Abstract
Long-day exposure of the grass Lolium temulentum may regulate flowering via changes in gibberellin (GA) levels. Therefore, we have examined both GA levels and expression of a MYB transcription factor that is specific to the GA signal transduction pathway in monocots. This MYB gene from L. temulentum shows over 90% nucleotide identity with the barley and rice GAMYB genes, and, like them, gibberellic acid (GA3) up-regulates its expression in the seed. Furthermore, cDNAs of both the barley and L. temulentum GAMYB show the same simple patterns of hybridization with digests of L. temulentum genomic DNA. Compared with vegetative shoot apices of L. temulentum, the in situ mRNA expression of LtGAMYB does not change during the earliest steps of “floral” initiation at the apex. However, by 100 h (the double-ridge stage of flowering) its expression increased substantially and was highest in the terminal and lateral spikelet sites. Thereafter, expression declined overall but then increased within stamen primordia. Prior to increased LtGAMYB expression, long-day exposure sufficient to induce flowering led to increased (5- to 20-fold) levels of GA1 and GA4 in the leaf. Thus, increases first in GA level in the leaf followed by increased expression of LtGAMYB in the apex suggest important signaling and/or response roles in flowering.
GAs have long been considered to play a role in flowering. Such a conclusion is supported by the observation that a number of LD plants, including Lolium temulentum, flower in response to applied GA (Lang, 1965). Also, within the shoot apex of L. temulentum the levels of bioassayed GA-like substances are elevated after exposure to florally inductive LDs (Pharis et al., 1987), as are endogenous GAs in leaves of some species (Graebe, 1987, and refs. therein). Thus, with exposure to LDs, increased GA content in the leaf with its subsequent transport to the shoot apex could represent one component in the regulation of the vegetative to floral transition at the apex of the LD plant L. temulentum.
A genetic approach to defining the role for GA in LD-induced flowering, although not pursued for L. temulentum, has been particularly informative with other LD plants. For example, ga1-3, a dwarf mutant of Arabidopsis with very reduced GA levels, never flowered under noninductive SDs, but GA application or exposure to LD conditions led to rapid flowering (Wilson et al., 1992). Conversely, the spy(spindly) and elo(elongated) mutants of Arabidopsis, which have the appearance of being treated with a high level of GA, flower early (Jacobsen and Olszewski, 1993; Halliday et al., 1996; Jacobsen et al., 1996). Thus, not only does GA promote flowering but also apparently substitutes for LD conditions.
At the molecular level little is known about how GAs regulate flowering, but the expression of at least one gene could be regulated by GA during flowering. The LEAFY gene of Arabidopsis is known to regulate early floral events and its promoter is responsive to GA (Blázquez et al., 1997, 1998). In addition, applied GA can rescue the weak flowering of leafy mutants (Okamuro et al., 1996). A further candidate gene is the GAMYB transcription factor. In the cereal aleurone the GAMYB protein is a transcriptional activator in GA regulation (Gubler et al., 1995); it acts by binding to a GA-response element (TAACAAA) in the promoter of an α-amylase gene. A GAMYB gene could therefore be as important as LEAFY in GA transcriptional regulation of flowering. Here we show that there is a GAMYB homolog in L. temulentum, that it is expressed in shoot apices, and that its expression is up-regulated during the vegetative to floral transition in parallel with increased GA levels. Thus, GA acting via GAMYB may play roles in both the cereal aleurone and, separately, in the shoot apex during its transition to flowering.
MATERIALS AND METHODS
Plant Material
Lolium temulentum L. strain Ceres plants were grown vegetatively in SD conditions (8 h) as described previously (Evans et al., 1990). When these plants are 6 or more weeks old, exposure to a single LD (16-h incandescent lamp extension, 15 μmol m−2 s−1) leads to rapid inflorescence formation (flowering). Controls retained in SD conditions remain vegetative. Gocal (1997) has described the sequence, timing, and spatial arrangement of floral organ development of the material used here for in situ mRNA hybridization. After 6 weeks of growth the vegetative apex had accumulated up to eight leaf primordia below the shoot apical dome. Upon flowering, along with a rapid (2- to 4-d) production of more spikelets by the dome, the presumptive spikelet sites between the leaf primordia were activated, forming the characteristic double-ridge stage that is the first morphological sign of inflorescence development (5–7 d after LD conditions). Spikelet outgrowth (8–11 d) followed, then the formation of glume, lemma, and floret primordia, and, finally (21 d), anther primordia appeared.
Molecular Cloning of the L. temulentum GAMYB Homolog LtGAMYB
Gocal (1997) has described the molecular techniques used to identify and characterize the L. temulentum genes expressed at the shoot apex. LtGAMYB was identified from duplicate lifts of a PCR-based cDNA library constructed from L. temulentum LD III shoot apices and hybridized with a barley GAMYB (HvGAMYB) 5′ fragment (nucleotides 272–1121; Gubler et al., 1995). The filters were washed at intermediate stringency (0.5× SSPE; 0.2% SDS at 65°C). Cloned DNA fragments were sequenced in both directions with either dideoxy or dye primer ready-reaction sequencing kits (PRISM, Applied Biosystems). We used Genetics Computer Group software (version 8.0; Devereux et al., 1984) to do sequence analysis.
