mechanics by velocity vector imaging: description and initial clinical application of a novel method using high-frame rate B-mode echocardiographic images. Echocardiography. 2005 Nov;22(10):826–830. doi: 10.1111/j.1540-8175.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 18.D'Hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. 2000 Sep;1(3):154–170. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 19.Douglas PS, Hendel RC, Cummings JE, Dent JM, Hodgson JM, Hoffmann U, et al. ACCF/ACR/AHA/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR 2008 Health Policy Statement on Structured Reporting in Cardiovascular Imaging. J Am Coll Cardiol. 2009 Jan 6;53(1):76–90. doi: 10.1016/j.jacc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Langeland S, D'Hooge J, Claessens T, Claus P, Verdonck P, Suetens P, et al. RF-based two-dimensional cardiac strain estimation: a validation study in a tissue-mimicking phantom. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2004;51(11):1537–1546. doi: 10.1109/tuffc.2004.1367495. [DOI] [PubMed] [Google Scholar]
- 21.Ribbers H, Lopata RG, Holewijn S, Pasterkamp G, Blankensteijn JD, de Korte CL. Noninvasive two-dimensional strain imaging of arteries: validation in phantoms and preliminary experience in carotid arteries in vivo. Ultrasound Med Biol. 2007 Apr;33(4):530–540. doi: 10.1016/j.ultrasmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Sonka M, Liang W, Lauer RM. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Transactions on Medical Imaging. 2002 Oct;21(10):1271–1279. doi: 10.1109/TMI.2002.806288. [DOI] [PubMed] [Google Scholar]
- 23.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979 Mar;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Bland JM. Measurement in Medicine: The Analysis of Method Comparison Studies. J R Stat Soc Ser D Stat. 1983;32(3):307–317. [Google Scholar]
- 25.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- 26.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009 Jul;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safar ME. Arterial aging--hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010 Aug;7(8):442–449. doi: 10.1038/nrcardio.2010.96. [DOI] [PubMed] [Google Scholar]
- 28.Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010 Jul;10 Suppl 1:S213–S220. doi: 10.1111/j.1447-0594.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007 May 22;115(20):2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 30.Arnett DK, Boland LL, Evans GW, Riley W, Barnes R, Tyroler HA, et al. Hypertension and arterial stiffness: the Atherosclerosis Risk in Communities Study. ARIC Investigators. Am J Hypertens. 2000 Apr;13(4 Pt 1):317–323. doi: 10.1016/s0895-7061(99)00281-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Keith JC, Jr, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008 Fall;26(3):214–223. doi: 10.1111/j.1755-5922.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- 32.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995 Mar 1;91(5):1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 33.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006 Feb 7;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 34.Duprez DA, Jacobs DR, Lutsey PL, Bluemke DA, Brumback LC, Polak J, et al. Small artery elasticity, but neither carotid artery elasticity nor aorta distensibility, predicts cardiovascular events in an asymptomatic - Results of the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2010 March 9;55(10) Supplement 1 2010; A133.E1252. [Google Scholar]
- 35.Yang EY, Chambless L, Sharrett AR, Virani SS, Teng Z, Boerwinkle E, et al. Arterial wall characteristics are associated with incident cardiovascular disease: An analysis from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Coll Cardiol. 2010;55(10) Supplement 1 A163.E1529-A163.E. [Google Scholar]
- 36.Godia EC, Madhok R, Pittman J, Trocio S, Ramas R, Cabral D, et al. Carotid Artery Distensibility: A Reliability Study. J Ultrasound Med. 2007 September 1;26(9):1157–1165. doi: 10.7863/jum.2007.26.9.1157. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchini E, Bozec E, Gemignani V, Faita F, Giannarelli C, Ghiadoni L, et al. Assessment of carotid stiffness and intima-media thickness from ultrasound data: comparison between two methods. J Ultrasound Med. 2010 Aug;29(8):1169–1175. doi: 10.7863/jum.2010.29.8.1169. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki T, Fukuda S, Shimada K, Maeda K, Yoshida K, Sunada H, et al. Direct measurement of wall stiffness for carotid arteries by ultrasound strain imaging. J Am Soc Echocardiogr. 2009 Dec;22(12):1389–1395. doi: 10.1016/j.echo.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Bjallmark A, Lind B, Peolsson M, Shahgaldi K, Brodin LA, Nowak J. Ultrasonographic strain imaging is superior to conventional non-invasive measures of vascular stiffness in the detection of age-dependent differences in the mechanical properties of the common carotid artery. Eur J Echocardiogr. 2010 Apr 10; doi: 10.1093/ejechocard/jeq033. [DOI] [PubMed] [Google Scholar]
- 40.Yang EY, Dokainish H, Polsani VR, Virani SS, Ballantyne CM, Nambi V. Measurement of circumferential systolic carotid arterial strain in a healthy population using 2D speckle tracking. J Am Soc Echocardiogr. 2009 May;22(5):588. 2009. [Google Scholar]
- 41.Yang EY, Dokainish H, Virani SS, Polsani VR, Misra A, Pritchett AM, et al. Segmental analysis of circumferential systolic carotid artery strain in diabetics using 2D speckle tracking. J Am Soc Echocardiog. 2010;23(5):B33. [Google Scholar]
- 42.Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez Hernandez SA, Kroon AA, van Boxtel MP, Mess WH, Lodder J, Jolles J, et al. Is there a side predilection for cerebrovascular disease? Hypertension. 2003 Jul;42(1):56–60. doi: 10.1161/01.HYP.0000077983.66161.6F. [DOI] [PubMed] [Google Scholar]
- 44.Onbas O, Dane S, Kantarci M, Koplay M, Alper F, Okur A. Clinical importance of asymmetry and handedness differences in common carotid artery intima-media thickness. Int J Neurosci. 2007 Apr;117(4):433–441. doi: 10.1080/00207450600592230. [DOI] [PubMed] [Google Scholar]
- 45.Foerch C, Buehler A, von Kegler S, Sitzer M. Intima-media thickness side differences are limited to the common carotid artery. Hypertension. 2003 Dec;42(6):e17. doi: 10.1161/01.HYP.0000103164.73172.30. author reply e8. [DOI] [PubMed] [Google Scholar]
- 46.Nambi V, Chambless L, Folsom A, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010 Apr;55(15):1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, et al. Extended-Release Niacin or Ezetimibe and Carotid Intima–Media Thickness. New England Journal of Medicine. 2009;361(22):2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997 Dec 16;96(12):4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 49.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: A validation and repeatability study of a new technique. Journal of the American College of Cardiology. 1992;20(4):952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 50.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association Between Local Pulse Pressure, Mean Blood Pressure, and Large-Artery Remodeling. Circulation. 1999 September 28;100(13):1387–1393. doi: 10.1161/01.cir.100.13.1387. 1999. [DOI] [PubMed] [Google Scholar]
- 51.Waddell TK, Dart AM, Medley TL, Cameron JD, Kingwell BA. Carotid Pressure Is a Better Predictor of Coronary Artery Disease Severity Than Brachial Pressure. Hypertension. 2001 October 1;38(4):927–931. doi: 10.1161/hy1001.096107. 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.