Abstract
Cord blood–derived neural stem cells (NSCs) are proposed as an alternative cell source to repair brain damage upon transplantation. However, there is a lack of data showing how these cells are driven to generate desired phenotypes by recipient nervous tissue. Previous research indicates that local environment provides signals driving the fate of stem cells. To investigate the impact of these local cues interaction, the authors used a model of cord blood–derived NSCs co-cultured with different rat brain–specific primary cultures, creating the neural-like microenvironment conditions in vitro. Neuronal and astro-, oligo-, and microglia cell cultures were obtained by the previously described methods. The CMFDA-labeled neural stem cells originated from, non-transformed human umbilical cord blood cell line (HUCB-NSCs) established in a laboratory. The authors show that the close vicinity of astrocytes and oligodendrocytes promotes neuronal differentiation of HUCB-NSCs, whereas postmitotic neurons induce oligodendrogliogenesis of these cells. In turn, microglia or endothelial cells do not favor any phenotypes of their neural commitment. Studies have confirmed that HUCB-NSCs can read cues from the neurogenic microenvironment, attaining features of neurons, astrocytes, or oligodendrocytes. The specific responses of neurally committed cord blood–derived cells, reported in this work, are very much similar to those described previously for NSCs derived from other “more typical” sources. This further proves their genuine neural nature. Apart from having a better insight into the neurogenesis in the adult brain, these findings might be important when predicting cord blood cell derivative behavior after their transplantation for neurological disorders.
Keywords: neurogenesis, brain environment, glial cells
The discovery of active adult neurogenesis in the human brain has raised great hopes for possible replacement therapies in neurological disorders (Johansson et al. 1999; Nunes et al. 2003; Sanai et al. 2004; Quinones-Hinojosa et al. 2006; Jin et al. 2006; Curtis et al. 2007). However, the success of clinical stem cell–based strategies will depend on detailed understanding of stem cell biology and precise evaluation of their functional efficacy and safety in preclinical animal models. Assumptions regarding the inherent limitation of the mature central nervous system (CNS) in replacing lost neurons focus on the local cellular and molecular microenvironment as the primary impediment to neural repair (Cova et al. 2004; Lindvall and Kokaia 2005; Munoz et al. 2005; Pluchino et al. 2005). The past decade has witnessed groundbreaking advances in human somatic stem cell isolation from different sources (i.e., bone marrow, peripheral blood, cord blood, amniotic fluid) in terms of transplantation. These cells were able to differentiate into neural cells (Sanchez-Ramos et al. 2000; Buzanska et al. 2002; Mezey et al. 2003; Hermann et al. 2004; Walczak et al. 2004; De Coppi et al. 2007).
In vitro studies performed by our group previously documented unexpected phenotypic plasticity of progenitor cells present in the mononuclear cell fraction of cord blood. According to our data, freshly isolated human umbilical cord blood (HUCB) cells express Oct3/4, Sox2, Mdr1, and Rex1 genes, being the master regulators of the pluripotent stem cell state (Habich et al. 2006). During cell culture in particular conditions, HUCB cells start to express pro-neural genes (nestin, glial fibrillary acidic protein [GFAP], NF200), and then the wide panel of neural markers (β-tubulin III, MAP2, GFAP, S100β, 04, GalC) comes into view. From the repeated expansion of HUCB cells and selection of non-adherent proliferating cells, we have established the first, expanding, indefinitely growing human umbilical cord blood neural-like stem cell line (HUCB-NSCs) (Buzanska et al. 2006; Domanska-Janik et al. 2008). HUCB-NSCs retain their potential pluripotency, while executing exclusively neural-restricted gene expression, and differentiate in vitro into neural-type cells (Jurga et al. 2006).
Early efforts in investigating the contributions made by adult CNS after human stem cell transplantation gave little information because of limited cell survival rate due to host–recipient disparity (Kogler et al. 2004; Walczak et al. 2004; Kuh et al. 2005; Pan et al. 2005; Liu HY et al. 2006; Kozlowska et al. 2007). To better understand the local environmental cues on neural stem cells, it is helpful to re-create CNS microenvironmental conditions in vitro. According to data obtained from several studies, astrocytes and microglia are essential to neurogenesis. However, the cellular and molecular mechanisms regulating neurogenic and gliogenic processes are still a matter of debate. We previously demonstrated that astrocytes isolated from neonatal rats induce the neuronal differentiation of HUCB-NSCs (Jurga et al. 2006). In the present work, we further characterize the functional properties of other brain cells (i.e., microglia, neurons, and oligodendrocytes) determining the fate of HUCB-NSCs. Such effects are compared with those exerted by astrocytes.
Materials and Methods
HUCB-NSC Line Culture
HUCB-NSCs (Buzanska et al. 2006) were cultured in DMEM/F12 medium (GIBCO, Carlsbad, CA) supplemented with 2% FBS (GIBCO), ITS Insulin, Transferrin, Sodium selenite (1:100; GIBCO), and antibiotic-antimycotic solution (AAS; 1:100; Sigma, St Louis, MO). The cell culture was maintained at 37C, 5% CO2 in a fully humidified atmosphere; fed every 3–4 days; trypsinized every 10 days; and reseeded at the density of 5 × 104 cells/cm2.
Cell Labeling with CMFDA Cell Tracker
HUCB-NSCs cultured in vitro were collected by trypsinization into a 15-ml centrifuge tube at a density of 106 cells in 400 µl of DMEM/F12 medium. The cells were incubated with 10 µM 5-chloromethyl-fluorescein-diacetate (CMFDA) cell tracker (Molecular Probes, Eugene, OR) at 37C for 30 min, washed twice with medium, and counted using a fluorescent microscope.
