Summary
Androgen ablation therapy is the primary treatment for metastatic prostate cancer. However, this therapy is associated with several undesired side-effects, including increased risk of cardiovascular diseases. To study if termination of long-term androgen ablation and restoring testosterone level may suppress the growth of relapsed hormone-refractory prostate tumors, we implanted testosterone pellets in castrated nude mice carrying androgen receptor (AR)-positive LNCaP 104-R2 cells, which relapsed from androgen-dependent LNCaP 104-S cells after long-term androgen deprivation. 104-R2 tumor xenografts regressed after testosterone pellets implant. 24 out of 33 tumors adapted to elevation of testosterone level and relapsed as androgen-insensitive tumors. Relapsed tumors (R2Ad) expressed less AR and prostate-specific antigen (PSA). We then study the molecular mechanism lying underneath the androgenic regulation of prostate cancer cell proliferation. Androgen suppresses proliferation of 104-R2 by inducing G1 cell cycle arrest via reduction of Skp2 and c-Myc, and induction of p27Kip1. 104-R2 cells adapted to androgen treatment and the adapted cells, R2Ad, were androgen-insensitive cells with slower growing rate and low protein level of AR, high levels of c-Myc and Skp2, and low levels of p27Kip1. Nuclear AR and PSA expression is present in 104-R2 cells but not R2Ad cells when androgen is absent. Overexpression of AR in R2Ad cells regenerated an androgen-repressed phenotype, while knockdown of AR in 104-R2 cells generated an androgen-insensitive phenotype. Overexpression of Skp2 and c-Myc in 104-R2 cells blocked the growth inhibition caused by androgens. We concluded that androgens cause growth inhibition in LNCaP 104-R2 prostate cancer cells via AR, Skp2, and c-Myc.
Introduction
Prostate cancer is one of the most common carcinoma of men in western countries. In 1941, Charles Huggins reported that androgen ablation therapy caused regression of metastatic prostate cancer (1). Since then, androgen ablation therapy has become the primary treatment for metastatic prostate cancer (2). However, 80–90% of the patients who receive androgen ablation therapy ultimately develop recurrent tumors in 12–33 months. The median overall survival of patients after tumor relapse is 1–2 years (3, 4). Androgen deprivation therapy is associated with several undesired side-effects, including sexual dysfunction, osteoporosis, hot flashes, fatigue, gynecomastia, anemia, depression, cognitive dysfunction, and increased risk of diabetes and cardiovascular diseases (2, 5–7). Therefore, shortening the period of androgen ablation therapy may protect the patients.
LNCaP was a commonly used cell line established from a human lymph node metastatic lesion of prostatic adenocarcinoma (8). We previously established relapsed androgen-independent human LNCaP 104-R1 (passage > 80, about 12 months) and 104-R2 cells (passage > 150, about 23 months) from androgen-dependent LNCaP 104-S cells after culturing under long-term androgen-depleted conditions (9, 10). Compared with 104-S cells, 104-R1 and 104-R2 cells express higher AR protein and mRNA (9–11). Up-regulation of AR protein has been observed in many patients with recurrent hormone-refractory tumors (12–14). Proliferation of 104-R1 and 104-R2 cells is not dependent on androgen (i.e. androgen-independent) but is suppressed by physiological concentrations of androgen both in vitro and in vivo (i.e. androgen-responsive) (9–11, 15–17), in part by down-regulation of c-Myc and induction of cyclin-dependent kinase inhibitor p27Kip1, thereby causing G1 cell cycle arrest (9, 10). In this study, we investigated whether AR-positive relapsed prostate tumors in patients receiving long-term (more than three years) androgen ablation therapy might be suppressed by physiological concentration of androgens after termination of the androgen ablation therapy. For this purpose, we used late passage (passage 285) of LNCaP 104-R2 cells, which were derived from androgen-dependent LNCaP 104-S cells after 43 months of androgen deprivation, both in xenograft model and in cell culture to study the effect of androgens on these cancer cells.
Materials and Methods
Cell Culture
LNCaP 104-S and 104-R2 cells were passaged and maintained as described (9–11, 18, 19). R2Ad cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 1nM dihydrotestosterone (DHT), 10% FBS (Atlas, Fort Collins, CO) plus penicillin (100 U/ml) and streptomycin (100 ug/ml;Invitrogen). R1881 (17β-hydroxy-17α-methylestra-4,9,11-trien-3-one) was from Perkin Elmer (Boston, MA). Bicalutamide (Casodex) was from AstraZeneca Pharmaceuticals (Wilmington, DE).
Cell Proliferation Assay
Relative cell number was analyzed by measuring DNA content of cell lysates with the fluorescent dye Hoechst 33258 (Sigma, St. Louis, MO) as described previously(18, 19).