DNA Analysis
We followed the method of Dellaporta et al. (1983) to isolate genomic DNA from etiolated 2-week-old L. temulentum seedlings. Twenty micrograms of DNA was digested with BglII, EcoRI, or XhoI, fractionated in a 1% agarose gel, and blotted onto Hybond N membrane according to the manufacturer's instructions (Amersham). To provide evidence that the L. temulentum cDNA encoded the barley GAMYB homolog, we hybridized a 3′ conserved EcoRI/SacI fragment (nucleotides 1239–1930) to a blot that was washed at high stringency (0.1× SSPE; 0.2% SDS at 65°C).
RNA Analysis
L. temulentum seeds were sterilized and dehusked by shaking for 2 h in 50% H2SO4 and then rinsed in sterile water. The embryos were cut off with a scalpel and the remaining half seeds were hydrated overnight on moist filter paper and then incubated at 25°C in 2 mL of 10 mm CaCl2, 150 μg mL−1 cefatoxime, 50 units mL−1 nystatin, and either 0 or 10−6 m GA3. We used the method of Schuurink et al. (1996) to isolate RNA from the endosperm halves. For RNA analysis, we fractionated 10 μg of RNA in a 1% agarose gel containing formaldehyde and blotted it onto a nylon membrane. The blots were hybridized with a 32P-dCTP-labeled EcoRI/SacI LtGAMYB fragment. The blot was washed at high stringency and analyzed by autoradiography. We reprobed the blots with a barley α-amylase cDNA (1–28 from P. Matthews, CSIRO) and a 9-kb wheat rRNA clone, pTA71 (Gerlach and Bedbrook, 1979).
In Situ mRNA Hybridizations
The in situ hybridization results were obtained with shoot apex sections collected in one experiment (Lt434) and are therefore directly comparable. Similar timing of floral development was seen across experiments (Gocal, 1997). On harvesting, the apices were fixed, dehydrated, paraffin-embedded, sectioned, and used for in situ hybridization according to the method of Gocal (1997). The LtGAMYB-specific EcoRI/SacI fragment (see above) was subcloned into the EcoRI/EcoRV sites of pBluescript SK(+) and pBluescript KS(+) (Stratagene). Sense and antisense DIG-labeled, in vitro-transcribed riboprobes were synthesized from the T7 promoter of these subclones linearized with EcoRI. The hybridizing probe was detected colorimetrically using an anti-DIG Fab fragment conjugated to alkaline phosphatase. Photographs were taken with Nomarski optics on an Axioplan microscope (Zeiss). The film was Fujichrome 64T and all exposures were identical.
Analysis of Endogenous GAs
To identify endogenous GAs by full-scan GC-MS, we harvested 16 g dry weight of shoots from 3-week-old seedlings from plants grown in SD conditions. On the basis of this identification, we used GC-MS with single- and multiple-ion monitoring to follow changes in GA levels in just fully expanded leaves from 6-week-old plants.
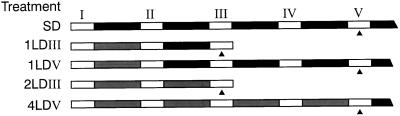
Leaves were harvested at a fixed time of the day after 0, 1, 2, or 4 d of LD treatment as shown in Figure 1. At harvest, the tissue was frozen in liquid nitrogen and ground with a mortar and pestle before lyophilization. The samples (6–7.5 g dry weight) were extracted in 250 mL of 80% (v/v) methanol at 4°C for 24 h and then filtered. The residue was re-extracted in 250 mL of 100% methanol for a further 24 h; following filtration we combined all of the methanolic fractions. Dideuterated GA standards (from L.N. Mander, Australian National University, Canberra) were added at the time of extraction. The amount of each GA added ranged from 1 to 20 ng g−1 dry weight of the sample and was estimated from preliminary assays so that in any sample, the ratio of deutero to protio ions was close to 1:1. Twelve thousand counts per minute each of high specific activity [3H]GA20, [3H]dihydro-GA19 (from J. Lenton, Long Ashton Research Station, Bristol, UK), and [3H]GA1 (from R.P. Pharis, University of Calgary, Alberta, Canada) were added to monitor GAs during purification. At these levels, none of these tritiated GAs could be detected by GC-MS. We extracted a sample for full-scan identification in 950 mL of 80% (v/v) methanol and added only the tritiated standards.
Figure 1.
Harvesting schedule (▴) in relation to the daily exposure to light (white bars), darkness (black bars), and to incandescent daylength extension (gray bars) for vegetative control plants exposed to SDs or to 1, 2, or 4 florally inductive LDs.
The extracts were reduced to an aqueous solution under reduced pressure at 35°C, then frozen overnight, thawed, and centrifuged. The supernatants were adjusted to pH 2.5 using 2 m HCl, and then filtered through a 0.2-μm nylon filter (Millipore) before ethyl acetate partitioning. Further purification through QAE Sephadex and C18 Sep-Pak was outlined in Green et al. (1997). Initial HPLC involved a 25-cm × 4.6-mm i.d. × 5-μm particle size C18 column (Allsphere ODS-2, Alltech, Deerfield, MI). Solvent A consisted of 10% methanol in 2 mm acetic acid, and solvent B was 100% methanol. A linear gradient from 20% B in A to 100% B over 40 min was used, with a flow rate of 1 mL min−1. We collected 1-min fractions and pooled and dried five groupings, based on the elution of radiolabeled GAs. Each of these groupings was then chromatographed isocratically at 1 mL min−1 with methanol containing 0.05% acetic acid using a 15-cm × 4.6-mm i.d. × 5-μm particle size N(CH3)2 column (Nucleosil, Alltech). We collected and pooled 1-min fractions, based on the tritiated standard elution times. Because of persistent impurities, some samples required a third HPLC step after methylation; in these cases we used the initial C18 column and conditions.
