Abstract
Acetylcholinesterase (AChE) is responsible for the hydrolysis of the neurotransmitter, acetylcholine, in the nervous system. The functional localization and oligomerization of AChE T variant are depending primarily on the association of their anchoring partners, either collagen tail (ColQ) or proline-rich membrane anchor (PRiMA). Complexes with ColQ represent the asymmetric forms (A12) in muscle, while complexes with PRiMA represent tetrameric globular forms (G4) mainly found in brain and muscle. Apart from these traditional molecular forms, a ColQ-linked asymmetric form and a PRiMA-linked globular form of hybrid cholinesterases (ChEs), having both AChE and BChE catalytic subunits, were revealed in chicken brain and muscle. The similarity of various molecular forms of AChE and BChE raises interesting question regarding to their possible relationship in enzyme assembly and localization. The focus of this review is to provide current findings about the biosynthesis of different forms of ChEs together with their anchoring proteins.
Keywords: acetylcholinesterase, butyrylcholinesterase, assembly, membrane trafficking, glycosylation
Introduction
Cholinesterases (ChEs) are serine hydrolases that preferentially act on choline esters. Vertebrates possess two types of cholinesterases (ChEs), corresponding to two distinct genes: acetyl cholinesterase (AChE, EC 3.1.1.7) and butyryl cholinesterase (BChE, EC 3.1.1.8). These two enzymes are distinguished on the basis of the substrate specificities and their sensitivities to selective inhibitors (Mendel and Rudney, 1943; Austin and Berry, 1953). The primary function of AChE is to efficiently hydrolyze the neurotransmitter acetylcholine (ACh) at cholinergic synapses (Massoulié et al., 1993), whereas the physiological function of BChE in vertebrates remains a question of different speculations. Studies of AChE knockout mice suggested that BChE can partially compensate for the absence of AChE in the nervous system (Xie et al., 2000; Duysen et al., 2001); therefore BChE could hydrolyze acetylcholine functionally. Poisoning by ChE inhibitors, such as insecticides or nerve gas, results in accumulation of ACh, and uncontrolled activation of cholinergic receptors, which causes cholinergic crisis and potentially leads to death (Feyereisen, 1995; Bajgar, 2004). On the other hand, controlled treatment with ChE inhibitors are used in therapeutics for patients suffering from myasthenia gravis (Brenner et al., 2008; Mehndiratta et al., 2011), Alzheimer’s disease (Giacobini, 2000; Stone et al., 2011), and Parkinson’s disease (Hutchinson and Fazzini, 1996; Emre et al., 2004).
In vertebrates, ACHE gene produces several types of coding sequences differing in an alternative choice of splice acceptor sites in the 3′ region. This process generates different AChE isoforms, named AChER, AChEH, and AChET (Massoulié, 2002). They contain the same catalytic domain, but are associated with distinct C-terminal peptides. In contrast, BCHE gene produces single type of transcript and generates single type of isoform BChET (Blong et al., 1997). AChE and BChE are well-known for their multiple molecular forms that have their specific localizations: AChER is a soluble monomer that is up-regulated in the brain under stress stimulation (Kaufer et al., 1998; Perrier et al., 2005); AChEH is a glycosylphosphatidylinositol-anchored dimer that is mainly expressed in red blood cells (Li et al., 1991); AChET and BChET are present in collagen-tailed forms at the neuromuscular junction (nmj) and hydrophobic-tailed forms in the brain (Legay et al., 1995; Blong et al., 1997; Massoulié et al., 2005). The molecular forms of AChET and BChET in brain and muscle are of particular interest because they are associated with their anchoring proteins: collagen Q (ColQ) or proline-rich membrane anchor (PRiMA). Complexes with ColQ represent the collagen-tailed or asymmetric (A) forms in muscle (Krejci et al., 1997), while complexes with PRiMA represent membrane-bound tetrameric globular form (G4), mainly in brain (Perrier et al., 2002, 2003; Xie et al., 2009) and muscle (Xie et al., 2007). In addition, mixed cholinesterases, ColQ-linked AChE–BChE A12 hybrid enzyme (Tsim et al., 1988a) and PRiMA-linked AChE–BChE G4 hybrid enzyme (Chen et al., 2010) that contain both AChE and BChE homodimers in a single molecule are being found in avian system. Here, we summarized the recent studies on the assembly of oligomeric AChE and BChE, as well as the regulation of AChE and/or BChE biosynthesis in neurons and muscles.
Molecular Forms of AChE and BChE in Brain and Muscle
PRiMA-linked globular forms of ChEs
In Xenopus oocytes, COS-7 cells, neuroblastoma cells, and muscle cells, the expression of PRiMA has been identified as a limiting factor in organizing G4 AChE and targeting it to the cell membrane (Perrier et al., 2002; Xie et al., 2007), as well as in directing its membrane raft localization (Xie et al., 2010a). In the absence of PRiMA, the G4 AChE, or the G4 BChE, could not be formed. In the brain, the catalytic subunit contained in G4 AChE is AChET (Inestrosa et al., 1994). The expression of PRiMA mRNA and protein are increased with an increment of G4 AChE during the development of brain and spinal cord (Leung et al., 2009; Xie et al., 2010b). The PRiMA-linked AChE is also present at the nmj, in which both motor neuron and muscle are the major suppliers. In rat muscles, the protein expression of PRiMA and AChET, as well as the G4 AChE, are dramatically increased during development (Leung et al., 2009). The parallel expression of PRiMA and G4 AChE during the development strengthens the importance of PRiMA in directing, and/or regulating the formation of G4 enzyme. The PRiMA-linked G4 BChE however has not been well studied, and therefore the expression profile of which in brain or muscle are not fully revealed.