Astrocyte, Oligodendrocyte, or Microglial Cell–Enriched Cultures
The brain hemispheres of neonatal Wistar rats were used to prepare mixed glial primary cell culture. All the procedures were approved by our Ethics Committee on Animal Care and Use. Briefly, the dissected tissue was placed in Ca2+- and Mg2+-free HBSS (GIBCO) and dispersed mechanically with a Pasteur pipette and 22-µm needles. The cells were filtered using 41-µm Millipore (Billerica, MA) membranes, spun down (1000 rpm, 10 min), and plated into 75-cm2 culture flasks precoated with 0.1 mg/ml poly-L-lysine at the density of 2 × 105/cm2 in DMEM + 10% FBS medium. Half of the medium volume was replaced with fresh medium every 2 days. After 12–14 days, when the cells became confluent, the particular types of neural cells were isolated according to a modified procedure (McCarthy and de Vellis 1980), based on their different adhesion properties. Accordingly, the cell cultures were rinsed with complete medium and shaken first for 1 hr on an orbital shaker (160 rpm) at 37C to remove the microglial fraction and then, after the medium replacement, for an additional 15–18 hr with the aim of gently detaching oligodendrocytes. Floating microglial cells obtained by mild shaking for 1 hr at 37C were transferred to a 75-cm2 new culture flask for 1 hr, at a density of 1.5–2 × 105/cm2. After 1 hr, the cultures were washed with fresh medium to remove non-adhering cells and cultured in DMEM + 10% FBS for 1 week to become confluent. Adherent cells after trypsynization were replated on poly-L-lysine-coated coverslips placed in 24-well culture plates at a density of 105 cells/cm2and cultured for 7 DIV (days in vitro). Oligodendrocyte progenitors obtained by this sequential dislodging method were spun down (1000 rpm, 10 min), mechanically dispersed with a 22-µm needle in F12/DMEM medium with ITS supplement, and than filtered through 41-µm Millipore membranes. The cells were seeded at a density of 2 × 105/cm2 on poly-L-lysine-coated coverslips placed in 24-well plates (Nunc, Rochester, NY) and cultured for 7 days in DMEM/F12 medium without serum to obtain the differentiated GalC+ oligodendrocytes. After removing the floating cells, the adherent astrocyte fraction was trypsinized and replated on poly-L-lysine-coated coverslips placed in 24-well tissue culture plates (105 cells/cm2) in 500 µl DMEM + 10% FBS medium. On day 7, cytosine arabinose (20 µM) was added to eliminate proliferating cells. After 3 days of culture, we changed the medium to DMEM + 10% FBS medium and kept the cells growing for an additional 2 days until fully recovered.
Neuronal Cell-Enriched Cultures
The neuronal primary cell cultures were prepared from the brains of neonatal Wistar rats according to the previously described procedure (Dotti et al. 1988; Goslin and Banker 1989; Brewer et al. 1993) with slight modifications. Neonatal rat hippocampus and cortex were dissected from the brains, treated with 0.25% trypsin (GIBCO) for 15 min at 37C, and dissociated by trituration. Then the dissociated cells were incubated in the mixture of DNA-se (Roche, Basel, Switzerland), MgSO4, and trypsin inhibitor (Sigma) in HBSS (GIBCO) for 3 min, and the mixture of cells was also triturated 10–15 times using the Pasteur pipette. When the non-dispersed tissue settled down, the supernatant was collected and centrifuged for 1–2 min, 200 g, at 4C. The cells from the pellet were gently resuspended in NB (GIBCO) medium supplemented with B27 (GIBCO) and L-glutamine (Sigma) and plated on poly-L-lysine-treated glass coverslips placed in 24-well plates, at a density of 2–3 × 105cells/cm2. On day 2, cytosine arabinose (10 µM) was added to eliminate proliferating cells. Then after 2 DIV, the medium was changed to neurobasal medium containing B27 and L-glutamine. The cells were kept for 7–10 days, to obtain the confluent cell culture.
Endothelial (t-END) Cell Culture
The endothelial transformed t-END line cells isolated from the thymus capillaries of mice were plated on poly-L-lysine 25-cm2dishes and cultured for 7 days in DMEM + 10% FBS medium to reach the confluence. The cells were replated at a density of 2 × 105 cells/cm2on poly-L-lysine-coated coverslips placed in 24-well tissue culture and cultivated for 5 more days in DMEM/F12 + 10% FBS + AAS medium to reach the confluence.
Differentiation of HUCB-NSCs in the Presence of Astrocytes, Microglia, Neurons, Oligodendrocytes, or Endothelial t-END Cells
HUCB-NSCs labeled with CMFDA were seeded on a monolayer of mature rat brain cells or t-END cells at a density of 5 × 104 cells/cm2 and co-cultured in the presence of neurons, astrocytes, microglia, oligodendrocytes or t-END cells for 7 days. The fresh medium was added every 3 days. Then, the 7-day co-cultures were fixed in 4% paraformaldehyde (PF) for 20 min. Concomitantly, HUCB-NSCs were cultured in media for 7 days and served as respective controls.