Samples were methylated and derivatized (Green et al., 1997) before injection onto a 25-m × 0.22-mm i.d. × 0.25-μm film thickness fused silica column (BPX-5, SGE, Austin, TX). GC conditions were as in Green et al. (1997). For GA identification in L. temulentum we analyzed a portion of each sample in the full-scan mode, and reanalyzed the remainder of the sample in the multiple-ion-monitoring mode (at least eight characteristic ions). We co-injected all samples with Parafilm to determine the Kovats Retention Index, and used authentic GAs (from L.N. Mander) for comparison. We also compared the full scans with a PC-based GA spectral library (Gaskin and MacMillan, 1991). For quantification we used the following pairs of characteristic ions (deutero ion/protio ion): 508/506 (GA1), 286/284 (GA4), 596/594 (GA8), 300/298 (GA9), 436/434 (GA19), 420/418 (GA20), 508/506 (GA29), 434/432 (GA44), and 450/448 (GA53). With the exception of GA53, calibration curves were used. For GA53, we made appropriate corrections to account for contributions to the area of the 450 ion from endogenous GA53.
RESULTS
L. temulentum GAMYB Gene
In initial experiments, barley 5′ or 3′ HvGAMYB probes were hybridized to a L. temulentum PCR-based cDNA gel blot. The largest hybridizing band was 2.8 kb, approximately the expected size for a GAMYB gene. Subsequently, two plaques in 360,000 from a PCR-based cDNA library showed specific hybridization. These were partially overlapping cDNAs encoded by the same gene. A complete sequence was obtained by primer extension of these cDNAs. The primer pair was designated as 001-Hind (5′-d [ATAAGCTTGAGATGTACCGGGTGAAGAGCGAGAGC]-3′), based on the barley GAMYB start codon sequence (Gubler et al., 1995), and Lo8 (5′-d[AAAGACCATTCCCATTCAGA]-3′), based on the L. temulentum MYB-like gene outside the R2/R3 repeat. Two rounds of amplification consisting of 30 and 20 cycles, respectively, were performed with Pfu DNA polymerase. The single band found on electrophoresis of the reaction product was blunt-end cloned and three inserts were sequenced. All three gave an identical reaction product of 567 bp, which overlapped by 114 bp with the 5′ end of each cDNA clone isolated in the library screen. The nucleotide sequence of the first nine amino acids of the LtGAMYB sequence is uncertain because it is identical to that of the primer.
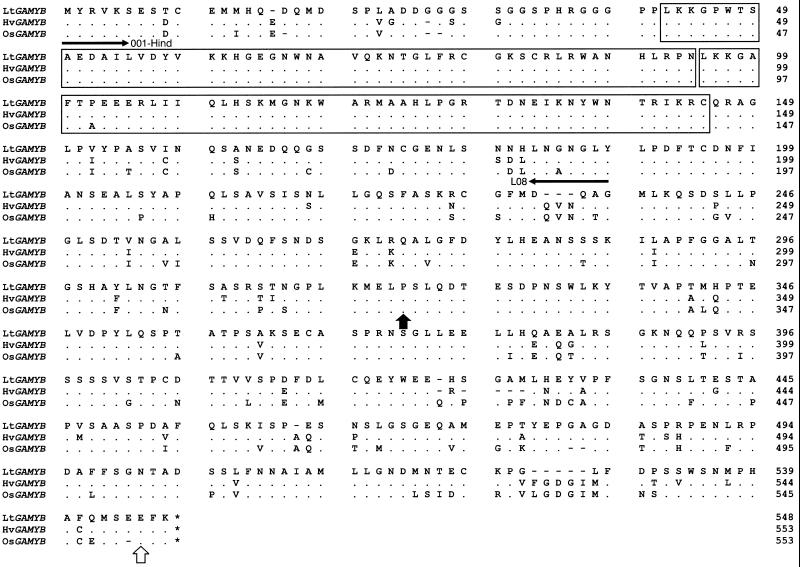
The nucleotide sequence of the LtGAMYB clone was lodged with GenBank (accession no. AF114162). It is 91% identical to both the barley and rice GAMYB sequences, with their proteins being 95% and 94% similar, respectively (Fig. 2). Within the DNA-binding R2/R3 repeat region (underlined in Fig. 2), the amino acid sequence of LtGAMYB is 99% identical to that of barley or rice, indicating that their binding specificities are likely to be very similar. Furthermore, when L. temulentum genomic DNA was cut with three restriction enzymes and analyzed by DNA gel blotting, the L. temulentum and barley probes both hybridized at high stringency to the same single bands for DNA digested with EcoRI or XhoI (Fig. 3). The presence of two bands when the DNA was cut with BglII indicates an internal BglII site. It is uncertain why there is greater hybridization of the barley probe with the lower band compared with that of the homologous probe; perhaps there was an uneven transfer of DNA to these blots. We cannot say whether there are more GAMYB-related genes in L. temulentum, but at the high level of stringency used, cDNA probe specificity can be expected for DNA and RNA hybridization (Fig. 3).