ColQ-linked asymmetric forms of ChEs
Asymmetric forms of AChE and BChE are characterized by the presence of collagen-like tail, which is formed by the triple helical association of three ColQ subunits (Feng et al., 1999). The cDNA encoding ColQ has been cloned in Torpedo (Krejci et al., 1991), rat (Krejci et al., 1997), human (Ohno et al., 1998), quail, and chicken (Ruiz and Rotundo, 2009a). The presence of the collagen-tailed forms of ChEs has been found in all classes of vertebrates, but not in invertebrates. They are specifically expressed in muscles and regulated by physiological activity (Sketelj and Brzin, 1985; Deprez et al., 2003; Lau et al., 2008). In human and rat, COLQ gene has two transcripts, ColQ-1 and ColQ-1a: they are differentiatedly expressed in slow-twitch (ColQ-1) and fast-twitch (ColQ-1a) muscles (Ohno et al., 1998; Krejci et al., 1999). The differentiated expression patterns may account for the synaptic and non-synaptic expression profile of ColQ-linked AChE in fast slow-twitch and fast-twitch muscles, respectively (Lee et al., 2004; Crne-Finderle et al., 2005; Choi et al., 2007).
Existence of AChE–BChE hybrid ChEs
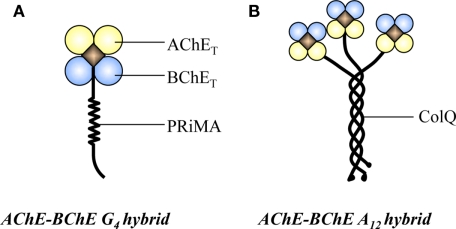
Most of our knowledge concerning ChEs derives from the studies on the classical AChE and BChE homogenous oligomers. However, the sequence similarity between AChET and BChET has been further emphasized regarding the existence of hybrid A12 forms in new-born chicken muscle (Tsim et al., 1988a), as well as a hybrid G4 from in the brain (Chen et al., 2010) and embryonic muscles of chicken (Chen et al., 2009). In these hybrid enzymes, both AChET and BChET are attached to the same anchoring protein, ColQ for A12 form and PRiMA for G4 form (Figure 1). The hybrid enzyme exists as a single type, with equivalent number of AChE and BChE catalytic subunits in a single molecule. Interestingly, the expression of these hybrid molecules in chicken brain and muscle was found to be developmentally regulated. In 1-day-old chicken muscle, the predominant form of AChE is A12 hybrid form; however, the proportion of BChE subunits in the hybrid molecules progressively disappear during the muscle development from embryonic, hatching to adult, and the homogeneous asymmetric AChE becomes the sole form upon the muscle maturation (Tsim et al., 1988b). On the other hand, a continuous increase of AChE–BChE G4 hybrid expression was observed in chicken brain, while an obvious decrease was found in the leg muscles. To date, we do not have conclusive idea about the underlining regulatory mechanism of these hybrid enzymes. We believe that AChE–BChE hybrid enzymes could take part in cholinergic functions, which includes (i) they carry the catalytic activities of AChE and BChE in hydrolyzing ACh; (ii) they are associated with the anchoring protein, PRiMA or ColQ, which can anchor the hybrid enzymes onto the plasma membrane in the brain or nmj in the muscles; and (iii) the level of BChET indirectly regulates the expression of homogenous G4 AChE.
Figure 1.
Two types of AChE–BChE hybrid enzyme. (A) The PRiMA-linked G4 AChE–BChE hybrid in chicken brain. (B) The ColQ-linked A12 AChE–BChE hybrid in chicken muscle.
In fact, the notion of having the existence of AChE–BChE hybrid enzyme is not new. An abnormal ChE species in the serum of patients suffering from carcinomas was reported (Zakut et al., 1988). This abnormal ChE species in human serum was inhibited by both AChE inhibitor BW284c51 and BChE inhibitor iso-OMPA. In addition, a collagen-tailed asymmetric hybrid AChE has been found relatively abundant in young chicken muscle (Tsim et al., 1988b), but which tends to disappear at the adult stage (Tsim et al., 1988b). Moreover, a hybrid tetramer having AChE and BChE activity was also found in cyst fluids derived from a human astrocytoma (García-Ayllón et al., 2001). However, none of the physiological function of these abnormal ChEs has been elucidated.
Assembly Mechanism of AChE and BChE
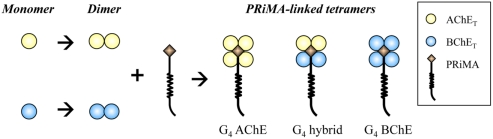
The presence of G4 and A12 hybrid ChEs raised interesting questions about the organization of the subunits in a hybrid ChE complex. The assembly of AChE and BChE in cells could provide a good model in revealing the protein assembly of oligomers. By using DNA transfected cell cultures, the organization of different subunits in the PRiMA-linked ChE tetramers has been studied (Chen et al., 2010). Interestingly, AChET and BChET could not form hybrid dimer in the absence of anchoring protein; on the other hand, a single type of hybrid tetramer was clearly observed when the two catalytic subunits were co-expressed with PRiMA. Therefore, a “2 + 2” model is proposed for the organization of the four catalytic subunits in the PRiMA-linked ChE tetramers (Figure 2). After protein synthesis, AChET and BChET spontaneously form AChE homodimer and BChET homodimer first. When two dimers (two AChET dimers, or two BChET dimers, or one AChET dimer plus one BChET dimer) encounter the anchoring protein, PRiMA, and thus ChE tetramers, e.g., G4 AChE, G4 BChE, and G4 AChE/BChE hybrid, are formed.
Figure 2.
Model for the assembly of PRiMA-linked AChE and BChE tetramers. When AChET and BChET subunits are expressed together, they form their own homodimers spontaneously. PRiMA recruits two homodimers together to form a PRiMA-linked tetramers, e.g., G4 AChE, G4 BChE. G4 hybrid.