Immunocytochemistry of HUCB-NSCs
HUCB-NSCs co-cultured in the presence of rat brain cells, previously fixed with 4% PFA, were blocked with 10% normal goat serum (NGS; Sigma) for NG2, O4, and GalC or with 10% normal goat serum and 0.1% Triton X-100 for all other antibodies used. The following primary antibodies (Lyck et al. 2008) were applied for the overnight incubation at 4C: mouse monoclonal antibodies directed anti-: human nestin (diluted 1:200; R&D Systems, Minneapolis, MN), NF-200 (1:500; Sigma), β-tubulin III (diluted 1:1000; Sigma), MAP-2 (diluted 1:1000; Sigma), O4 (diluted 1:100; Sigma), GalC (diluted 1:150; Chemicon, Temecula, CA), and Ki67 (diluted 1:100; Novocastra, Bannockburn, IL) and rabbit polyclonal antibodies anti: GFAP (diluted 1:1000; Cappel, Cochranville, PA), S-100β (diluted 1:2000; Swant, Switzerland), fibronectin (diluted 1:100; DAKO, Carpinteria, CA), NG2 (diluted 1:100; Chemicon), and vWF (diluted 1:200; Sigma). After washing with PBS, the following secondary antibodies were applied for 60 min at room temperature: goat anti-mouse IgG2b for β-tubulin III; goat anti-mouse IgG1 for NF-200, MAP-2, Ki67, and nestin; goat anti-mouse IgG3 for GalC; goat anti-mouse IgM for O4; and goat anti-rabbit IgG (H+L) for NG2, S-100β, and GFAP. Except for IgG3, all the secondary antibodies were conjugated either to Alexa Fluor 546 or Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and used in 1:1000 dilutions. For IgG3, the Texas Red or FITC-conjugated secondary antibody was applied (1:100; SouthernBiotech, Birmingham, AL). Cell nuclei were stained with 5 µM Hoechst 33258 (Sigma) for 20 min. After final wash, the slides were mounted in Fluoromount-G (Southern-Biotech). As control, the first antibodies were omitted during immunocytochemical staining.
Microscopy and Cell Counting
The live growing cells or prefixed immunochemically labeled cultures were observed either in phase contrast or in ultraviolet (UV) light under a fluorescent microscope using Axiovert 25 or Axioscope 2 (Carl Zeiss, Stuttgart, Germany), respectively. The images were captured by the Videotronic CCD-4230 camera coupled with the microscope and processed using the computer-based programmable image analyzer KS 300; KS RUN (Carl Zeiss). To obtain detailed images of the cells, a confocal laser scanning microscope (Zeiss LSM 510) was used. An argon laser (488 nm) and helium-neon laser (543 nm) were used for the excitation of FITC and Texas Red, respectively. Following acquisition, the images were processed using the Zeiss LSM 510 software package version 2.8 and Corel Draw version 11.0 (Corel, Ottawa, Ontario, Canada).
Layout of the Experiment and Statistical Analysis of the Data
Each treatment (enrichment of the medium with a particular type of neural cells) and appropriate controls were replicated 4–10 times for nearly 4 years. Mostly, these were separate runs of the experiment (29 in total), and therefore in the analysis, we treated them as independent. Within replication, each marker was assigned to three wells on a 24-well plate, and 200 live cells were inspected for each well and immunopositive cells counted.
The effect of neural cells was expressed as the odds ratio (Agresti 1990) of differentiation in experimental and control conditions. The odds ratio (OR) was computed as P̄E(1-P̄EC) / P̄EC(1-P̄EE), where P̄EE and P̄EC stand for average proportion in the experimental and control group, respectively. We used percentile bootstrap (Manly 1997) to obtain confidence intervals for odds ratios. There was no major discrepancy between the pictures obtained.
For graphical presentation of the effect of neural cells compared to control, we found 95% confidence intervals (CIs) for the difference of percentages using the Satterthwaite (Welch) method (Zar 1999). The picture was compared with the results obtained by Dunnett, Student, and permutation methods (Proc ANOVA, TTEST and NPAR1WAY; SAS Institute Inc. 2007); differences were practically immaterial. On the graphs, we plotted pairs of bars (experiment and appropriate control). Half of the CI length was superimposed on the shorter bar, and if it did not become longer after that, the remaining difference was considered the least probable difference between the true percentages at the 95% confidence level.
Results
Characteristic of HUCB-NSCs Growing in LS and HS Media
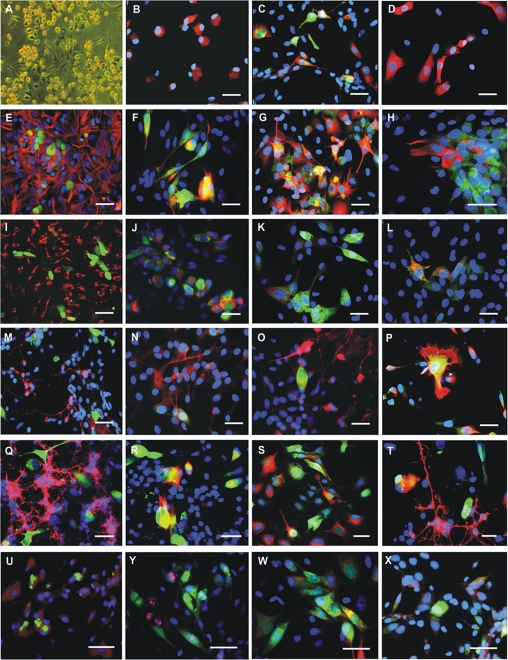
HUCB-NSCs cultured in medium form a mixed population of partly floating round-shaped cells and partly adherent spindle-shaped cells with short, bipolar processes (Fig. 1A). The proliferation of HUCB-NSCs was 30% on average, as depicted by Ki67+ cells. Immunocytochemical staining for neural specific markers revealed 44% of nestin+ (expressed in neural stem cells), 29% of NF200+ (an immature neuronal marker), 20% of TUJ1+ (more mature neuronal marker), 43% of S100β+ (a mature astrocytic marker), and 1% of O4+ (a mature oligodendrocytic marker) cells (Fig. 1B–D).
Figure 1.