Figure 2.
Predicted amino acid sequence of LtGAMYB and comparison with the sequences for the barley (Hv) and rice (Os) GAMYB homologs (Gubler et al., 1995, 1997). From the nucleotide sequence, the position of EcoRI ( ) and SacI ( ) restriction sites conserved with those in HvGAMYB are indicated with arrows. The stop codon is indicated with an asterisk. The R2 and R3 repeats are shown as blocks. Dashes indicate gaps to maximize alignment. Dots show identical amino acids.
Figure 3.
L. temulentum genomic DNA gel-blot analysis using homologous 3′-end-specific probes from LtGAMYB and HvGAMYB. Genomic DNA (20 μg) was digested with BglII, EcoRI, and XhoI. After blotting, the digested DNA was hybridized with an EcoRI/SacI restriction fragment from either LtGAMYB or HvGAMYB. This restriction fragment corresponds to the nucleotide sequence encoding the amino acids between the arrowheads in Figure 2.
Seed Expression of LtGAMYB
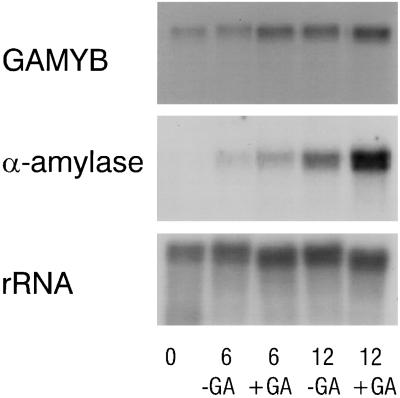
Because HvGAMYB was initially isolated as a transactivator of an α-amylase gene in barley aleurone tissue, it was important to determine whether LtGAMYB was also expressed in L. temulentum de-embryonated half-seeds (caryopsese) and whether GA up-regulated this mRNA. Therefore, we incubated the embryoless L. temulentum half-seeds in the presence or absence of GA3 for 6 or 12 h prior to α-amylase enzyme activity assays and extraction of RNA. After 12 h, GA3 had stimulated α-amylase activity in these half-seeds 2- to 3-fold (data not shown). To examine changes in mRNA levels, we probed an RNA gel blot with the gene-specific 32P-dCTP-labeled LtGAMYB EcoRI/SacI fragment (nucleotides 962–1638) or with the barley α-amylase cDNA probe. LtGAMYB and α-amylase mRNAs were expressed in half-seeds incubated without hormone and were weakly up-regulated after GA treatment (Fig. 4). As Evans et al. (1994) found previously, there was a high background α-amylase enzyme activity in untreated half-seeds. If these L. temulentum seeds contained sufficient endogenous GAs to cause this increase in mRNAs over the 12-h period, then only a weak response to applied GA might be expected.
Figure 4.
Effect of 10−6 m GA3 on GAMYB and α-amylase gene expression in L. temulentum de-embryonated half-seeds. RNA was isolated at 0, 6, or 12 h and blotted; the blots were probed with the EcoRI/SacI probe from LtGAMYB, a barley α-amylase cDNA, and a wheat rDNA clone (pTA71).
Overall, the very high sequence identity, the presence of a single copy sequence in genomic DNA, and the GA up-regulation of expression in half-seeds all confirm that LtGAMYB is the functional equivalent of HvGAMYB.
Inflorescence Expression of LtGAMYB
To determine the pattern of LtGAMYB expression during floral evocation and development, we examined the spatial and temporal localization of LtGAMYB mRNA within the inflorescence by in situ hybridization (Fig. 5). At an equivalent concentration of riboprobe, the antisense-probed sections all showed considerable hybridization (Fig. 5, A and C–F), but the sense probe barely hybridized at any time point for SD or LD apices. The comparison of sense and antisense probes (Fig. 5, B versus C) for LD VI apices is the most exacting because it involved the most intense hybridization of the antisense probe.
Figure 5.
In situ localization of LtGAMYB transcripts in L. temulentum shoot apices using longitudinal sections of apices harvested at different developmental stages and hybridized using DIG-labeled, in vitro-transcribed LtGAMYB riboprobes synthesized from an EcoRI/SacI fragment (indicated in Fig. 2). All sections were hybridized with the antisense riboprobe except B, which was hybridized with the sense control riboprobe. Concentration of the probe and the anti-DIG antibody and the duration of the color development were identical. Photographs were taken using Nomarski optics with identical exposures. A, Six-week-old vegetative apex; B and C, double-ridge stage; D, lateral spikelet meristems at the glume stage; E, spikelets at floret stage; and F, a stamen primordium on the flank of a floret site. Bars = 100 μm. am, Apical meristem; f, floret; g, glume; gp, glume primordium; l, lemma; lp, leaf primordium; pr, provascular strand; sm, spikelet meristem; s, stamen primordium; tsm, terminal spikelet meristem.