T-peptide is necessary for the oligomerization of AChET and BChET
AChET and BChET have a catalytic domain of approximately 500 amino acids, followed by a C-terminal t-peptide of 40 and 41 residues, respectively. The t-peptide on AChET and BChET presents a considerable sequence similarity, with 24 identical residues, including seven aromatic residues and one cysteine near the C-terminus, which are conserved in human, cat, rabbit, mouse, cow, rat, chicken, and Torpedo (Massoulié et al., 1993).
The t-peptide was reported to play an important role in the biosynthesis of ChEs, particularly in the protein folding and exportation. The presence of aromatic residues in the t-peptide induces the misfolding of newly synthesized AChET polypeptides, and this effect depends on the hydrophobic character of these residues, because the same effect occurs when they are replaced by leucines (Falasca et al., 2005). In the absence of a proline-rich attachment domain (PRAD)-containing anchoring protein, the t-peptide enhanced a pool of AChET molecules toward endoplasmic reticulum (ER)-associated degradation (Belbeoc’h et al., 2003).
The major function of t-peptide is directing the assembly of tetramers of AChET (Bon and Massoulié, 1997) and BChET (Blong et al., 1997), as well as the association with the structure proteins, ColQ and PRiMA (Krejci et al., 1997; Perrier et al., 2002; Bon et al., 2004). The t-peptide is also named as tryptophan (W) amphiphilic tetramerization (WAT) domains, which contains a sector with seven aromatic residues that are strictly conserved between AChET and BChET. The association between AChET or BChET catalytic subunits and anchoring proteins, ColQ and PRiMA, is mostly based on the interaction between four WAT domains on the t-peptides and a PRAD on ColQ or PRiMA (Bon et al., 1997; Dvir et al., 2004; Noureddine et al., 2007). A crystallographic analysis of the complex of synthetic t-peptide and PRAD peptide indicated that four α-helical t-peptides form coiled-coil structure around the PRAD, which is arranged in a poly-proline II helix (Dvir et al., 2004). In addition, the formation of disulfide bonds through the cysteine residues near the end of the t-peptides stabilizes the quaternary association, and in fact this association appears to be critical in the case of AChE dimer formation. Dimers could be hardly observed after mutagenesis of the C-terminal cysteine residues in H or T-peptides of Torpedo and rat AChE (Morel et al., 2001; Chen et al., 2010).
The importance of t-peptide in the assembly of AChET and BChET was also reported by DNA mutagenesis studies. The truncated mutants, AChEΔT and BChEΔT, in which the t-peptides were deleted from the catalytic subunits, produced only monomers (Duval et al., 1992). AChET and AChEBChE-T, BChET and BChEAChE-T, in which the catalytic domain of each enzyme was swapped with the t-peptide of each other, presented similar assembly ability to form oligomers (Liang et al., 2009; Chen et al., 2010).
The FHB domain is involved in the selection of catalytic subunits during dimerization
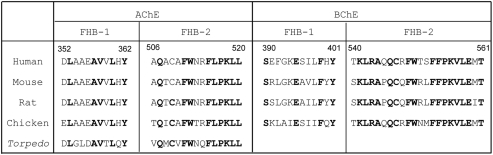
Although the nature of AChET and BChET oligomers depends on the presence of the t-peptides, the catalytic domains also influence the oligomerization patterns. The X-ray crystallography studies of Torpedo AChE dimers showed that the contact zone between two AChEH subunits could be a “four-helix bundle” (FHB), formed by two α helices from each catalytic domain (Sussman et al., 1991). In addition, rat AChET was also demonstrated to dimerize through FHB inter-subunit contact zone (Morel et al., 2001). Based on these findings, we compared the predicted FHB sequences that are responsible for the dimeric contact zone of AChET and BChET from different species. Indeed, FHB domains are highly conserved across different species for either AChE or BChE, including human, mouse, rat, chicken, and Torpedo (Figure 3). On the other hand, the similarity between FHB domains of AChET and BChET is very low. An inter-species hybrid dimer could be formed between human AChET and chicken AChET, but not between mammalian AChET and BChET, in transfected HEK293T cells (Chen et al., 2010). The selectivity of dimerization seems to be based on the feature that the FHB domains of AChE are highly conserved among different species of vertebrates, but are distinguished from vertebrate BChETs. Moreover, another hybrid dimer, between human AChEBChE-T and chicken AChET, which contained the similar FHB but different t-peptides, was formed when they were co-expressed together in HEK293T cultured cells (Chen et al., 2010). This further confirmed that the catalytic domains, possibly the FHB domains, should play a critical role in the selection of subunits during the dimerization of ChEs.
Figure 3.
Comparison of FHB sequences of AChET and BChET among different species. The sequences of the two alpha helices (FHB-1 and FHB-2) forming the dimeric contact zone of AChE and BChE are shown. The residues conserved across species are highlighted in bold. The amino acid sequences of human, mouse, rat, chicken, and Torpedo AChE catalytic subunits were deduced from nucleotide sequences accessed from GeneBank™ AAA68151, CAA39867, EDM13278, P36196, and CAA27169, respectively. The amino acid sequences of human, mouse, rat, and chicken BChE catalytic subunits were deduced from nucleotide sequences accessed from GeneBank™ AAA99296.1, AAH99977, NP_075231, and NP_989977, respectively.
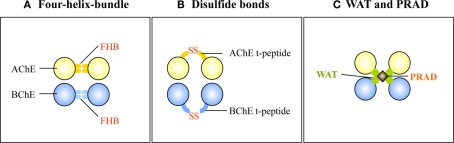
According to our current knowledge, the oligomerization of AChET or BChET with their associated anchoring proteins could rely on three types of interactions (Figure 4): (i) the FHB interaction for the formation of dimer through hydrophobic interaction; (ii) intercatenary disulfide bonds between the t-peptide of AChET or BChET subunits; and (iii) tight hydrophobic interaction between the WAT domains of AChET or BChET subunits and the PRAD on PRiMA or ColQ.