Human umbilical cord blood neural-like stem cells (HUCB-NSCs) in a culture (A–D), and neural marker expression in HUCB-NSCs co-cultured with neonatal rat brain cells: astrocytes (E–H), microglia (I–L), neurons (M–P), oligodendrocytes (Q–T), and endothelial cells (U–Y). Specific immunostaining for nestin (B), NF200 and TUJ1 (C), or S-100β (D) in the HUCB-NSC population. Cells stained with Texas red after immune reactions were detected simultaneously, and Hoechst 33258 staining showed their nuclei (blue). Neonatal rat astrocyte primary culture monolayer immune labeled with anti–glial fibrillary acidic protein (GFAP) antibody (red) co-cultured with HUCB-NSCs (green by CMFDA) (E). Neural differentiation of HUCB-NSCs cultured with rat astrocytes. HUCB-NSC (green by CMFDA and red by Texas red after phenotype-specific immune reaction) can be detected in the presence of all cells forming astrocyte primary culture shown after using Hoechst 33258 (blue). Co-localization of red and green labeling appears yellow after overlaying these two images. NF200 (F) and TUJ1 (G) expressing cells display neuron-like morphology with axonal projection. S-100β expression (red) is detected yellow in some green prelabeled HUCB-NSC (H). Microglia of rat primary culture stained with anti-ED1 antibody (red) co-cultured with HUCB-NSCs (green by CMFDA) (I). Immunophenotyping of NF200 (J), TUJ1 (K), and S-100β (L) positive cells (red) in HUCB-NSCs (green prelabeled with CMFDA) co-cultured with rat microglia. Neonatal rat postmitotic neuron primary culture monolayer immunostained with anti-TUY1 antibody (red) co-cultured with HUCB-NSCs (green by CMFDA) (M). Immunolabeling for NF200 (N), TUJ1 (O), and O4 (P) positive cells (red) in HUCB-NSCs (green prelabeled with CMFDA) cultured in the presence of mature rat neurons. Oligodendrocyte-enriched rat primary culture stained with anti-NG2 antibody (red) co-cultured with HUCB-NSCs (green by CMFDA) (Q). Immune reaction depicting NF200 (R), TUJ1 (S), and GalC (T) positive cells (red) among HUCB-NSCs (green prelabeled CMFDA) co-cultured with rat oligodendrocytes. Endothelial cells (t-END line) monolayer stained with anti-vWF antibody (red) co-cultured with HUCB-NSCs (green by CMFDA) (U). Immunostaining for Ki67 (Y), TUJ1 (W), and S-100β (X) positive cells (red) in HUCB-NSC population (green prelabeled with CMFDA) cultured in the presence of t-END cells. Scale bars = 20 µm.
Phenotypes of HUCB-NSCs Co-cultured with Astrocytes from Neonatal Rat Brain
The feeder layer of primary astrocytes derived from neonatal rat brain was enriched with astrocytes (94% GFAP+ cells). The marginal content of microglia (2% ED1+ cells), neurons (1% TUJ1+ cells), oligodendrocytes (1% O4+ cells), and fibroblasts (2% fibronectin+ cells) was also noticed. After 24 hr of co-culture with astrocytes, HUCB-NSCs remained round, but during the next 48 hr, they exhibited typical morphology of neuron-like cells. The co-culture of HUCB-NSCs with astrocytes caused an evident decrease in nestin+ cells compared with HUCB-NSCs cultured in medium (1% vs 29%). Within 7 days of co-culture, HUCB-NSCs acquired mature neural markers. The presence of neonatal rat brain astrocytes stimulated the differentiation of HUCB-NSCs into neuronal cells while lowering differentiation in astrocyte-like cells. A high number of human cells co-cultured with astrocytes expressed neuronal cytoskeleton marker β-tubulin III (76%). Another cytoskeleton protein, NF200, was also immunodetected in these cells (65%). NF200, an early marker, was found in non-differentiated cells and was also coexpressed along with β-tubulin III marker. MAP2, specific for mature neurons, was expressed by 51% of HUCB-NSC descendents. The MAP2 protein was coexpressed with β-tubulin III in some cells and localized to neuronal processes. S100β+ and O4+ cells appeared in a marginal amount (0.9% and 0.5%, respectively). Interestingly, we demonstrated that HUCB-NSCs retained the potential to proliferate (24%) when primary astrocytes provided the feeder layer (Fig. 1E–H and Fig. 2).
Figure 2.
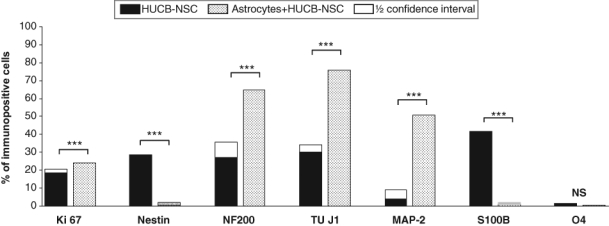
Effects of astrocytes on the proliferation and differentiation of human umbilical cord blood neural-like stem cells (HUCB-NSCs) into various types of neural cells. For each experiment–control pair, superimposed on the shorter bar is a half confidence interval for the difference of percentages. The remaining gap between bars reflects the least probable difference at the 95% confidence level. ****p<0.0001.
Phenotypes of HUCB-NSCs Co-cultured with Microglia from Neonatal Rat Brain
The microglia-enriched feeder layer consisting of microglial cells (93% ED1+ cells), astrocytes (2% GFAP+ cells), neurons (2% TUJ1+ cells), oligodendrocytes (1% O4+ cells), and fibroblasts (2% fibronectin+ cells) created different conditions for HUCB-NSC differentiation than that provided by astrocytes. HUCB-NSCs co-cultured with microglia acquired a neural phenotype expressing neuronal as well as astrocytic markers. The close vicinity of microglia slightly increased the differentiation rate of HUCNB-NSCs toward neurons as well as astrocytes in comparison with HUCB-NSCs cultured alone (40% vs 30% of TUJ1+ cells; 13% vs 4% of MAP2+ and 56% vs 42% of S100β+ cells). There was no preference to direct HUCB-NSCs to differentiate along a certain neural cell lineage. Conversely, a decrease in the number of nestin+ cells was observed in HUCB-NSCs co-cultured with microglia compared to HUCB-NSCs cultured in media (5% vs 29%). In addition, the Ki67 immunostaining revealed that the microglia feeder layer preserved the proliferation activity of HUCB-NSCs observed in the control (16% vs 19%) (Fig. 1I–L and Fig. 3).