The expression of the LtGAMYB message was detected throughout the vegetative SD shoot apex; it was highest in the apical dome at the tip of the shoot apex and within the developing leaf primordia at its base (Fig. 5A). Compared with the SD apices, expression remained unchanged in the shoot apex during floral evocation over the first 12 to 30 h (LD II and LD III) after LD exposure (data not shown). We detected the greatest expression within the double-ridge apex (Fig. 5C, LD VI), predominantly in the apical dome but also within the lateral spikelet meristems. The original leaf primordia evident as the lower bulge on the “double ridge” (Fig. 5C) showed much less LtGAMYB expression. In advanced double-ridge apices 8 to 11 d after the LD transition (LD IX–LD XII), expression declined but was still detected within spikelet sites (Fig. 5D), developing glume primordia, and the apical dome (data not shown). At later times (LD XXX), expression was still evident in the developing glume and lemma primordia, as well as in the floret meristems that initiate in their axils (Fig. 5E). Of the floral organs that initiate from the floret meristem, the highest expression was within the stamen primordia (Fig. 5F). LtGAMYB was also expressed within the provascular tissue, especially that leading to the developing glumes and lemma during late stages of development.
GA Levels in L. temulentum Leaves
From full-scan GC-MS and/or multiple-ion monitoring analysis involving more than eight selected ions, we have identified a number of endogenous GAs in L. temulentum (Table I). We based their identification on: (a) matches in their mass spectra with authentic standards; (b) common Kovats Retention Index Values; and (c) their co-chromatography on HPLC.
Table I.
Identification of GAs from L. temulentum leaves by GC-MS
| Putative and Ref GA | HPLC Fraction
|
KRI | GA Library Matcha | Multiple Ion Monitoring Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C18 | N(CH3)2 | ||||||||||||||
| % of M+ ion area | |||||||||||||||
| 594(M+) | 579 | 535 | 504 | 448 | 379 | 281 | 238 | 207 | |||||||
| Lt GA8 | 8–11 | 29–31 | 2685 | 93 | 100 | 4 | 3 | 1 | 12 | 5 | 3 | 6 | 13 | ||
| GA8 std | – | – | 2685 | 93 | 100 | 5 | 4 | 1 | 14 | 7 | 3 | 6 | 10 | ||
| 506(M+) | 491 | 447 | 389 | 375 | 303 | 235 | 207 | ||||||||
| Lt GA29 | 8–11 | 39–42 | 2586 | 91 | 100 | 8 | 6 | 5 | 7 | 13 | 4 | 21 | |||
| GA29 std | – | – | 2586 | 91 | 100 | 10 | 5 | 6 | 8 | 15 | 3 | 14 | |||
| 506(M+) | 491 | 448 | 376 | 375 | 357 | 313 | 235 | 207 | |||||||
| Lt GA1 | 14–17 | 33–35 | 2585 | 78 | 100 | 7 | 12 | 10 | 6 | 2 | 4 | 4 | 11 | ||
| GA1 std | – | – | 2586 | 95 | 100 | 8 | 14 | 9 | 6 | 2 | 4 | 3 | 13 | ||
| 504(M+) | 489 | 475 | 460 | 445 | 431 | 387 | 370 | 311 | 238 | 208 | |||||
| Lt GA3 | 14–17 | 42–44 | 2612 | 86 | 100 | 6 | 4 | 4 | 7 | 5 | 4 | 6 | 5 | 4 | 23 |
| GA3 std | – | – | 2613 | 87 | 100 | 5 | 2 | 3 | 5 | 3 | 4 | 4 | 4 | 3 | 21 |
| 418(M+) | 403 | 390 | 375 | 359 | 347 | 301 | 235 | 207 | |||||||
| Lt GA20 | 22–25 | 36–39 | 2450 | 76 | 100 | 12 | 3 | 46 | 9 | 2 | 10 | 3 | 16 | ||
| GA20 std | – | – | 2451 | 94 | 100 | 13 | 3 | 48 | 11 | 2 | 11 | 5 | 19 | ||
| 432(M+) | 417 | 401 | 373 | 345 | 329 | 313 | 297 | 238 | 207 | ||||||
| Lt GA44 | 26–29 | 26–28 | 2778 | 93 | 100 | 13 | 5 | 20 | 3 | 1 | 1 | 1 | 32 | 85 | |
| GA44 std | – | – | 2778 | 94 | 100 | 14 | 6 | 21 | 3 | 2 | 1 | 1 | 32 | 74 | |
| 462(M+) | 434 | 402 | 374 | 345 | 315 | 285 | 258 | 239 | 207 | ||||||
| Lt GA19 | 26–29 | 66–70 | 2555 | 95 | 9 | 100 | 25 | 48 | 18 | 11 | 14 | 11 | 24 | 17 | |
| GA19 std | – | – | 2558 | 90 | 8 | 100 | 25 | 52 | 20 | 12 | 15 | 12 | 24 | 19 | |
| 448(M+) | 433 | 416 | 389 | 373 | 357 | 329 | 275 | 251 | 235 | 207 | |||||
| Lt GA53 | 30–35 | 16–18 | 2443 | 91 | 100 | 9 | 16 | 30 | 8 | 5 | 8 | 2 | 21 | 14 | 62 |
| 450(M+) | 435 | 418 | 391 | 375 | 359 | 331 | 277 | 253 | 237 | 209 | |||||
| [2H2]GA53 std | – | – | 2442 | – | 100 | 12 | 18 | 33 | 11 | 6 | 10 | 4 | 26 | 17 | 79 |
| 330(M+) | 298 | 286 | 270 | 243 | 228 | 226 | 217 | 211 | 183 | 159 | |||||
| Lt GA9 | 30–35 | 32–34 | 2317 | 68 | 12 | 100 | 7 | 50 | 43 | 5 | 35 | 14 | 8 | 12 | 13 |
| GA9 std | – | – | 2317 | 93 | 16 | 100 | 9 | 56 | 42 | 6 | 39 | 16 | 12 | 17 | 17 |
HPLC fractions were selected by comparison with prior separations of standards (std). To determine the Kovats Retention Index (KRI) for GC-MS, all samples were co-injected with Parafilm. Multiple ion monitoring data are presented.