Figure 4.
Three types of interaction involved in the oligomerization of AChE and BChE. (A) The FHB domains of AChET or BChET direct the dimer formation. (B) The t-peptides of AChET or BChET form intercatenary disulfide bonds. (C) WAT domains of AChET and BChET interact with PRAD on PRiMA or ColQ.
N-glycosylation is not required for the assembly of AChET but is required for the membrane trafficking
AChET and BChET are well-known highly glycosylated enzymes, which carry various amounts of N-linked carbohydrate side chains attached to their core polypeptides (Liao et al., 1992; Kolarich et al., 2008). Mature human AChET monomer possesses three potential N-linked glycosylation sites (Soreq et al., 1990; Velan et al., 1993). Mature human BChET carries nine potential N-linked glycosylation sites (Lockridge et al., 1987). These N-linked glycosylation sites, both in AChE and BChE, are highly conserved in mammals, which implies the physiological importance of these glycans for ChEs.
Glycosylation is proposed to be used as a marker for the progression of ChE forms through different subcellular compartments, since it is known that the glycans added in ER are remodeled and matured in Golgi apparatus. In chicken muscles, the assembly of catalytically active dimer and tetramer occurred in the rough ER, with a subset of tetramers being further assembled with ColQ in Golgi apparatus into asymmetric forms (Rotundo, 1984). Once assembled, these catalytically active AChE oligomers were stable, acquired complex oligosaccharides in Golgi apparatus, and were transported to plasma membrane or secreted into medium (Rotundo, 1984). All of these exported AChE molecules contain complex oligosaccharides, because they could bind to lectins such as wheat germ agglutinin and ricin, and were endoglycosidase H (Endo H) resistant (Rotundo et al., 1989). In contrast, 70–80% of the newly synthesized AChE polypeptide chains in chicken muscle appeared to be catalytically inactive and Endo H sensitive, and they were degraded intracellularly with a half-life of about 1.5 h (Rotundo, 1988). Recently, Ruiz and Rotundo (2009a,b) reported that the expression of AChE in quail muscles is regulated by muscle activity through post-translational controls: the over-expression of ER molecular chaperons, such as calnexin, ER protein 72, and protein disulfide isomerase results in an increase of catalytic active ColQ-linked AChE in quail muscles.
The biological function of the glycans on ChEs was elucidated by site mutagenesis studies. Elimination of N-glycosylation sites did not interfere with the ability of AChET to form a soluble dimer (Velan et al., 1993), or to assembly with PRiMA to form a PRiMA-linked AChE tetramer (Chen et al., 2011). It appears therefore that the oligosaccharide side chains do not affect the structural elements that are responsible for the interaction of different subunits. Indeed, none of the N-glycosylation sites on AChE is close to or within the FHB domain implied in dimerization of AChE subunits (Sussman et al., 1991; Morel et al., 2001), or the t-peptide that allows the oligomerization of AChET with the anchoring proteins, e.g., ColQ and PRiMA (Bon et al., 2004). Moreover, the glycosylation of AChET can greatly affects the protein folding and membrane trafficking. When the glycosylation is eliminated, the folding of AChET fails, leading to a severe lost of the enzymatic activity (Chen et al., 2011). In the absence of glycosylation, the secreted G1 and G2 AChE are dramatically reduced in transfected cells, and the PRiMA-linked G4 AChE is retained in ER and fails to be exported to plasma membrane (Chen et al., 2011).
The importance of N-glycosylation in the biosynthesis of AChE could explain the abnormality of glycosylation status in some pathological conditions. Proper glycosylation of AChE is important for normal brain function. Accumulation of molecular forms of AChE with altered patterns of glycosylation has been observed in the brain and cerebrospinal fluid of Alzheimer’s patients (Sáez-Valero et al., 1999, 2000). Moreover, characteristics of AChE found in the senile plaques are different from those in normal brain with a higher degree of glycosylation, which is proposed to be one of the factors facilitating formation of amyloid fibrils in the senile plaques (Mimori et al., 1997). These abnormalities in the glycosylation of AChE are very specific for Alzheimer’s disease and are not detected in other dementia illness, which suggests that glycosylation of AChE may have a diagnostic value.
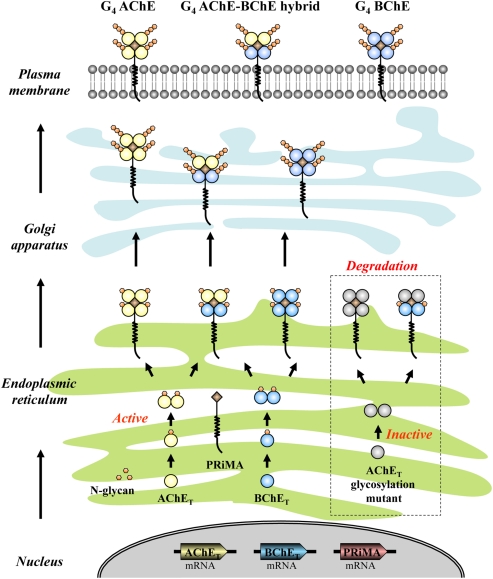
To summarize the assembly and membrane processing of G4 ChEs, a model is being proposed as Figure 5. After the mRNAs are translated into peptides, AChET and BChET polypeptides undergo initial glycosylation, presumably in a co-translation manner. Shortly after the synthesis, the glycosylated AChET and BChET are assembled into homodimers spontaneously. When these homodimers encounter PRiMA, they form PRiMA-linked G4 AChE, or G4 BChE, or AChE–BChE G4 hybrid in ER. Afterward, PRiMA targets these G4 complexes to Golgi apparatus where AChET and BChET subunits have further glycosylation. This trafficking allows the G4 enzymes to be fully functional and finally become anchored onto the plasma membrane. During the whole process, the proper glycosylation of AChET is the key point for the membrane targeting. Without glycosylation, AChET polypeptides cannot fold properly, resulting in inactive AChE molecules. These un-glycosylated and inactive AChET molecules can still form PRiMA-linked G4 AChE and AChE–BChE G4 hybrid, but both of them are retained in ER, failing to be exported to Golgi apparatus, and finally they are subjected to degradation most possibly.