Figure 3.
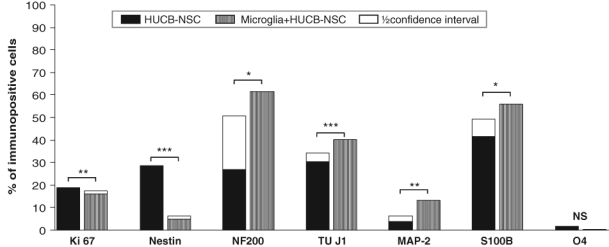
Effects of microglia on the proliferation and differentiation of human umbilical cord blood neural-like stem cells (HUCB-NSCs) into various types of neural cells. For each experiment–control pair, superimposed on the shorter bar is a half confidence interval for the difference of percentages. The remaining gap between bars reflects the least probable difference at the 95% confidence level. *p<0.05. **p<0.01. ***p<0.001. ****p<0.0001.
Phenotypes of HUCB-NSCs Co-cultured with Neurons from Neonatal Rat Brain
The primary neuronal culture consisted mainly of cells with TUJ1 expression (90%–95%). Moreover, 30% of TUJ1+ cells expressed concomitantly MAP2, a mature neuronal marker. Low cell contamination of astrocytes (4% GFAP+ cells) and microglia (1% ED1+ cells) was noticed. Co-culture with neonatal rat brain neurons appeared to be the best for promotion of HUCB-NSC commitment into oligodenroglia-like cells. After 7 DIV, HUCB-NSCs differentiated mostly into O4+ cells (65%). The percentage of TUJ1+ and MAP2+ cells retained almost the same level of expression as compared with control HUCB-NSCs (26% vs 30% and 5% vs 4%, respectively). However, the number of S100β+ cells in HUCB-NSCs co-cultured with neurons was about twice lower in comparison with HUCB-NSCs kept in media (23% vs 42%). Interestingly, the mitotic activity depicted by Ki67 immunostaining of HUCB-NSCs cultured in the presence of rat neurons rose significantly to that maintained in media conditions (28% vs 19% of positive cells) (Fig. 1M–P and Fig. 4).
Figure 4.
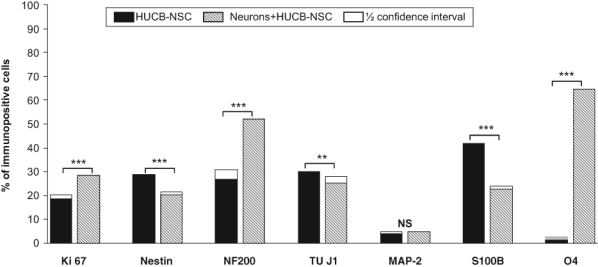
Effects of postmitotic neurons on the proliferation and differentiation of human umbilical cord blood neural-like stem cells (HUCB-NSCs) into various types of neural cells. For each experiment–control pair, superimposed on the shorter bar is a half confidence interval for the difference of percentages. The remaining gap between bars reflects the least probable difference at the 95% confidence level.
Phenotypes of HUCB-NSCs Co-cultured with Oligodendrocytes from Neonatal Rat Brain
To obtain mature oligodendrocyte cell culture, the NG-2 progenitors were isolated from neonatal rat brains and cultured in vitro for 7 days toward mature GalC+ cells. The immunocytochemical characteristics confirmed both the maturation and purity of this oligodendrocyte primary culture (98% O4+ cells, half of them concomitantly expressing GalC). After 1 week in serum-free medium, the cell content unveiled the presence of sparse neurons (2% TUJ1+ cells). Rat oligodendrocytes were shown to efficiently stimulate HUCB-NSC differentiation. Immunocytochemical analysis of HUCB-NSCs cultured in the presence of oligodendrocytes revealed that the majority of the HUCB-NSCs differentiated into neurons. The number of NF-200+ cells increased markedly as compared to HUCB-NSCs cultured in media (81% vs 27%). The observed results were accompanied by the expansion of TUJ1+ cells (88% vs 30%). Moreover, MAP2+ mature neurons could be observed occasionally. The glial phenotypes were acquired by the minor population of HUCB-NSCs co-cultured with rat oligodendrocytes. The percentage of cells with S100β expression decreased noticeably in comparison with HUCB-NSCs cultured in media (7% vs 42%). Conversely, a slight increase in O4+ cells was noted (6% vs 1%). Nestin expression was observed in less than 1% of HUCB-NSCs co-cultured with rat oligodendrocytes as compared with the controls (29%), which together with a significant decrease in Ki67+ (12% vs 19%) indicates the strong stimulating influence of the oligodendrocytes on HUCB-NSC terminal neuronal differentiation (Fig. 1Q–T and Fig. 5).
Figure 5.
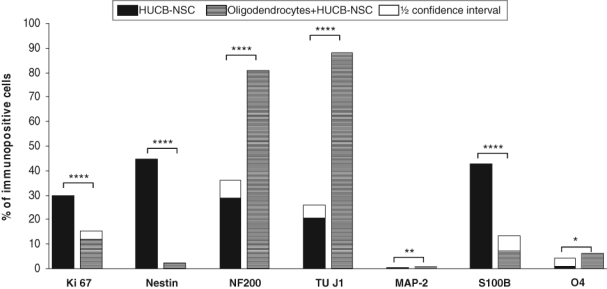
Effects of oligodendrocytes on the proliferation and differentiation of human umbilical cord blood neural-like stem cells (HUCB-NSCs) into various types of neural cells. For each experiment–control pair, superimposed on the shorter bar is a half confidence interval for the difference of percentages. The remaining gap between bars reflects the least probable difference at the 95% confidence level.