Full scans were also obtained and the percentage match with a PC-based GA library is shown (Gaskin and MacMillan, 1991).
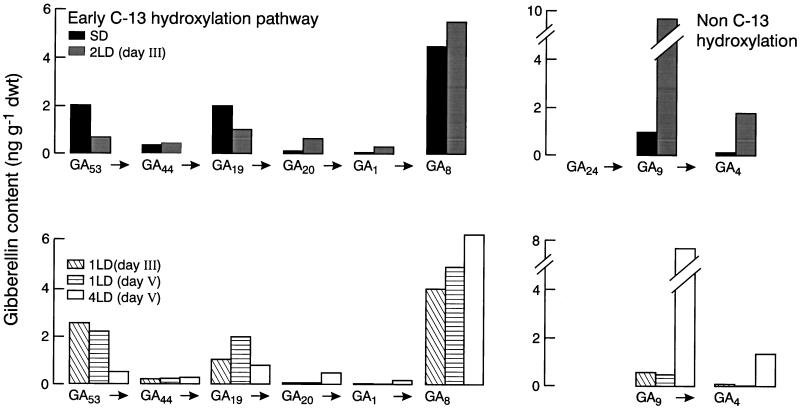
The GC-MS-single-ion monitoring analysis of changes in GA content of leaves exposed to various florally inductive LD conditions appears in Figure 6 for the various times of harvest. By 48 h after beginning exposure to LD conditions, there were increases of 5- to 20-fold in the content of GA1 and GA4 (Fig. 6). In L. temulentum both of these GAs were active for stem elongation (Evans et al., 1990) and they were, respectively, the biosynthetic products of conversion of GA19 to GA20 to GA1 and of GA24 to GA9 to GA4 (Graebe, 1987). The reduction in precursor levels due to LD exposure, particularly of GA19, implies an increase in its metabolism.
Figure 6.
Effect of LD exposure on leaf GA content in L. temulentum. Levels are shown for biosynthetic precursors, active GAs (GA1, GA4), and one catabolite (GA8) for the two common biosynthetic pathways in plants that differ in carbon-13 hydroxylation. The timing of harvests relative to exposure to various SD or LD was outlined in Methods. GA24 was not quantified. dwt, Dry weight.
Floral development in L. temulentum responds proportionately to increasing the number of LD, and we assume that GA metabolism did change in the leaf following the 1st LD, but we were unable to detect it in this study. In a repeat experiment GA biosynthesis did appear to increase in the leaf after 1 LD (data not shown). It could be expected that GA levels at the shoot apex would increase with those in the leaf, and our earlier bioassay studies indicated such change (Pharis et al., 1987). Recently, we have applied highly sensitive GC-MS techniques to measure GAs in the shoot apex; and we have found reproducible evidence of increases after 2 LD (R.W. King and T. Moritz, unpublished data).
DISCUSSION
In the grass L. temulentum, we have identified and characterized LtGAMYB, a homolog of the barley GA-regulated MYB gene. LtGAMYB is expressed in vegetative tissue and we assume that it is important for maintaining normal vegetative growth. Its enhanced expression during the early stages of floral development (double-ridge stage; LD VI) implies that GAMYB is important for floral development (more so than for floral evocation) and that GA levels at the apex increase at that time. To our knowledge, this is the first report of an increase in MYB gene expression at flower initiation. However, unrelated MYB genes play roles in flower coloration (Jackson et al., 1991) and regulate petal epidermal cell shape (Noda et al., 1994). Even more significant is the link we can make between flowering, LtGAMYB expression, and evidence that GA levels, both endogenous (Fig. 6) and applied (Pharis et al., 1987; Evans et al., 1990), may regulate flowering of L. temulentum.
GA not only regulates flowering, but also seed germination, α-amylase production in the cereal aleurone, stem elongation, and many more processes (Graebe, 1987). It is now known that GAMYB is one component of the transduction pathway from GA to responses involving aleurone function (Gubler et al., 1995, 1997). In the present study, by cloning LtGAMYB, we were able to extend these findings to the grass L. temulentum, which has the additional benefit that the timing of its flowering responses has been precisely defined in our previous physiological studies (Evans and King, 1985; King et al., 1993). The very high degree of identity of LtGAMYB with existing barley and rice GAMYB genes (Fig. 2), together with the fact that the 3′-end-specific barley and L. temulentum probes both hybridize with the same single-copy sequence within L. temulentum genomic DNA (Fig. 3), identifies this L. temulentum MYB-like gene as the L. temulentum GAMYB homolog. The fact that expression of the L. temulentum homolog is up-regulated in de-embryonated half-seeds in response to GA3 reinforces this claim (Fig. 4). It follows that, just as GAMYB is proposed to mediate some GA-controlled germination-related processes, it may also provide a critical link between endogenous GA and the transcriptional activation of a cascade of genes involved in flowering.