Figure 5.
Proposed model for the assembly and membrane processing of G4 ChEs. G4 AChE, G4 BChE, and AChE–BChE G4 hybrid molecules are assembled in ER where both AChET and BChET subunits have initial glycosylation. These G4 complexes are subsequently transported to Golgi apparatus where the catalytic subunits can have further glycosylation, and finally anchored onto the plasma membrane. The AChET glycosylation mutant, in which the glycosylation is completely abolished, is still able to assembly with PRiMA and BChET to form G4 AChE and G4 hybrid. However, both of them are retained in ER, which possibly will be subjected to the degradation pathway.
Summary
The assembly of ChEs constitutes a fascinating model to study numerous biological processes, such as post-translational modification, protein–protein interactions, membrane trafficking, and protein degradation. The physiological function of ChEs depends on the catalytic property of the enzymes and the restricted subcellular localization. In brain and muscles, ChEs display extremely rich molecular polymorphisms, possessing soluble, membrane-bound and basal lamina-anchored forms, and additionally, hybrid ChEs containing both AChE and BChE catalytic subunits also curiously exist. During the assembly of these ChE complexes, dimer is believed to be the precursor for the PRiMA-linked tetramers and ColQ-linked asymmetric forms. The dimer formation of AChE or BChE depends on recognition between the FHB domains in their catalytic domains, and the assembly of tetramers with PRiMA or ColQ requires the interaction of WAT domain on the C-terminal t-peptides with the PRAD domain on PRiMA or ColQ. N-linked glycans of AChE are employed as both maturation and quality control tags that dictate the destination of the enzyme being exported or not, which inspires that the control of glycosylation may be as a means in regulating the level of functional AChE in pathological conditions. However, there are still several questions out there, which have not been resolved. These questions are: (i) the possible control mechanism in directing the formation of different forms of ChEs; (ii) the regulatory mechanism for the protein trafficking of different states of ChEs; (iii) the fate of the active and inactive ChEs; and (iv) the possible non-cholinergic function of different forms of ChEs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research in Tsim’s laboratory was supported by grants from the Research Grants Council of Hong Kong (662407, 660409, 662911, F-HK21/06T) and the Croucher Foundation (CAS-CF07/08.SC03). The authors are grateful to Drs. Jean Massoulié and Suzanne Bon from Institut de Biologie de l’Ecole Normale Supérieure, Paris, France for their advices during the study.
Abbreviations
A, asymmetric; ACh, acetylcholine; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; ChEs, cholinesterases; ColQ, collagen tail; Endo H, endoglycosidase H; ER, endoplasmic reticulum; FHB, four-helix bundle; G4, tetrameric globular form; nmj, neuromuscular junction; PRAD, proline-rich attachment domain; PRiMA, proline-rich membrane anchor; WAT, tryptophan amphiphilic tetramerization.
References
- Austin L., Berry W. K. (1953). Two selective inhibitors of cholinesterase. Biochem. J. 54, 695–700 [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. (2004). Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 38, 151–216 10.1016/S0065-2423(04)38006-6 [DOI] [PubMed] [Google Scholar]
- Belbeoc’h S., Massoulié J., Bon S. (2003). The C-terminal t peptide of acetylcholinesterase enhances degradation of unassembled active subunits through the ERAD pathway. EMBO J. 22, 3536–3545 10.1093/emboj/cdg360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blong R. M., Bedows E., Lockridge O. (1997). Tetramerization domain of human butyrylcholinesterase is at the C-terminus. Biochem. J. 327, 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S., Coussen F., Massoulié J. (1997). Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem. 272, 3016–3021 10.1074/jbc.272.5.3016 [DOI] [PubMed] [Google Scholar]
- Bon S., Dufourcq J., Leroy J., Cornut I., Massoulié J. (2004). The C-terminal t peptide of acetylcholinesterase forms an alpha helix that supports homomeric and heteromeric interactions. Eur. J. Biochem. 271, 33–47 10.1046/j.1432-1033.2003.03892.x [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. (1997). Quaternary associations of acetylcholinesterase. I. Oligomeric associations of T subunits with and without the amino-terminal domain of the collagen tail. J. Biol. Chem. 272, 3007–3015 10.1074/jbc.272.5.3007 [DOI] [PubMed] [Google Scholar]
- Brenner T., Nizri E., Irony-Tur-Sinai M., Hamra-Amitay Y., Wirguin I. (2008). Acetylcholinesterase inhibitors and cholinergic modulation in Myasthenia Gravis and neuroinflammation. J. Neuroimmunol. 201–202, 121–127. 10.1016/j.jneuroim.2008.05.022 [DOI] [PubMed] [Google Scholar]