Western Blotting Analysis
Western blots were performed as previously described (16–19). Antibodies were against AR (AN-21 polyclonal rabbit antibody) (9–11), PSA (DAKO Elostrup, Denmark), p27Kip1 (BD Biosciences, Lexington, KY), p21Cip, and Skp2 (Santa Cruz, Santa Cruz, CA), c-Myc (Abcam, Cambridge, MA), and β-actin (Chemicon, Temecula, CA). Measurement of β-actin expression was used as a loading control in all experiments.
Cell Cycle Analysis
Cell cycle distribution analysis of LNCaP 104-S, 104-R2, or R2Ad cells treated with different concentration of R1881 (0, 0.1, 10 nM) for 96 hours was performed as described previously (10, 11, 20).
Preparation of Nuclear and Cytoplasmic Fractions of LNCaP Cells
LNCaP cells were washed twice with cold PBS and incubated with 1 ml of 10 mM Tris HCl pH 7.5, 150 mM NaCl, 1 mM EDTA for 2–3 min. Cells were removed by scrapping and transferred suspension to a 1.5ml tube on ice. Cells were pelleted at 3000 × g at 4°C for 2 min. The cells pellet was resuspended in 0.5 ml of cold cell lysis buffer (50 mM Tris pH 7.5, 5 mM MgCl2, 0.4% Nonidet P40, 1mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin). After gentle mixing, nuclei were centrifuged at 3000 × g for 2 min. Supernatant was collected as the cytoplasmic fraction and resuspended in cell lysis buffer. Nuclei in the pellet were resuspended in 0.5 ml cell lysis buffer and centrifuged at 3000 × g for 2 min. Washed nuclei were resuspended in cell lysis buffer.
Tumor Xenografts in Athymic Mice
Experiments involving mice were approved by the University of Chicago Institutional Animal Care and Use Committee. Male Balb/c nu/nu mice (NCI Frederick, MD), 6–8 weeks of age, were injected subcutaneously in both flanks with 1 × 106 LNCaP 104-R2 cells suspended in 0.5ml of Matrigel (BD Bioscience, Franklin Lakes, New Jersey) 14 days after mice were castrated. Tumors were measured weekly using calipers and volume was calculated using the formula volume = length × width × height × 0.52 (16, 17). Where indicated mice were implanted subcutaneously with 20 mg pellets of testosterone or cholesterol.
Real-Time Quantitative Polymerase Chain Reaction
RNA was isolated and specific mRNAs were quantified as described (16–19, 21, 22). The sequences of primers and probes for androgen receptor (AR) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were described previously (11, 17, 21). Primer and probe sequences used for PSA quantification were described by Gelmini et al (23). All transcript levels were normalized to GAPDH levels in each sample.
Protein over-expression and knockdown in LNCaP cells
Overexpression and shRNA knockdown of AR were preformed as previously described (11). Ectopic expression of Skp2 and c-Myc was achieved by infecting LNCaP 104-R2 cells with pMV7 or pBabe retroviruses carrying the cDNA of the Skp2 and c-Myc, respectively. Antibiotic-resistant (G418 and puromycin) colonies were expanded and screened for increased target protein expression by Western blot. Cells infected with retrovirus carrying empty vectors were used as controls.
Results
Regression of LNCaP 104-R2 xenografts
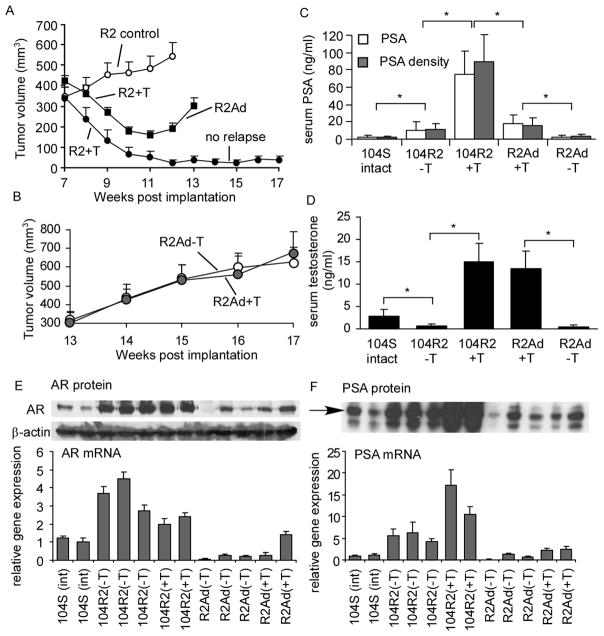
Six weeks after inoculation of LNCaP 104-R2 passage 285 (P285) cells, mice in the treatment group were implanted subcutaneously with testosterone pellets to mimic elevation of serum testosterone in patients after termination of androgen ablation therapy. Control mice were implanted subcutaneously with cholesterol pellets. Tumors in treatment group started to regress within a week, while all tumors in the control group continued to grow (Fig. 1A). Six weeks after pellets implantation, 24 out of 33 tumors relapsed and began to increase in size while 9 of the regressed tumors never relapse. Removal of the testosterone pellet did not affect the growth of these adapted R2Ad tumors (Fig. 1B), indicating that growth of R2Ad tumors was androgen-insensitive. Testosterone pellet implantation in mice bearing 104-R2 tumors initially increased the serum PSA level 5-fold (Fig. 1C, 1D), but as the growth of 104-R2 tumors was suppressed by testosterone and R2Ad tumors developed, the serum PSA level decreased 4-fold. Removal of the testosterone pellet from the mice bearing R2Ad tumors decreased the serum PSA level further to a level similar to that in intact mice bearing 104-S tumors (Fig. 1C, 1D).
