Abstract
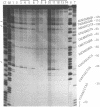
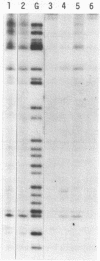
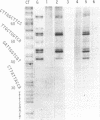
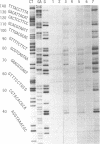
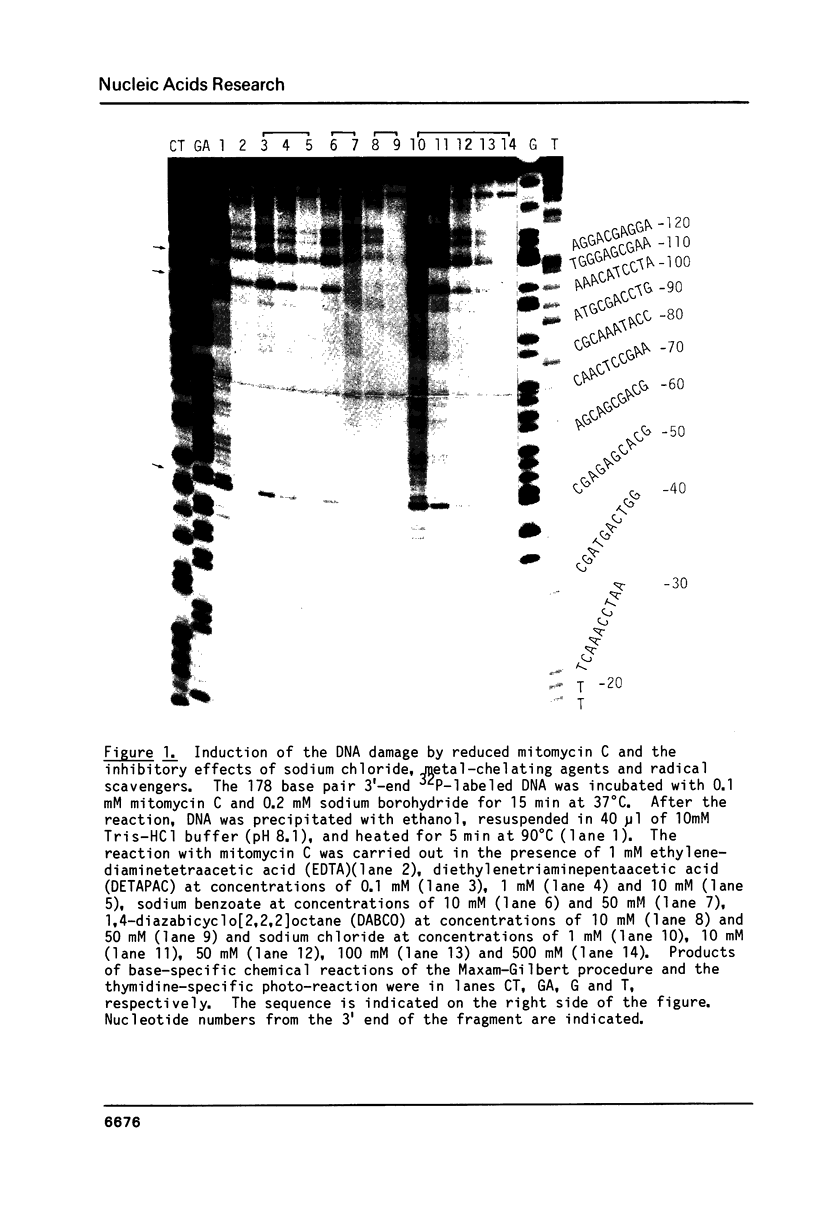
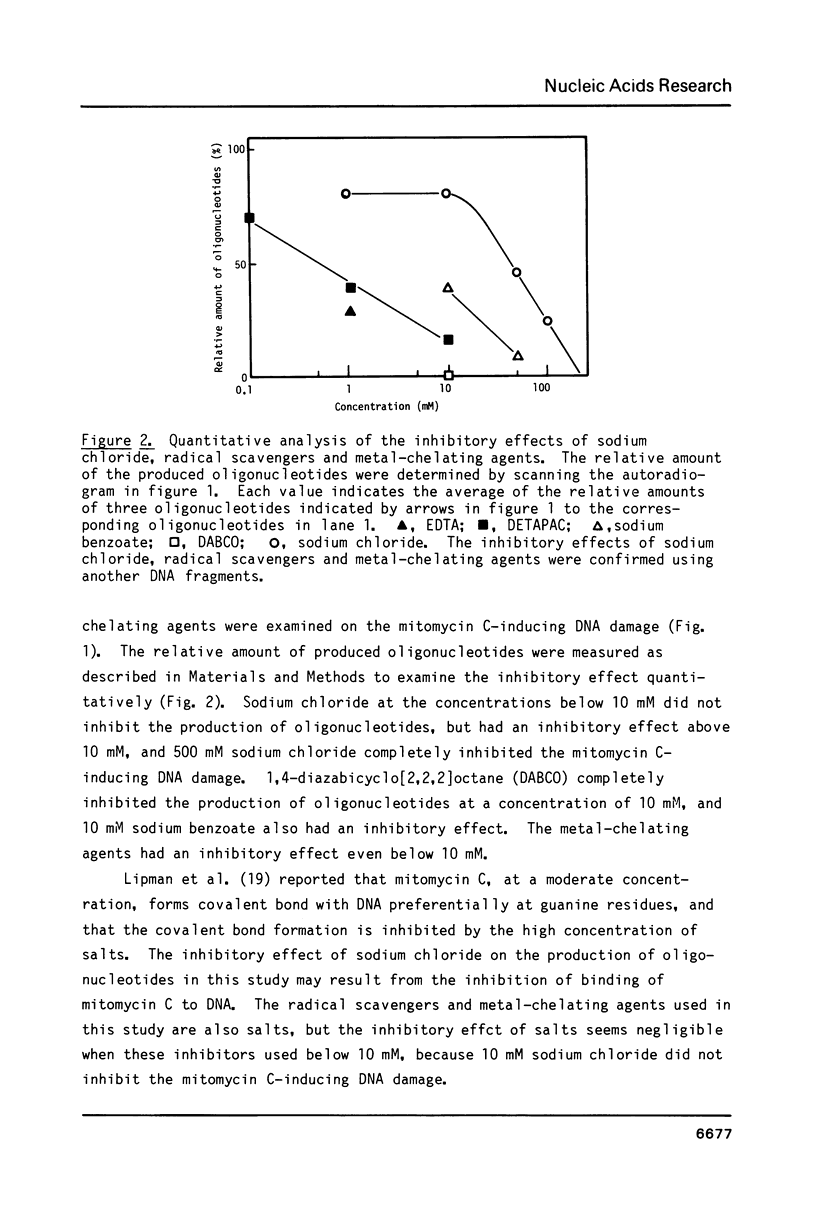
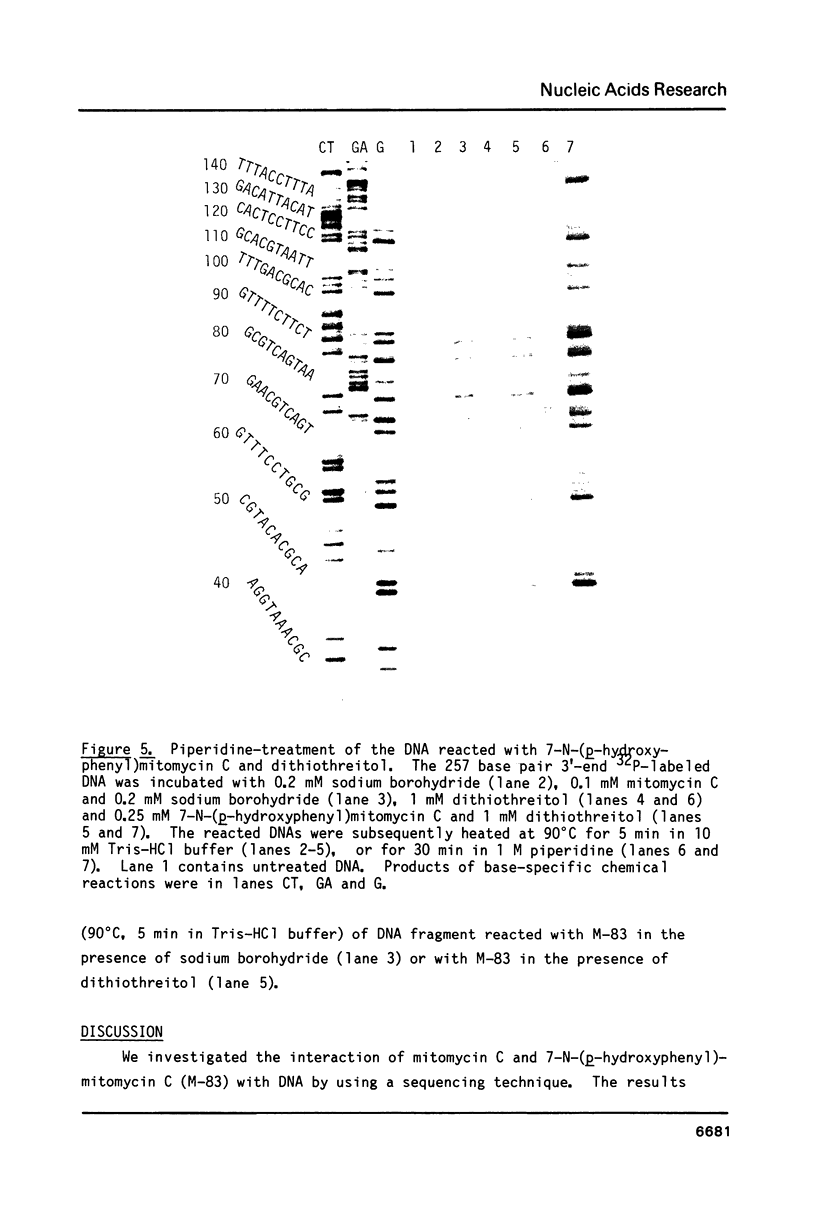
Mitomycin C reduced with sodium borohydride induced the DNA damage at deoxyguanosines preferentially in dinucleotide sequence G-T. The DNA damage produced strand breaks when subsequently heated. The DNA damage scarcely occurred when the end-labeled DNA was preincubated with ethidium bromide or actinomycin D before the addition of mitomycin C and the reducing agent. Fully reduced mitomycin C did not induce the DNA damage. The mitomycin C-inducing DNA damage seems to require the intercalation of the partially reduced mitomycin C of short life time, probably semiquinone radical, between DNA base pairs. The inhibitory effects of sodium chloride and radical scavengers suggested that the requirement of the covalent bond formation of mitomycin C to DNA and the involvement of oxygen radicals in the DNA damage. 7-N-(p-hydroxyphenyl)mitomycin C, which is reported to show a higher antitumor activity and a lower toxicity than mitomycin C, was readily reduced with dithiothreitol and induced the sequence-specific DNA damage, whereas mitomycin C was not.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. N. Formation of alkali labile linkages in DNA by hedamycin and use of hedamycin as a probe of protein-DNA complexes. Nucleic Acids Res. 1982 Aug 11;10(15):4581–4594. doi: 10.1093/nar/10.15.