- Chen V. P., Choi R. C., Chan W. K., Leung K. W., Guo A. J., Chan G. K., Luk W. K., Tsim K. W. (2011). The assembly of PRiMA-linked acetylcholinesterase: glycosylation is required for enzymatic activity but not for oligomerization. J Biol Chem. 286, 32948–32961 10.1074/jbc.M110.175273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V. P., Xie H. Q., Chan W. K., Leung K. W., Chan G. K., Choi R. C., Bon S., Massoulié J., Tsim K. W. (2010). The PRiMA-linked cholinesterase tetramers are assembled from homodimers: hybrid molecules composed of acetylcholinesterase and butyrylcholinesterase dimers are up-regulated during development of chicken brain. J. Biol. Chem. 285, 27265–27278 10.1074/jbc.M110.101469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V. P., Xie H. Q., Leung K. W., Choi R. C., Tsim K. W. (2009). “The molecular assembly of acetylcholinesterase and butyrylcholinesterase: the formation of PRiMA-linked G4 hybrid,” in Joint Scientific Meeting of the 27th Hong Kong Society of Neurosciences and the 6th Biophysical Society of Hong Kong, Hong Kong, 144 [Google Scholar]
- Choi R. C., Ting A. K., Lau F. T., Xie H. Q., Leung K. W., Chen V. P., Siow N. L., Tsim K. W. (2007). Calcitonin gene-related peptide induces the expression of acetylcholinesterase-associated collagen ColQ in muscle: a distinction in driving two different promoters between fast- and slow-twitch muscle fibers. J. Neurochem. 102, 1316–1328 10.1111/j.1471-4159.2007.04630.x [DOI] [PubMed] [Google Scholar]
- Crne-Finderle N., Pregelj P., Sketelj J. (2005). Junctional and extrajunctional acetylcholinesterase in skeletal muscle fibers. Chem. Biol. Interact. 157–158, 23–27. 10.1016/j.cbi.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Deprez P., Inestrosa N. C., Krejci E. (2003). Two different heparin-binding domains in the triple-helical domain of ColQ, the collagen tail subunit of synaptic acetylcholinesterase. J. Biol. Chem. 278, 23233–23242 10.1074/jbc.M301384200 [DOI] [PubMed] [Google Scholar]
- Duval N., Krejci E., Grassi J., Coussen F., Massoulié J., Bon S. (1992). Molecular architecture of acetylcholinesterase collagen-tailed forms; construction of a glycolipid-tailed tetramer. EMBO J. 11, 3255–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen E. G., Li B., Xie W., Schopfer L. M., Anderson R. S., Broomfield C. A., Lockridge O. (2001). Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J. Pharmacol. Exp. Ther. 299, 528–535 [PubMed] [Google Scholar]
- Dvir H., Harel M., Bon S., Liu W. Q., Vidal M., Garbay C., Sussman J. L., Massoulié J., Silman I. (2004). The synaptic acetylcholinesterase tetramer assembles around a polyproline II helix. EMBO J. 23, 4394–4405 10.1038/sj.emboj.7600425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M., Aarsland D., Albanese A., Byrne E. J., Deuschl G., De Deyn P. P., Durif F., Kulisevsky J., van Laar T., Lees A., Poewe W., Robillard A., Rosa M. M., Wolters E., Quarg P., Tekin S., Lane R. (2004). Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 351, 2509–2518 10.1056/NEJMoa041470 [DOI] [PubMed] [Google Scholar]
- Falasca C., Perrier N., Massoulié J., Bon S. (2005). Determinants of the t peptide involved in folding, degradation, and secretion of acetylcholinesterase. J. Biol. Chem. 280, 878–886 10.1074/jbc.M409201200 [DOI] [PubMed] [Google Scholar]
- Feng G., Krejci E., Molgo J., Cunningham J. M., Massoulié J., Sanes J. R. (1999). Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J. Cell Biol. 144, 1349–1360 10.1083/jcb.144.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R. (1995). Molecular biology of insecticide resistance. Toxicol. Lett. 82–83, 83–90. 10.1016/0378-4274(95)03470-6 [DOI] [PubMed] [Google Scholar]
- García-Ayllón M. S., Sáez-Valero J., Muñoz-Delgado E., Vidal C. J. (2001). Identification of hybrid cholinesterase forms consisting of acetyl- and butyrylcholinesterase subunits in human glioma. Neuroscience 107, 199–208 10.1016/S0306-4522(01)00355-4 [DOI] [PubMed] [Google Scholar]
- Giacobini E. (2000). Cholinesterase inhibitors stabilize Alzheimer’s disease. Neurochem. Res. 25, 1185–1190 10.1023/A:1007679709322 [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Fazzini E. (1996). Cholinesterase inhibition in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatr. 61, 324–325 10.1136/jnnp.61.3.324-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa N. C., Moreno R. D., Fuentes M. E. (1994). Monomeric amphiphilic forms of acetylcholinesterase appear early during brain development and may correspond to biosynthetic precursors of the amphiphilic G4 forms. Neurosci. Lett. 173, 155–158 10.1016/0304-3940(94)90172-4 [DOI] [PubMed] [Google Scholar]
- Kaufer D., Friedman A., Seidman S., Soreq H. (1998). Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393, 373–377 10.1038/30741 [DOI] [PubMed] [Google Scholar]
- Kolarich D., Weber A., Pabst M., Stadlmann J., Teschner W., Ehrlich H., Schwarz H. P., Altmann F. (2008). Glycoproteomic characterization of butyrylcholinesterase from human plasma. Proteomics 8, 254–263 10.1002/pmic.200700720 [DOI] [PubMed] [Google Scholar]
- Krejci E., Coussen F., Duval N., Chatel J. M., Legay C., Puype M., Vandekerckhove J., Cartaud J., Bon S., Massoulié J. (1991). Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 10, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci E., Legay C., Thomine S., Sketelj J., Massoulie J. (1999). Differences in expression of acetylcholinesterase and collagen Q control the distribution and oligomerization of the collagen-tailed forms in fast and slow muscles. J. Neurosci. 19, 10672–10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci E., Thomine S., Boschetti N., Legay C., Sketelj J., Massoulié J. (1997). The mammalian gene of acetylcholinesterase-associated collagen. J. Biol. Chem. 272, 22840–22847 10.1074/jbc.272.36.22840 [DOI] [PubMed] [Google Scholar]