Figure 1.
Regression and relapse of 104-R2 xenografts in athymic mice. (A) Castrated mice were injected subcutaneously with 104-R2 P285 cells. After allowing tumors to grow for 7 weeks, mice were separated into control groups (open circle, 7 mice with 11 tumors, no pellet implantation) and treatment group (filled circle and filled rectangular, 18 mice with 33 tumors, implanted with 20 mg testosterone pellets). Tumors in a subgroup of the treated mice (filled circle, 9 tumors from 6 mice) regressed and did not relapse by the end of the experiment (week 17). Tumors in other treated mice (24 tumors from 15 mice) relapsed 5 weeks after androgen treatment (week 12). (B) The relapsed R2Ad tumors were separated into a control group (pellet removed, open circle, 7 mice with 12 tumors) and a treatment group (pellet remained implanted, filled circle, 7 mice with 10 tumors). Points represent mean of tumor volumes; bars represent standard error. Mice carrying LNCaP 104-S tumors (9 weeks after cancer cell injection, 18 mice), 104-R2 tumors without testosterone pellet implant (control group in (A), week 8 and other 104-R2 tumors, 14 mice), 104-R2 tumors implanted with testosterone pellets (treatment group in (A), week 8, 9 mice), R2Ad tumors after testosterone pellet removal (in (B), week 17, 7 mice), and R2Ad tumors with testosterone pellets (in (B), week 17, 7 mice) were assayed for serum PSA (C) or testosterone level (D). Columns represent mean for 3 to 8 serum samples; bars represent standard deviation. AR (E) and PSA (F) protein and mRNA levels were measured in different tumors.β-actin was measured and used as a loading control. Columns represent mean for 3 replicates; bars represent standard deviation.
On average, during the progression from 104-R2 to R2Ad tumors, AR mRNA reduced 7-fold (Fig. 1E). AR protein level in 104-R2 tumors was dramatically increased as compared to 104-S tumors, while AR protein in R2Ad tumors was reduced to a level comparable to that in 104-S tumors (Fig. 1E). PSA mRNA and protein in 104-R2 tumors in castrated mice without testosterone pellets was 4 to 6-fold higher than that of 104-S tumors in intact mice (Fig. 1F). After testosterone pellet implantation, the PSA mRNA and protein increased another 2 to 3-fold. R2Ad tumor endogenous PSA mRNA and protein decreased slightly after removal of testosterone pellets (Fig. 1F).
Establishment of LNCaP R2Ad cells
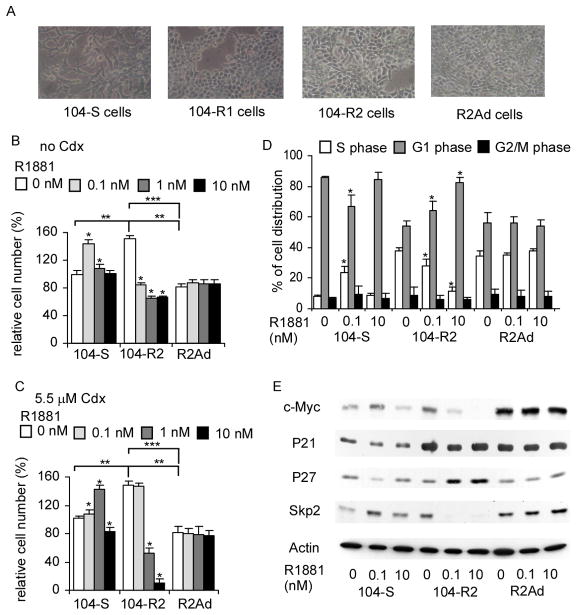
To study the molecular mechanism of androgenic growth inhibition, we cultured LNCaP 104-R2 P285 in medium containing physiological concentration of androgen to generate R2Ad cells. 104-R2 P285 cells treated with 10 nM synthetic androgen R1881 for 6 to 8 days remained attached but enlarged. By day 14, slowly growing colonies appeared. After passaging, the enlarged 104-R2 cells could not re-attach. Colony counts indicated that the attached cells, called R2Ad cells, arose from 104-R2 populations at a frequency of approximately 1 in 1000. The morphologies of 104-R1, 104-R2, and R2Ad cells were similar, but were very different from 104-S cells (Fig. 2A). The proliferation rate of the R2Ad cells was very slow during the first 3 passages, but increased afterwards. 