Phenotypes of HUCB-NSCs Co-cultured in the Presence of Endothelial Cells
Endothelial cells used as a contact cell culture system with HUCB-NSCs do not favor any phenotypes of human cell neural commitment. Nevertheless, in the presence of endothelial cells derived from the thymus capillary of mice, the higher percentage of TUJ1+ and S100β+ cells was found in HUCB-NSCs after 7 DIV co-culture in comparison with HUCB-NSCs cultured in media (41% vs 30% and 60% vs 42%, respectively). Concomitantly, the slight decrease in the percentage of nestin+ cells was observed (21% vs 29%). At the same time, HUCB-NSCs cultured in the presence of t-END cells revealed higher mitotic potential (Ki67+) than control HUCB-NSCs (27% vs 19%). (Fig. 1U–X and Fig. 6).
Figure 6.
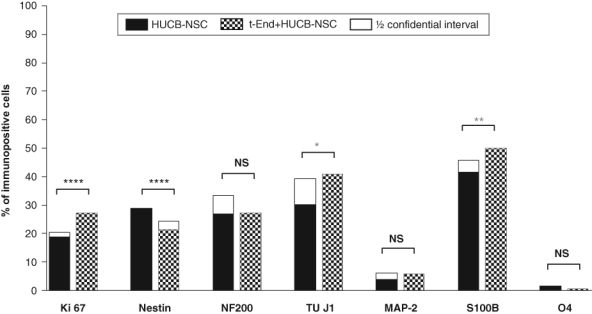
Effects of endothelial cells on the proliferation and differentiation of human umbilical cord blood neural-like stem cells (HUCB-NSCs) into various types of neural cells. For each experiment–control pair, superimposed on the shorter bar is a half confidence interval for the difference of percentages. The remaining gap between bars reflects the least probable difference at the 95% confidence level.
Discussion
The present study explored the possibility that distinct cells in brain parenchyma have a different influence on neural stem cell lineage commitments. To evaluate better the contributions of astrocytes, microglia, neurons, and oligodendrocytes to HUCB-NSCs, we used a contact cell culture system to observe in vitro the morphological changes and the expression of immunophenotypes of HUCB-NSC descendents in such cultures with different rat brain cells.
Astrocytes were shown to promote survival and induce neurogenesis by both adult neural (Barker and Ullian 2010; Környei et al. 2005; Lim and Alvarez-Buylla 1999; Markiewicz and Lukomska 2006; Song et al. 2002) and embryonic stem cells (Nakayama et al. 2003) in vitro. Extending our previous observation (Jurga et al. 2006), we found that astrocytes from the neonatal rat brain increase neurogenesis from cultured HUCB-NSCs, promoting their proliferation and neuronal maturation. Our data show an increase in the number of neuronal cells (NF-200+, TUJ1+, MAP-2+ cells) produced from HUCB-NSCs on a feeder layer of neonatal rat astrocytes as compared to those cultured in parallel in media alone. The expression of NF200 and TUJ1, the neuronal markers during the 7-day-long experiments, reveals constant production of these cells in co-culture with astrocytes. Moreover, we observed there was more than a 10-fold increase in the percentage of mature neurons (MAP2+). In contrast to the observed influence on the neuronal commitment of HUCB-NSCs, the neonatal rat astrocytes did not promote their differentiation into astrocytes. This was in concordance with other findings. Song et al. (2002) showed that astrocytes from the hippocampus of newborn rats increase the rate of proliferation of adult neural stem cells and steer their progeny to become neurons rather than glia. The authors demonstrated that there is a significant regional specificity in the ability of astrocytes to induce neurogenesis. Astrocytes purified from hippocampus retain the potential to encourage neuronal differentiation, but spinal cord astrocytes, one of the non-neurogenic regions, are ineffective in promoting neurogenesis from adult stem cells. Culturing dissociated postnatal or adult cells isolated from the mice subventricular zone (SVZ) on the astrocyte monolayer supported extensive neurogenesis similar to that observed in vivo (Lim and Alvarez-Buylla 1999). SVZ precursors proliferated rapidly on astrocytes to form aggregates containing neuroblasts. The data provided by Nakayama et al. (2003) showed that aggregate formation and predifferentiation of neural stem cells rendered these cells responsive to astroglia-derived neurogenic signals. Although these observations confirm that astrocytes have the potential to induce neuron maturation, little is known about the factors responsible for this effect. Lim and Alvarez-Buylla (1999) have shown that direct contact with astrocytes is necessary for the proliferation of SVZ neuronal precursors and differentiation into neuroblasts.
The contribution of microglia to the modulation of neurogenesis has been less explored. Until recently, microglial cells have been ignored as part of the environment that could affect the proliferation and differentiation of NSCs. However, CNS inflammation sustained by microglia has been associated with the inhibition of neurogenesis (Ekdahl et al. 2003; Liu Z et al. 2007). Lately, both pro- and anti-neurogenic effects have been reported, reflecting the complexity of microglial activation (Cacci et al. 2008; Walton et al. 2006; Ziv et al. 2006). It was shown that acutely activated microglia reduced the NSC survival, prevented neuronal differentiation, and strongly increased glial differentiation (Cacci et al. 2008; Monje et al. 2003), whereas chronically activated microglia were permissive to neuronal differentiation and still supported glial maturation (Battista et al. 2006; Butovsky et al. 2006). We demonstrate that in vitro exposure of HUCB-NSCs to microglia derived from neonatal rat brain stimulates both neuronal and glial differentiation. When induced to differentiate, a progressive reduction in nestin-expressing progenitors was observed. Our observations are consistent with the data reporting the generation of new neurons from NSCs and the maintenance of astrocytes by unstimulated or chronically stimulated microglia (Cacci et al. 2008). Simultaneous pro-neurogenic and pro-gliogenic effect of neonatal rat brain microglia on HUCB-NSCs observed in our studies might be the effects of distinct microglial functional profiles on NSC differentiation.