- Lau F. T., Choi R. C., Xie H. Q., Leung K. W., Chen V. P., Zhu J. T., Bi C. W., Chu G. K., Tsim K. W. (2008). Myocyte enhancer factor 2 mediates acetylcholine-induced expression of acetylcholinesterase-associated collagen ColQ in cultured myotubes. Mol. Cell. Neurosci. 39, 429–438 10.1016/j.mcn.2008.07.018 [DOI] [PubMed] [Google Scholar]
- Lee H. H., Choi R. C., Ting A. K., Siow N. L., Jiang J. X., Massoulié J., Tsim K. W. (2004). Transcriptional regulation of acetylcholinesterase-associated collagen ColQ. J. Biol. Chem. 279, 27098–27107 10.1074/jbc.M405271200 [DOI] [PubMed] [Google Scholar]
- Legay C., Huchet M., Massoulié J., Changeux J. (1995). Developmental regulation of acetylcholinesterase transcripts in the mouse diaphragm: alternative splicing and focalization. Eur. J. Neurosci. 7, 1803–1809 10.1111/j.1460-9568.1995.tb00699.x [DOI] [PubMed] [Google Scholar]
- Leung K. W., Xie H. Q., Chen V. P., Mok M. K., Chu G. K., Choi R. C., Tsim K. W. (2009). Restricted localization of proline-rich membrane anchor (PRiMA) of globular form acetylcholinesterase at the neuromuscular junctions – contribution and expression from motor neurons. FEBS J. 276, 3031–3042 10.1111/j.1742-4658.2009.07022.x [DOI] [PubMed] [Google Scholar]
- Li Y., Camp S., Rachinsky T. L., Getman D., Taylor P. (1991). Gene structure of mammalian acetylcholinesterase. Alternative exons dictate tissue-specific expression. J. Biol. Chem. 266, 23083–23090 [PubMed] [Google Scholar]
- Liang D., Blouet J. P., Borrega F., Bon S., Massoulié J. (2009). Respective roles of the catalytic domains and C-terminal tail peptides in the oligomerization and secretory trafficking of human acetylcholinesterase and butyrylcholinesterase. FEBS J. 276, 94–108 10.1111/j.1742-4658.2008.06756.x [DOI] [PubMed] [Google Scholar]
- Liao J., Heider H., Sun M. C., Brodbeck U. (1992). Different glycosylation in acetylcholinesterases from mammalian brain and erythrocytes. J. Neurochem. 58, 1230–1238 10.1111/j.1471-4159.1992.tb11333.x [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. (1987). Complete amino acid sequence of human serum cholinesterase. J. Biol. Chem. 262, 549–557 [PubMed] [Google Scholar]
- Massoulié J. (2002). The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals 11, 130–143 10.1159/000065054 [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S., Perrier N., Falasca C. (2005). The C-terminal peptides of acetylcholinesterase: cellular trafficking, oligomerization and functional anchoring. Chem. Biol. Interact. 157–158, 3–14. 10.1016/j.cbi.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. (1993). Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 41, 31–91 10.1016/0301-0082(93)90040-Y [DOI] [PubMed] [Google Scholar]
- Mehndiratta M. M., Pandey S., Kuntzer T. (2011). Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst. Rev. 2, CD006986. [DOI] [PubMed] [Google Scholar]
- Mendel B., Rudney H. (1943). Studies on cholinesterase: 1. Cholinesterase and pseudo-cholinesterase. Biochem. J. 37, 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori Y., Nakamura S., Yukawa M. (1997). Abnormalities of acetylcholinesterase in Alzheimer’s disease with special reference to effect of acetylcholinesterase inhibitor. Behav. Brain Res. 83, 25–30 10.1016/S0166-4328(97)86041-X [DOI] [PubMed] [Google Scholar]
- Morel N., Leroy J., Ayon A., Massoulié J., Bon S. (2001). Acetylcholinesterase H and T dimers are associated through the same contact. Mutations at this interface interfere with the C-terminal T peptide, inducing degradation rather than secretion. J. Biol. Chem. 276, 37379–37389 10.1074/jbc.M103574200 [DOI] [PubMed] [Google Scholar]
- Noureddine H., Schmitt C., Liu W., Garbay C., Massoulié J., Bon S. (2007). Assembly of acetylcholinesterase tetramers by peptidic motifs from the proline-rich membrane anchor, PRiMA: competition between degradation and secretion pathways of heteromeric complexes. J. Biol. Chem. 282, 3487–3497 10.1074/jbc.M607221200 [DOI] [PubMed] [Google Scholar]
- Ohno K., Brengman J., Tsujino A., Engel A. G. (1998). Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc. Natl. Acad. Sci. U.S.A. 95, 9654–9659 10.1073/pnas.95.16.9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier A. L., Massoulié J., Krejci E. (2002). PRiMA: the membrane anchor of acetylcholinesterase in the brain. Neuron 33, 275–285 10.1016/S0896-6273(01)00584-0 [DOI] [PubMed] [Google Scholar]
- Perrier N. A., Khérif S., Perrier A. L., Dumas S., Mallet J., Massoulié J. (2003). Expression of PRiMA in the mouse brain: membrane anchoring and accumulation of “tailed” acetylcholinesterase. Eur. J. Neurosci. 18, 1837–1847 10.1046/j.1460-9568.2003.02914.x [DOI] [PubMed] [Google Scholar]