0.1 nM and 1 nM R1881 increased growth of 104-S cells by 50% and 10%, respectively (Fig. 2B). In the absence of androgen, 104-R2 cells proliferated 50% faster than 104-S cells; however, 0.1, 1, and 10 nM R1881 caused 45%, 57%, and 57% inhibition of 104-R2 cell proliferation (Fig. 2B). In the presence of 5.5 μM bicalutamide, 0.1 and 1 nM R1881 increased 104-S cell population by 6% and 41%, respectively, indicating that the most optimal growing condition for 104-S cells shifted from 0.1 nM R1881 to 1 nM R1881 when co-treated with bicalutamide (Fig. 2C). R1881 at 10 nM suppressed proliferation of 104-S cells when 5.5 μM bicalutamide was present. Bicalutamide at 5.5 uM blocked the suppression of 0.1 nM R1881 treatment in 104-R2 cell but could not block the suppressive effect of androgens at higher concentrations. These results may reflect the low affinity of bicalutamide for AR. In the presence of 5.5 μM bicalutamide, 1 nM and 10 nM R1881 inhibited 64% and 93% of 104-R2 cell proliferation, respectively (Fig. 2D). It is interesting that co-treatment of anti-androgen bicalutamide and high concentration of androgen almost completely inhibited the proliferation of LNCaP 104-R2 cells. Co-treatment with the anti-androgen bicalutamide (Casodex) blocked the androgenic effect of R1881, confirming that the effect of androgen on cellular proliferation was mediated through AR. The proliferation rate of R2Ad cells was only 53% of parental 104-R2 cells (Fig. 2B). Neither R1881 nor bicalutamide affected the proliferation of R2Ad cells, suggesting that R2Ad cells were androgen-insensitive (Fig. 2B, 2C).
Figure 2.
Androgen effect on proliferation and cell cycle of 104-R2 cells. (A) Morphology of LNCaP 104-S, 104-R1, 104-R2, and R2Ad cells under maintenance conditions. Proliferation of 104-S, 104-R2, and R2Ad cells growing in 10% CS-FBS DMEM (B) or 10% CS-FBS DMEM plus 5.5 μM Casodex (C) and treated with increasing concentrations of R1881 determined by 96-well proliferation measuring total DNA content per well using Hoechst 33258 fluorescence. Relative cell numbers were normalized to that of 104-S cells without R1881 or bicalutamide treatment. Asterisk (*) represents cell number is statistically significantly different (P<0.05) compared to the same cell type without R1881 treatment, double asterisks (**) or triple asterisks (***) represent that in the absence of R1881, cell numbers of 104-R2 and R2Ad were statistically significantly different (P<0.05) compared to that of 104-S cells or compared to each other, respectively. Columns represent mean for 24 replicates; bars represent standard deviation. (D) Cell cycle distribution of 104-S, 104-R2, and R2Ad cells treated with 0, 0.1, and 10 nM R1881 for 96 hours was determined by flow cytometry. Asterisk (*) indicates statistically significant difference (P < 0.05) compared to control. (E) Protein expression of c-Myc, p21Cip , p27Kip1, Skp2, and β-actin in 104-S, 104-R2, and R2Ad cells treated with 0, 0.1, and 10 nM R1881 for 96 hours was determined by Western blotting.
Cell cycle regulation by androgens in LNCaP 104-S, 104-R2, and R2Ad cells
R1881 increased the percentage of cells in S phase and decreased the percentage of cells in G1 phase in 104-S cells but R1881 caused G1 cell cycle arrest in 104-R2 cells (Fig. 2D). S-phase population of 104-R2 cells in the absence of R1881 was 4.8 fold higher than that of 104-S cells. In the presence of R1881, S-phase population in 104-R2 cells dropped 21% and was slightly higher than that of 104-S cells. However, R1881 had no effect on cell cycle distribution of R2Ad cells (Fig. 2D). R1881 increased c-Myc and S-phase kinase-associated protein 2 (Skp2) while decreased p27Kip1 protein in 104-S cells. In contrast, R1881 decreased c-Myc and Skp2 but increased p27Kip1 protein in 104-R2 cells (Fig. 2E). R1881 did not alter the protein abundence of c-Myc, p27Kip1, and Skp2 in R2Ad cells. R1881 had very little effect on p21Cip in any of these LNCaP sublines (Fig. 2E).