Until now, limited information has been available on neuronal cell contribution to the regulation of neurogenesis. Wu et al. (2003) presented a study of the olfactory epithelium in which the generation of new neurons by neuronal progenitors was inhibited by a signal from neurons themselves. They identified growth and differentiation factor 11 (GDF11) as a feedback inhibitory signal of neurogenesis. GDN11, also known as bone morphogenetic protein 11 (BMP11), was shown to play a general role in modulating neural differentiation (Ge et al. 2005). In our experiments, neuron-enriched cultures are not sufficient to promote neuron and astrocyte differentiation. Notably, the fraction of oligodendrocytes (O4+) increased significantly under these conditions, indicating that primary neurons promote oligogenesis from HUCB-NSCs. The same pattern was reported by Song et al. (2002). Within 6 days of co-culture with neurons, most neural stem cells isolated from rat hippocampus acquired mature oligodendrocytic markers, such as RIP. Neurons are known to control the development of myelinated glial cells. There are studies suggesting that axons promote oligodendrocyte development by helping to drive proliferation of oligodendrocyte precursor cells (OPCs) or by promoting the survival of mature, myelinating oligodendrocytes (Barres and Raff 1999; Simons and Trajkovic 2006).
Not only was the influence on the oligodendrocyte survival rate reported, but the auto-regulation of neurogenesis by mature neurones was highlighted as well (Hastings and Gould 2003). In our studies, the number of mature neurons (TUJ1+, MAP2+) in the co-culture of HUCB-NSCs on a feeder layer of primary neurons was comparable with those cultured in medium alone.
Oligodendrocytes were reported to influence neurons multidirectionally: They induce local accumulation of neurofilaments within axons, regulate axonal caliber and stability, generate myelin, and are potent to either promote or inhibit neuronal survival and development (Allen and Barres 2005; Fields and Stevens-Graham 2002; Göritz et al. 2007; Kinrade and Hidalgo 2004; Simons and Trajkovic 2006). Differentiated oligodendrocytes are known to secrete glial cell line–derived neurotrophic factor (GDNF) and insulin-like growth factor 1 (IGF-1), which regulate both the neuronal survival and axonal length, by activating two intracellular signaling pathways: the PI3kinase/Akt and MAPkinase/Erk (Atwal et al. 2000; Wilkins et al. 2003). Other neurotrophic factors (midkine, transforming growth factor [TGF]–β2, hepatocyte growth factor [HGF], actin A, brain-derived neurotrophic factor [BDNF]) stimulate neurite outgrowth in vitro (Zhang YW et al. 2006), as well as increase the survival rate of transplanted dopamine neurons (Sortwell et al. 2000). On the other hand, the molecules expressed by oligodendrocytes (Nogo, MAG, OMgp) have been shown to inhibit axon outgrowth (Ng et al. 1996; He and Koprivica 2004; Wang et al. 2010). Glial-restricted progenitors have been shown to be proliferatively active even in the adult brain (Magnus et al. 2008; Tamura et al. 2007; White et al. 2010). CNS insults such as brain or spinal cord injury trigger the mobilization of endogenous progenitor cells that migrate and finally differentiate into myelin-forming cells (Nielsen et al. 2006; Nistor et al. 2005). The presence of newly formed oligodendrocytes differently influences the scar local microenvironment, presumably enhancing or inhibiting regeneration and functional recovery. The transplanted HUCBs have been shown to differentiate into myelin-forming cells, which secrete neurotrophin-3 (NT-3) and BDNF (Dasari et al. 2007). The emerging evidence, however, indicates that just after spinal cord injury, Nogo-A mRNA is upregulated around the lesion, probably contributing to the failure of axon regeneration (Hunt et al. 2003; Zhang S et al. 2008). Furthermore, the protein has been shown to inhibit migration and differentiation of the oligodendrocyte precursor cells, which is another obstacle to functional recovery following CNS injury (Su et al. 2007; Syed et al. 2008).
In our experiments, direct contact with myelin-forming cells accelerated differentiation of HUCB-NSCs mostly into immature neurons (NF200+, TUJ1+), indicating promotion of neurogenesis. This observation may correspond to the hypothesis suggesting the active role of oligodendrocytes in tissue restoration. Accordingly, the oligodendrocytes might induce neurogenesis from endogenous stem cells (recruited by factors of local inflammation) and stimulate their differentiation to currently required cell replacement. In view of our research, the oligodendrocyte function in the neuronal promotion of adult stem cells links them to the cell population that regulates neurogenesis.
Accumulating evidence suggests the essential role the endothelial cells play in the regulation of stem cell self-renewal and differentiation. The first hint that endothelial cells are implicated in neurogenesis dates back to 1999, when Leventhal et al. showed that co-culture of adult rat SVZ explants with endothelial cells resulted in significantly more neurons (Leventhal et al. 1999). It has been demonstrated that in the presence of endothelial cells, adult neural stem cells undergo proliferative divisions to produce undifferentiated progenitors with multipotential capacity (Mathieu et al. 2008; Shen et al. 2004). The presence of endothelial cells inhibits neural stem cell differentiation with the increase of the number of cells expressing nestin (Shen et al. 2004; Guo et al. 2008; Plane et al. 2010; Suzuki et al. 2010). On the other hand, the presence of endothelial cells increases the number of neural progenitors having the “side population” phenotype, which have been shown to contain quiescent cells (Mathieu et al. 2006).