Our claim that GAMYB expression is important for flowering implies that GA levels increase at the shoot apex in association with LD-induced flowering of L. temulentum. Quantitation of endogenous levels of “biologically active” GAs at the shoot apex during monocot inflorescence development has been assessed only by bioassay in barley (Nicholls, 1974), L. temulentum (Pharis et al., 1987), oat (Kaufman et al., 1976), and rice (Osada et al., 1973). In such apices the level of GA3-like activity was high at the double-ridge stage and decreased thereafter; a second peak of GA-like activity was then observed during stamen development in barley (Nicholls, 1974), oat (Kaufman et al., 1976), and rice (Osada et al., 1973). Our evidence showing that LD exposure led to increased active GAs in the leaf (Fig. 6) indicates that shoot apex GA levels could be high just before the double-ridge stage, when LtGAMYB expression increases at the apex (Fig. 5). This information fits with the timing of increased LtGAMYB expression and with our earlier evidence that the florigenic effect of applied GA3 on excised apices remained high until the double-ridge stage of floral development (LD VI; King et al., 1993). During stamen development GA action may also be important (Sawhney, 1992), and this agrees with the enhanced expression of LtGAMYB in stamen primordia (Fig. 5F). Thus, LtGAMYB may mediate the processes of floral development in response to endogenous GAs. We have previously argued that GAs may not regulate earlier processes involving floral evocation (King et al., 1993); however, this issue has yet to be examined.
For years it has been known that LD conditions enhance the GA biosynthesis of dicot species (Zeevaart, 1971; Gilmour et al., 1986; Talon and Zeevaart, 1990; Zeevaart and Gage, 1993; Wu et al., 1996). For monocots a LD-regulated increase in GA levels in leaves has been shown previously for Poa pratensis (Junttila et al., 1997) and now also for L. temulentum (Fig. 6). It is possible that in L. temulentum, LD conditions activate specific biosynthetic steps, including those involving the 20-oxidase enzyme(s) regulating both the early 13-hydroxylation pathway and the non-13-hydroxylation pathway (Fig. 6), as was reported for spinach (Gilmour et al., 1986). On the other hand, the increases in GA1 and GA4 suggest increased activity of 3β-hydroxylases or a more general up-regulation of early steps of GA biosynthesis. Reduced catabolism of 3β-hydroxylated GAs could also cause a buildup of GA1 and GA4, an explanation we cannot assess.
Enhanced expression of floral identity genes related to APETALA1 and cell-cycle-regulatory genes in L. temulentum, including CDC2 (Gocal, 1997), are associated with the very earliest (d 1–4) events of floral evocation. Later (d 5–6), when LtGAMYB expression was greatest, cell division became intensive but the two responses were not linked. For example, after a further 3 to 6 d there was much reduced LtGAMYB expression (late double-ridge/early glume stage; Fig. 5D), yet cell division was still intensive. There are many phases of plant development at which GAMYB expression could be important, but the dramatic contrast between the high level of expression of LtGAMYB at the double-ridge stage and the weaker expression during other floral stages and in vegetative apices may indicate that LtGAMYB plays an important developmental role over the period associated with inflorescence primordia formation. We envisage that such a specific developmental role of LtGAMYB could involve activation of the expression of the floral transcription factor related to the LEAFY gene of Arabidopsis. In L. temulentum apices, LtGAMYB expression (Fig. 5) precedes the expression of the L. temulentum LEAFY homolog (Gocal, 1997). Furthermore, there may be a GAMYB-binding site within the LEAFY promoter (e.g. for Arabidopsis, the sequence CAACTGTC; accession no. M91208). However, it remains to be determined whether this site is functionally important for GA-induced gene expression in Arabidopsis, and, if so, whether it is mediated through a GAMYB. Whether this site is conserved in the promoter of the L. temulentum LEAFY gene also remains to be determined.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Peter Chandler, Lloyd Evans, Jake Jacobsen, and Masumi Robertson for their helpful comments during the preparation of this manuscript. Greg Gocal would like to thank those at the Australian National University and CSIRO who were so helpful during the course of his Ph.D.