Role of Androgen receptor, Skp2, and c-Myc in androgenic suppression
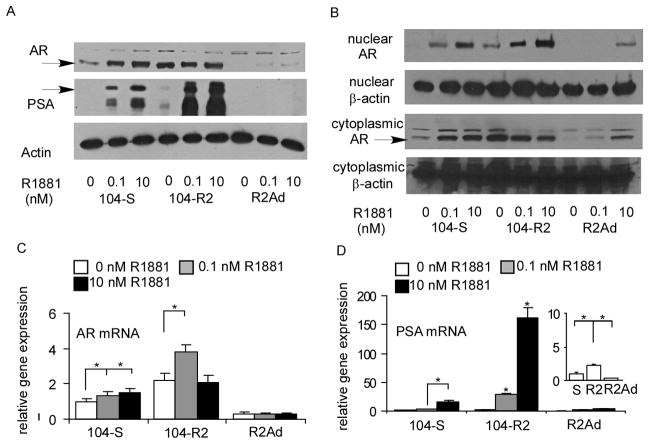
AR protein expression level was higher in 104-R2 cells than in 104-S cells in the absence of androgen. AR expression level was barely detectable in R2Ad cells (Fig. 3A). AR translocates to the nucleus upon binding androgen to activate gene transcription, we thus compared the subcellular localization of AR in response to androgen in these cells. In the absence of androgen, no AR was detected in nuclei of 104-S cells but a significant amount of AR was present in the nuclei of 104-R2 cells (Fig. 3B). R1881 treatment increased the amount of nuclear AR in 104-S, 104-R2, and R2Ad cells. However, AR was undetectable in the nuclei of R2Ad cells treated with 0 or 0.1 nM R1881, suggesting that nuclear AR was no longer required for proliferation and survival of R2Ad cells. R1881 treatment increased the amount of cytoplasmic AR in 104-S and R2Ad cells but not 104-R2 cells (Fig. 3B). R1881 slightly increased expression of AR mRNA in 104-S and 104-R2 cells but not R2Ad (Fig. 3C). AR mRNA level was very low in R2Ad cells and may be responsible for the low level of expression of AR in these cells. The effect of androgen on the various cells lines may reflect transcriptional activity of AR. Transcriptional activity of AR in 104-S, 104-R2, and R2Ad cells was assessed by examining androgenic induction of protein and mRNA expression of AR target gene, prostate specific antigen (PSA). PSA protein levels increased with R1881 treatment but were much higher in 104-R2 than in 104-S cells (Fig. 3A). The expression level of PSA mRNA correlated with protein expression levels in 104-S, 104-R2, and R2Ad cells treated with R1881 (Fig. 3D). When R1881 was absent, the PSA mRNA level was higher in 104-R2 cells compared to 104-S or R2Ad cells (Fig. 3D).
Figure 3.
Protein and mRNA expression of AR and PSA in LNCaP sublines. Expression of AR and PSA in total cell lysates (A) and AR expression in cytoplasmic and nuclear fractions of cell lysates (B) in 104-S, 104-R2, and R2Ad cells treated with 0, 0.1, and 10 nM of R1881 for 96 hours. β-actin was used as loading control. Expression of AR (C) and PSA (D) mRNA was detected with quantitative real-time PCR in 104-S, 104-R2, and R2Ad cells treated with 0, 0.1, and 10 nM of R1881 for 96 hours. Relative PSA mRNA expression of 104-S, 104-R2, and R2Ad without androgen treatment is shown enlarged as an inset in panel (F). Asterisk (*) indicates statistically significant difference (P < 0.05) compared to control.
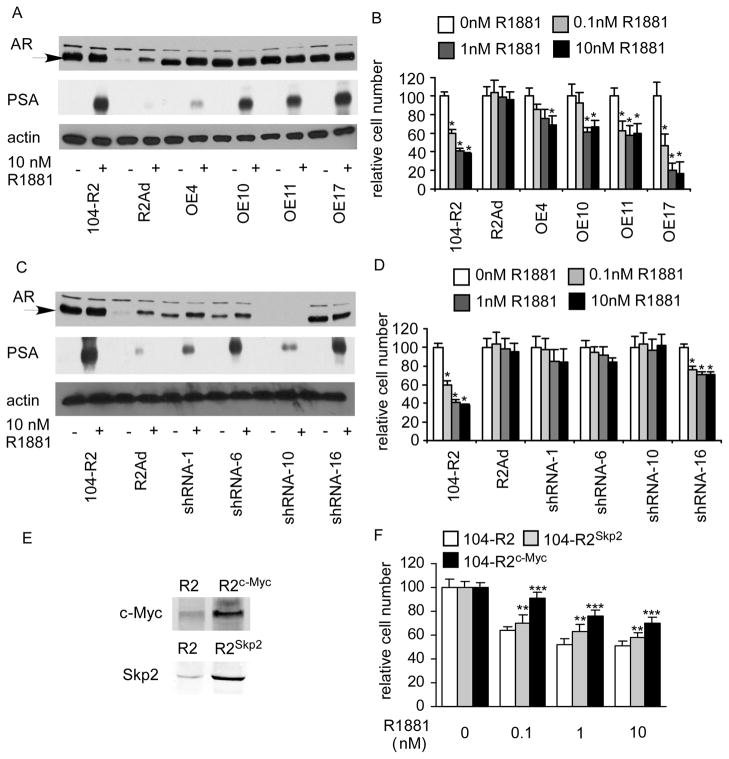
To determine if the AR expression level was responsible for the response of R2Ad cells to androgen, R2Ad cells were transduced with a pLNCX2-AR retroviral expression vector. This restored androgenic stimulation of PSA expression (Fig. 4A) and androgen-repression of cell proliferation (Fig. 4B). The knockdown of AR in by shRNA in clone 1, 6, 10 was about 90%, 90%, and 100%, respectively, while knockdown of AR in clone 16 was about 50% (Fig. 4C). All shRNA AR-knockdown clones expressed reduced level of PSA (Fig. 4C). Therefore, knockdown of AR by shRNA in 104-R2 cells reduced androgen-stimulated PSA expression. Clone 10, which expressed no detectable AR protein level, expressed the least PSA; in contrast, clone 16, which expressed 50% of AR protein compared to control, expressed about half amount of PSA compared to control. Proliferation of clone 1, 6, 10 was androgen-insensitive (Fig. 4D). Proliferation of clone 16 was suppressed by androgen, however, the androgenic suppression was much less compared to control 104-R2 cells. These observations indicated that reduction of 50% AR protein expression in 104-R2 was not enough to generate androgen-insensitive phenotype but partially blocked the growth inhibition caused by androgens, while reduction of 90% of AR protein expression in 104-R2 cells generated a totally androgen-insensitive phenotype. These results also suggested that down-regulation of AR allows the 104-R2 cells to escape from androgenic suppression. (Fig. 4C) and generated an androgen-insensitive phenotype (Fig. 4D). We next determine if Skp2 or c-Myc is essential for growth inhibition caused by androgen. Overexpression of Skp2 and c-Myc both partially blocked the androgenic growth inhibition (Fig. 4E, 4F). Better rescue effect was observed in c-Myc overexpressed 104-R2 cells.