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama T., Goldberg I. H., Takeshita M., Grollman A. P. Nucleotide specificity in DNA scission by neocarzinostatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3603–3607. doi: 10.1073/pnas.75.8.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai R., Ashizawa T., Urakawa C., Morimoto M., Nakamura N. Antitumor activity of 7-N-phenyl derivatives of mitomycin C in the leukemia P388 system. Gan. 1980 Aug;71(4):560–562. [PubMed] [Google Scholar]
- Imai R., Morimoto M., Marumo H., Kobayashi T., Tsuruo T., Inaba M., Tsukagoshi S., Sakurai Y. Antitumor activity of 7-n-(p-hydroxyphenyl)-mitomycin C in experimental tumor systems. Gan. 1981 Dec;72(6):944–949. [PubMed] [Google Scholar]
- Kobayashi T., Inaba M., Tsukagoshi S., Sakurai Y., Imai R., Morimoto M. Comparison of the hematologic toxicity of 7-N-(p-hydroxyphenyl),-mitomycin C and mitomycin C. Gan. 1981 Dec;72(6):950–954. [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. J., Dabrowiak J. C., Vournakis J. N. Sequence specificity of actinomycin D and Netropsin binding to pBR322 DNA analyzed by protection from DNase I. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3260–3264. doi: 10.1073/pnas.80.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R., Weaver J., Tomasz M. Electrostatic complexes of mitomycin C with nucleic acids and polyanions. Biochim Biophys Acta. 1978 Dec 21;521(2):779–791. doi: 10.1016/0005-2787(78)90317-9. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO I., LARK K. G. ALTERED DNA ISOLATED FROM CELLS TREATED WITH MITOMYCIN C. Exp Cell Res. 1963 Oct;32:192–196. doi: 10.1016/0014-4827(63)90089-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remers W. A., Weiss M. J. The mitomycin antibiotics. Synthetic studies. XXI. Indoloquinone analogs with further variations at C-5. J Med Chem. 1968 Jul;11(4):737–742. doi: 10.1021/jm00310a601. [DOI] [PubMed] [Google Scholar]