Abbreviations:
- LD
long day
- SD
short day
LITERATURE CITED
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsisby activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT, King RW (1985) Lolium temulentum L. In AH Halevy, ed, CRC Handbook of Flowering, Vol III. CRC Press, Boca Raton, FL, pp 306–323
- Evans LT, King RW, Chu A, Mander LN, Pharis RP. Gibberellin structure and florigenic activity in Lolium temulentum, a long-day plant. Planta. 1990;182:97–106. doi: 10.1007/BF00239990. [DOI] [PubMed] [Google Scholar]
- Evans LT, King RW, Mander LN, Pharis RP, Duncan KA. The differential effects of C-16,17 dihydro gibberellins and related compounds on stem elongation and flowering in Lolium temulentum. Planta. 1994;193:107–114. [Google Scholar]
- Gaskin P, MacMillan J (1991) GC-MS of Gibberellins and Related Compounds: Methodology and a Library of Spectra. Cantocks Enterprises, University of Bristol, UK
- Gerlach WL, Bedbrook JR . Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979;7:1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zeevaart JAD, Schwenen L, Graebe JE. Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol. 1986;82:190–195. doi: 10.1104/pp.82.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GFW (1997) Molecular biology of floral evocation in Lolium temulentum. PhD thesis. Australian National University, Canberra
- Graebe JE. Gibberellin biosynthesis and control. Annu Rev Plant Physiol. 1987;38:419–465. [Google Scholar]
- Green LS, Mosleth Færgestad E, Poole A, Chandler PM. Grain developmental mutants of barley α-amylase production during grain maturation and its relation to endogenous gibberellic acid content. Plant Physiol. 1997;114:203–212. doi: 10.1104/pp.114.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a MYBgene in barley aleurone cells: evidence for MYB transactivation of a high-pI α-amylase gene promoter. Plant Cell. 1995;7:1879–1891. doi: 10.1105/tpc.7.11.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Watts RJ, Kalla R, Matthews P, Keys M, Jacobsen JV. Cloning of a rice cDNA encoding a transcription factor homologous to barley GAMYB. Plant Cell Physiol. 1997;38:362–365. doi: 10.1093/oxfordjournals.pcp.a029175. [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Devlin PF, Whitelam GC, Hanhart CJ, Koornneef M. The ELONGATED gene of Arabidopsisacts independently of light and gibberellins in the control of elongation growth. Plant J. 1996;9:305–312. doi: 10.1046/j.1365-313x.1996.09030305.x. [DOI] [PubMed] [Google Scholar]
- Jackson D, Culianez-Macia F, Prescott AG, Roberts K, Martin C. Expression patterns of myb genes from Antirrhinumflowers. Plant Cell. 1991;3:115–125. doi: 10.1105/tpc.3.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsisalter gibberellin signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila O, Heide OM, Lindgard B, Ernstsen A. Gibberellins and the photoperiodic control of leaf growth in Poa pratensis. Physiol Plant. 1997;101:599–605. [Google Scholar]
- Kaufman PB, Ghosheh NS, Nakosteen L. Analysis of native gibberellins in internode, nodes, leaves and inflorescence of developing Avenaplants. Plant Physiol. 1976;58:131–134. doi: 10.1104/pp.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Blundell C, Evans LT. The behaviour of shoot apices of Lolium temulentum in vitroas the basis of an assay system for florigenic extracts. Aust J Plant Physiol. 1993;20:337–348. [Google Scholar]
- Lang A. Physiology of flower initiation. In: Ruhland W, editor. Encyclopedia of Plant Physiology, Vol 15/1. Berlin: Springer-Verlag; 1965. pp. 1380–1536. [Google Scholar]
- Nicholls PB (1974) The effect of daylength on the development of the barley inflorescence and the endogenous gibberellin concentration. Bulletin 12. In RL Bieleski, AR Ferguson, MM Cresswell, eds, Mechanisms of Regulation of Plant Growth. The Royal Society of New Zealand, Wellington, pp 305–309
- Noda K-I, Glover BJ, Linstead P, Martin C. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature. 1994;369:661–664. doi: 10.1038/369661a0. [DOI] [PubMed] [Google Scholar]
- Okamuro JK, den Boer BGW, Lotys-Prass C, Szeto W, Jofuku KD. Flowers into shoots: photo and hormonal control of a meristem identity switch in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:13381–13386. doi: 10.1073/pnas.93.24.13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada A, Suge H, Shibukawa S, Noguchi I. Changes of endogenous gibberellins in rice plants as affected by growth stage and different growth conditions. Proc Crop Sci Soc Jpn. 1973;42:41–45. [Google Scholar]
- Pharis RP, Evans LT, King RW, Mander LN. Gibberellins, endogenous and applied, in relation to flower induction in the long-day plant Lolium temulentum. Plant Physiol. 1987;84:1132–1138. doi: 10.1104/pp.84.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney VK. Floral mutants in tomato: development, physiology, and evolutionary implications. Can J Bot. 1992;70:701–707. [Google Scholar]
- Schuurinck RC, Vain PV, Jones RL. Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiol. 1996;111:371–380. doi: 10.1104/pp.111.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Zeevaart JAD. Gibberellins and stem growth as related to photoperiod in Silene armeriaL. Plant Physiol. 1990;92:227–235. doi: 10.1104/pp.92.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thalianaunder short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li L, Gage DA, Zeevaart JAD. Molecular cloning and photoperiod-regulated expression of gibberellin 20-oxidase from the long-day plant spinach. Plant Physiol. 1996;110:547–554. doi: 10.1104/pp.110.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD. Effects of photoperiod on growth rate and endogenous gibberellins in the long-day rosette plant spinach. Plant Physiol. 1971;47:821–827. doi: 10.1104/pp.47.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Gage DA. ent-Kaurene biosynthesis is enhanced by long photoperiods in the long-day plants Spinacia oleracea L. and Agrostemma githagoL. Plant Physiol. 1993;101:25–29. doi: 10.1104/pp.101.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]