Figure 4.
Ectopic expression of AR, Skp2, and c-Myc in R2Ad cells as well as knockdown of AR expression in 104-R2 cells. Protein expression of AR, and PSA was assayed by Western blotting in 104-R2 cells, R2Ad cells, clones (OE4–17) of R2Ad cells overexpressing AR (A), and clones (RNAi 1–16) of 104-R2 cells with AR knockdown by shRNA (C) in the absence or presence of 10 nM R1881 for 96 hours. Effect of androgen on proliferation of these clones (B)(D) treated with 0, 0.1, 1, 10 nM R1881 for 96 hours was determined by 96-well proliferation assay. (E) Protein expression of Skp2 and c-Myc was assayed by western blotting in 104-R2 cells in the absence or presence of 10 nM R1881 for 96 hours. (F) Effect of androgen on these clones treated with 0, 0.1, 1, 10 nM R1881 for 96 hours was determined by proliferation assay.
Discussion
We used testosterone pellet and R1881 treatment to mimic elevation of serum testosterone level due to termination of long-term androgen ablation therapy. Using LNCaP 104-R2 P285 xenograft and cell culture as a model, we studied the effect of androgen on proliferation and cell cycle of relapsed AR-positive prostate cancer cells. We found that androgen caused G1 cell cycle arrest in 104-R2 P285 cells via androgen receptor, Skp2, and c-Myc. Overexpression both Skp2 and c-Myc (Fig. 4F) in 104-R2 partially block the growth inhibition caused by androgen treatment, which suggested that other signaling molecules may also be involved in the androgenic suppression. Elevation of serum testosterone in mice bearing 104-R2 tumors initially increased the serum PSA level, but as the growth of 104-R2 tumors was suppressed by testosterone and R2Ad tumors developed, the serum PSA level decreased several folds. Our observations may provide molecular mechanism to explain why two clinical studies observed that patients with relapsed prostate tumor expressing elevated PSA responded to termination of androgen ablation therapy (24, 25). While the serum testosterone level increased, the serum PSA level increased first then dropped and remained normal for a long period of time (24, 25).
Androgen binding induces a conformational change in the AR, which facilitates the formation of AR homodimer complexes and allows AR to enter the nucleus and bind to androgen-response elements (AREs) in the promoter regions of target genes (26). Nuclear localization of AR in the absence of androgen in 104-R2 cells may thus promote androgen-independent proliferation and survival (Fig. 3B). PSA is the most common marker used for detecting prostate cancer growth in patients (27). Nuclear AR may also be involved in PSA mRNA and protein expression in 104-R2 cells without androgen treatment (Fig. 3A). Ligands other than androgens or phosphorylation of AR may contribute to this androgen-independent AR transcription activity. The observations that androgen-independent 104-R2 cells expressed PSA in the absence of androgen (Fig. 3A) and that serum PSA level in castrated mice bearing 104-R2 tumors without a testosterone pellet implant was 8-fold higher than in intact mice with 104-S tumors (Fig. 1C) may be similar to the clinical situation where hormone refractory prostate cancer develops in patients after androgen deprivation therapy. Often, the serum levels of PSA in these patients increase several fold while the serum testosterone level is very low due to the androgen ablation therapy (28, 29).