- Saito I., Sugiyama H., Matsuura T., Ueda K., Komano T. A new procedure for determining thymine residues in DNA sequencing. Photoinduced cleavage of DNA fragments in the presence of spermine. Nucleic Acids Res. 1984 Mar 26;12(6):2879–2885. doi: 10.1093/nar/12.6.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz M., Mercado C. M., Olson J., Chatterjie N. The mode of interaction of mitomycin C with deoxyribonucleic acid and other polynucleotides in vitro. Biochemistry. 1974 Nov 19;13(24):4878–4887. doi: 10.1021/bi00721a002. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Novel assay of 7-alkylation of guanine residues in DNA. Application to nitrogen mustard, triethylenemelamine and mitomycin C. Biochim Biophys Acta. 1970 Aug 8;213(2):288–295. doi: 10.1016/0005-2787(70)90037-7. [DOI] [PubMed] [Google Scholar]
- Ueda K., Morita J., Komano T. Induction of single strand scission in bacteriophage phi X174 replicative form I DNA by mitomycin C. J Antibiot (Tokyo) 1981 Mar;34(3):317–322. doi: 10.7164/antibiotics.34.317. [DOI] [PubMed] [Google Scholar]
- Ueda K., Morita J., Komano T. Phage inactivation and DNA strand scission activities of 7-N-(p-hydroxyphenyl)mitomycin C. J Antibiot (Tokyo) 1982 Oct;35(10):1380–1386. doi: 10.7164/antibiotics.35.1380. [DOI] [PubMed] [Google Scholar]
- Ueda K., Morita J., Komano T. Sequence specificity of heat-labile sites in DNA induced by mitomycin C. Biochemistry. 1984 Apr 10;23(8):1634–1640. doi: 10.1021/bi00303a008. [DOI] [PubMed] [Google Scholar]