- Perrier N. A., Salani M., Falasca C., Bon S., Augusti-Tocco G., Massoulié J. (2005). The readthrough variant of acetylcholinesterase remains very minor after heat shock, organophosphate inhibition and stress, in cell culture and in vivo. J. Neurochem. 94, 629–638 10.1111/j.1471-4159.2005.03140.x [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. (1984). Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc. Natl. Acad. Sci. U.S.A. 81, 479–483 10.1073/pnas.81.2.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L. (1988). Biogenesis of acetylcholinesterase molecular forms in muscle. Evidence for a rapidly turning over, catalytically inactive precursor pool. J. Biol. Chem. 263, 19398–19406 [PubMed] [Google Scholar]
- Rotundo R. L., Thomas K., Porter-Jordan K., Benson R. J., Fernandez-Valle C., Fine R. E. (1989). Intracellular transport, sorting, and turnover of acetylcholinesterase. Evidence for an endoglycosidase H-sensitive form in Golgi apparatus, sarcoplasmic reticulum, and clathrin-coated vesicles and its rapid degradation by a non-lysosomal mechanism. J. Biol. Chem. 264, 3146–3152 [PubMed] [Google Scholar]
- Ruiz C. A., Rotundo R. L. (2009a). Dissociation of transcription, translation, and assembly of collagen tailed acetylcholinesterase in skeletal muscle. J. Biol. Chem. 284, 21488–21495 10.1074/jbc.M109.038471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C. A., Rotundo R. L. (2009b). Limiting role of protein disulfide isomerase in the expression of collagen-tailed acetylcholinesterase forms in muscle. J. Biol. Chem. 284, 31753–31763 10.1074/jbc.M109.038471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Valero J., Barquero M. S., Marcos A., McLean C. A., Small D. H. (2000). Altered glycosylation of acetylcholinesterase in lumbar cerebrospinal fluid of patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatr. 69, 664–667 10.1136/jnnp.69.5.664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Valero J., Sberna G., McLean C. A., Small D. H. (1999). Molecular isoform distribution and glycosylation of acetylcholinesterase are altered in brain and cerebrospinal fluid of patients with Alzheimer’s disease. J. Neurochem. 72, 1600–1608 10.1046/j.1471-4159.1999.721600.x [DOI] [PubMed] [Google Scholar]
- Sketelj J., Brzin M. (1985). Asymmetric molecular forms of acetylcholinesterase in mammalian skeletal muscles. J. Neurosci. Res. 14, 95–103 10.1002/jnr.490140109 [DOI] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y., Zakut H. (1990). Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc. Natl. Acad. Sci. U.S.A. 87, 9688–9692 10.1073/pnas.87.24.9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. G., Casadesus G., Gustaw-Rothenberg K., Siedlak S. L., Wang X., Zhu X., Perry G., Castellani R. J., Smith M. A. (2011). Frontiers in Alzheimer’s disease therapeutics. Ther. Adv. Chronic Dis. 2, 9–23 10.1177/2040622310382817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. (1991). Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 253, 872–879 10.1126/science.1678899 [DOI] [PubMed] [Google Scholar]
- Tsim K. W., Randall W. R., Barnard E. A. (1988a). An asymmetric form of muscle acetylcholinesterase contains three subunit types and two enzymic activities in one molecule. Proc. Natl. Acad. Sci. U.S.A. 85, 1262–1266 10.1073/pnas.85.4.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsim K. W., Randall W. R., Barnard E. A. (1988b). Synaptic acetylcholinesterase of chicken muscle changes during development from a hybrid to a homogeneous enzyme. EMBO J. 7, 2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velan B., Kronman C., Ordentlich A., Flashner Y., Leitner M., Cohen S., Shafferman A. (1993). N-glycosylation of human acetylcholinesterase: effects on activity, stability and biosynthesis. Biochem. J. 296, 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. Q., Choi R. C., Leung K. W., Chen V. P., Chu G. K., Tsim K. W. (2009). Transcriptional regulation of proline-rich membrane anchor (PRiMA) of globular form acetylcholinesterase in neuron: an inductive effect of neuron differentiation. Brain Res. 1265, 13–23 10.1016/j.brainres.2009.01.065 [DOI] [PubMed] [Google Scholar]
- Xie H. Q., Choi R. C., Leung K. W., Siow N. L., Kong L. W., Lau F. T., Peng H. B., Tsim K. W. (2007). Regulation of a transcript encoding the proline-rich membrane anchor of globular muscle acetylcholinesterase. J. Biol. Chem. 282, 11765–11775 10.1074/jbc.M608265200 [DOI] [PubMed] [Google Scholar]
- Xie H. Q., Liang D., Leung K. W., Chen V. P., Zhu K. Y., Chan W. K., Choi R. C., Massoulié J., Tsim K. W. (2010a). Targeting acetylcholinesterase to membrane rafts: a function mediated by the proline-rich membrane anchor (PRiMA) in neurons. J. Biol. Chem. 285, 11537–11546 10.1074/jbc.M110.153676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. Q., Leung K. W., Chen V. P., Chan G. K., Xu S. L., Guo A. J., Zhu K. Y., Zheng K. Y., Bi C. W., Zhan J. Y., Chan W. K., Choi R. C., Tsim K. W. (2010b). PRiMA directs a restricted localization of tetrameric AChE at synapses. Chem. Biol. Interact. 187, 78–83 10.1016/j.cbi.2010.02.018 [DOI] [PubMed] [Google Scholar]
- Xie W., Stribley J. A., Chatonnet A., Wilder P. J., Rizzino A., McComb R. D., Taylor P., Hinrichs S. H., Lockridge O. (2000). Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesterase. J. Pharmacol. Exp. Ther. 293, 896–902 [PubMed] [Google Scholar]
- Zakut H., Even L., Birkenfeld S., Malinger G., Zisling R., Soreq H. (1988). Modified properties of serum cholinesterases in primary carcinomas. Cancer 61, 727–737 [DOI] [PubMed] [Google Scholar]