The level of p21Cip in 104-R2 and R2Ad cells was higher than that in 104-S cells (Fig. 2B). p21Cip positivity is reported to be correlated with lower disease-free survival in prostate cancer patients (30) and elevation of p21Cip protein expression is associated with progression to hormone refractive growth in both prostate cancer patients and a mouse xenograft model (31, 32), indicating that 104-R2 and R2Ad cells represent a more advanced disease phenotype compared to 104-S cells. Skp2 is an F-box protein that targets p27Kip1 for degradation (33) and the observed changes in Skp2 and p27kip1 are consistent with the function of Skp2 (Fig. 2E). c-Myc protein, a well-known proto-oncoprotein, is an important transcriptional regulator of the androgenic response and cell proliferation in prostate cancer (9, 34). c-Myc mRNA and protein expression increase during progression of prostate cancer (28, 35). c-Myc was found to be down-stream of AR and overexpression of c-Myc promoted androgen-independent growth of LNCaP cells (36). c-Myc levels in R2Ad cells were several fold higher than that in 104-S and 104-R2 cells (Fig. 2E). Elevation of c-Myc may compensate for the requirement of AR-signaling for cell proliferation in R2Ad cells.
LNCaP cells express a mutant AR (T877A) that displays relaxed ligand binding specificity (37, 38), however, androgenic suppression is not limited to LNCaP cells. PC-3 is a commonly used human prostate cancer cell line established from a bone-derived metastasis but does not express AR (39). Our unpublished data about experiment using PC-3 cells overexpressing wild-type AR indicated that androgen also suppressed these cells via reduction of Skp2 and c-Myc, suggesting that the molecular mechanism we observed is possibly an universal phenomenon in AR-positive castration-resistant prostate cancer cells. Other groups also observed that physiological concentrations of androgens caused growth inhibition and G1 cell cycle arrest caused in PC-3 cells overexpressing full length wild-type AR (40–42) or ARCaP cells. ARCaP is another AR-positive prostate cancer cell line derived from the ascites fluid of a patient with advanced metastatic disease (43–45). Their observations suggested that the androgenic suppression phenomenon was not limited to LNCaP cells. We believe that our study may have potential application in clinical treatment of AR-positive castration-resistant prostate cancers.
Acknowledgments
This work was supported by CS-100-PP-12 (NHRI), DOH100-TD-C-111-014 (DOH), and NSC 99-2320-B-400-015-MY3 (NSC) in Taiwan for C.-P.Chuu and US NIH grants CA58073 for S. Liao.
Footnotes
Disclosure of Conflicts of Interest
There is no conflict of interest for all authors.
References
- 1.Huggins C, SR, Hodges C. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:15. [Google Scholar]
- 2.Seruga B, Tannock IF. Intermittent androgen blockade should be regarded as standard therapy in prostate cancer. Nat Clin Pract Oncol. 2008;5:574–6. doi: 10.1038/ncponc1180. [DOI] [PubMed] [Google Scholar]
- 3.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–79. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 4.Fowler JE, Jr, Bigler SA, Kolski JM, Yee DT. Early results of a prospective study of hormone therapy for patients with locally advanced prostate carcinoma. Cancer. 1998;82:1112–7. [PubMed] [Google Scholar]
- 5.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 6.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 7.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horoszewicz JS, Leong SS, Chu TM, et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–32. [PubMed] [Google Scholar]
- 9.Kokontis J, Takakura K, Hay N, Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994;54:1566–73. [PubMed] [Google Scholar]
- 10.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–53. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 11.Kokontis JM, Hsu S, Chuu CP, et al. Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity. Prostate. 2005;65:287–98. doi: 10.1002/pros.20285. [DOI] [PubMed] [Google Scholar]
- 12.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 13.Ford OH, 3rd, Gregory CW, Kim D, Smitherman AB, Mohler JL. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003;170:1817–21. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi Y, Uemura H, Fujinami K, et al. Fluorescence in situ hybridization evaluation of c-myc and androgen receptor gene amplification and chromosomal anomalies in prostate cancer in Japanese patients. Prostate. 2000;43:225–32. doi: 10.1002/(sici)1097-0045(20000515)43:3<225::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci U S A. 1996;93:11802–7. doi: 10.1073/pnas.93.21.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082–4. doi: 10.1158/0008-5472.CAN-04-3992. [DOI] [PubMed] [Google Scholar]
- 17.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66:6482–6. doi: 10.1158/0008-5472.CAN-06-0632. [DOI] [PubMed] [Google Scholar]
- 18.Chuu CP, Chen RY, Hiipakka RA, et al. The liver X receptor agonist T0901317 acts as androgen receptor antagonist in human prostate cancer cells. Biochem Biophys Res Commun. 2007;357:341–6. doi: 10.1016/j.bbrc.2007.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuu CP, Chen RY, Kokontis JM, Hiipakka RA, Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009;275:86–92. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643–8. [PubMed] [Google Scholar]
- 21.Fukuchi J, Hiipakka RA, Kokontis JM, et al. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 2004;64:7682–5. doi: 10.1158/0008-5472.CAN-04-2647. [DOI] [PubMed] [Google Scholar]
- 22.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–9. doi: 10.1158/0008-5472.CAN-04-2332. [DOI] [PubMed] [Google Scholar]
- 23.Gelmini S, Tricarico C, Petrone L, et al. Real-time RT-PCR for the measurement of prostate-specific antigen mRNA expression in benign hyperplasia and adenocarcinoma of prostate. Clin Chem Lab Med. 2003;41:261–5. doi: 10.1515/CCLM.2003.040. [DOI] [PubMed] [Google Scholar]
- 24.Mathew P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: the case for a prospective trial. Ann Oncol. 2008;19:395–6. doi: 10.1093/annonc/mdm568. [DOI] [PubMed] [Google Scholar]
- 25.Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol. 2009;56:97–103. doi: 10.1016/j.eururo.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 27.Young CY, Montgomery BT, Andrews PE, Qui SD, Bilhartz DL, Tindall DJ. Hormonal regulation of prostate-specific antigen messenger RNA in human prostatic adenocarcinoma cell line LNCaP. Cancer Res. 1991;51:3748–52. [PubMed] [Google Scholar]
- 28.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin Cancer Res. 2003;9:5271–81. [PubMed] [Google Scholar]
- 29.Polito M, Minardi D, Recchioni A, Giannulis I, De Sio G, Muzzonigro G. Serum markers for monitoring of prostatic carcinoma. Prostate. 1997;33:208–16. doi: 10.1002/(sici)1097-0045(19971101)33:3<208::aid-pros10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar FH, Li Y, Sakr WA, et al. Relationship of p21(WAF1) expression with disease-free survival and biochemical recurrence in prostate adenocarcinomas (PCa) Prostate. 1999;40:256–60. doi: 10.1002/(sici)1097-0045(19990901)40:4<256::aid-pros7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Fizazi K, Martinez LA, Sikes CR, et al. The association of p21((WAF-1/CIP1)) with progression to androgen-independent prostate cancer. Clin Cancer Res. 2002;8:775–81. [PubMed] [Google Scholar]
- 32.Zhou JR, Yu L, Zerbini LF, Libermann TA, Blackburn GL. Progression to androgen-independent LNCaP human prostate tumors: cellular and molecular alterations. Int J Cancer. 2004;110:800–6. doi: 10.1002/ijc.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 34.Vellaichamy A, Dezso Z, JeBailey L, et al. “Topological significance” analysis of gene expression and proteomic profiles from prostate cancer cells reveals key mechanisms of androgen response. PLoS One. 2010;5:e10936. doi: 10.1371/journal.pone.0010936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grad JM, Dai JL, Wu S, Burnstein KL. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol. 1999;13:1896–911. doi: 10.1210/mend.13.11.0369. [DOI] [PubMed] [Google Scholar]
- 36.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–31. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldscholte J, Ris-Stalpers C, Kuiper GG, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 38.Kokontis J, Ito K, Hiipakka RA, Liao S. Expression and function of normal and LNCaP androgen receptors in androgen-insensitive human prostatic cancer cells. Altered hormone and antihormone specificity in gene transactivation. Receptor. 1991;1:271–9. [PubMed] [Google Scholar]
- 39.Chuu CP, Kokontis JM, Hiipakka RA, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci. 2007;14:543–53. doi: 10.1007/s11373-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 40.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 41.Litvinov IV, Antony L, Isaacs JT. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate. 2004;61:299–304. doi: 10.1002/pros.20187. [DOI] [PubMed] [Google Scholar]
- 42.Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–11. [PubMed] [Google Scholar]
- 43.Zhau HY, Chang SM, Chen BQ, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A. 1996;93:15152–7. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cinar B, Koeneman KS, Edlund M, Prins GS, Zhau HE, Chung LW. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res. 2001;61:7310–7. [PubMed] [Google Scholar]
- 45.Hara T, Nakamura K, Araki H, Kusaka M, Yamaoka M. Enhanced androgen receptor signaling correlates with the androgen-refractory growth in a newly established MDA PCa 2b-hr human prostate cancer cell subline. Cancer Res. 2003;63:5622–8. [PubMed] [Google Scholar]