In our study, the co-culture of HUCB-NSCs with t-END cells triggered their proliferation (more Ki67+ cells were observed than in the control) while simultaneously priming them to differentiate. In fact, the great majority of HUCB-NSCs still expressed nestin, and the differentiation potential of HUCB-NSCs co-cultured with t-END cells was much lower than in the presence of astrocytes, microglia, neurons, or oligodendrocytes. It was recently reported that endothelial cells induce differentiation of neural stem cells into neurons and astrocytes but not into oligodendrocytes (Imura et al. 2008; Lai et al. 2008). The mechanism of this dual regulation remains elusive. Endothelial cells are known to enhance neurogenesis possibly through the secretion of different factors specifying the fate of neural stem cells. The profile of signaling molecules may induce a shift in the mixed population of proliferating and differentiating neural progenitors to promote their self-renewal or differentiation.
In conclusion, our studies have shown that HUCB-NSCs can read cues from the neurogenic microenvironment. This is further supported by the observations that HUCB-NSCs survive in the presence of rat brain cells and subsequently attain features of neurons and glia. Rat astrocytes and oligodendrocytes strongly promote HUCB-NSC neuronal differentiation. In turn, postmitotic neurons provide differentiating neural progenitors of human cord blood origin with distinctive pro-oligodendrogenic signals. However, microglia and endothelial cells do not promote any particular neural commitment of these cells. Our results strongly suggest that brain cellular microenvironment may provide key signals guiding stem/progenitor cells to differentiate into three main neural lineages. The specific responses of neurally committed cord blood–derived cells, reported in the present work, are very much similar to those described previously for NSCs derived from other, “more typical” sources. This further proves their genuine neural nature. Apart from having a better insight into the neurogenesis in the adult brain, these findings might be important when predicting the cord blood cell derivative behavior after their transplantation for neurological disorders.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The work reported here has been supported by Polish Ministry of Scientific Research and Higher Education grants No. 1309/P01/2006/31, 296/P01/2007/32 and 142/B/P01/2008/35.
References
- Agresti A. 1990. Categorical data analysis. New York: John Wiley [Google Scholar]
- Allen NJ, Barres BA. 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 15:542–548 [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. 2000. The TrkB-Shc site signals neuronal survival and local axon growth via MAPK/Erk pathways and PI3-kinase. Neuron. 27:265–277 [DOI] [PubMed] [Google Scholar]
- Barker AJ, Ullian EM. 2010. Astrocytes and synaptic plasticity. Neuroscientist. 16:40–50 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. 1999. Axonal control of the oligodendrocyte development. J Cell Biol. 147:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. 2006. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 23:83–93 [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Toricelli JR, Eveg EK, Price PJ. 1993. Optimized survival of hippocampal neurons in B27-suplemented Neurobasal™, a new serum-free medium combination. J Neurosc Res. 35:567–576 [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. 2006. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 31:149–160 [DOI] [PubMed] [Google Scholar]
- Buzanska L, Jurga M, Stachowiak EK, Stachowiak MK, Domanska-Janik K. 2006. Neural stem-like cell line derived from nonhematopoietic population of human umbilical cord blood. Stem Cell Dev. 15:391–406 [DOI] [PubMed] [Google Scholar]
- Buzanska L, Machaj ED, Zablocka B, Pojda Z, Domanska-Janik K. 2002. Human cord blood–derived cells attain neuronal and glial features in vitro. J Cell Sci. 115:2131–2138 [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. 2008. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 56:412–425 [DOI] [PubMed] [Google Scholar]
- Cova L, Ratti A, Volta M, Fogh I, Cardin V, Corbo M, Silani V. 2004. Stem cell therapy for neurodegenerative diseases: the issue of transdifferentiation. Stem Cells Dev. 13:121–131 [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Zetterstrom-Axel M, Wikkelso C, Holtas S, van Roon-Mom WMC, Bjork-Eriksson T, Nordborg C, et al. 2007. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 315:1243–1249 [DOI] [PubMed] [Google Scholar]
- Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, Rao JS, Dinh DH. 2007. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 24:391–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. 2007. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 25:100–106 [DOI] [PubMed] [Google Scholar]
- Domanska-Janik K, Buzanska L, Lukomska B. 2008. A novel, neural potential of non-hematopoietic human umbilical cord blood stem cells. Int J Dev Biol. 52:237–248 [DOI] [PubMed] [Google Scholar]
- Dotti C, Sullivan C, Banker G. 1988. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 8:1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. 2003. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 11:13632–13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. 2002. New insights into neuron-glia communication. Science. 298:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Hopkins DR, Ho WB, Greenspan DS. 2005. GDF11 forms a bone morphogenetic protein 1–activated latent complex that can modulate nerve growth factor–induced differentiation of PC12 cells. Mol Cell Biol. 25:5846–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göritz C, Thiebaut R, Tessier LH, Nieweg K, Moehle C, Buard I, Dupont JL, Schurgers L, Schmitz G, Pfrieger F. 2007. Glia-induced neuronal differentiation by transcriptional regulation. Glia. 55:1108–1122 [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. 1989. Experimental observations of the development of polarity by hippocampal neurons in culture. J Cell Biol. 108:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Shi D, Li W, Liang C, Wang H, Ye Z, Hu L, Wang HQ, Li Y. 2008. Proliferation and neurogenesis of neural stem cells enhanced by cerebral microvascular endothelial cells. Microsurgery. 28:54–60 [DOI] [PubMed] [Google Scholar]
- Habich A, Jurga M, Markiewicz I, Lukomska B, Bany-Laszewicz U, Domanska-Janik K. 2006. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp Hematol. 34:914–925 [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. 2003. Neurons inhibit neurogenesis. Nat Med. 9:264–266 [DOI] [PubMed] [Google Scholar]
- He Z, Koprivica V. 2004. The Nogo signalling pathway for regeneration block. Annu Rev Neurosci. 27:341–368 [DOI] [PubMed] [Google Scholar]
- Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, et al. 2004. Efficient generation of neural stem cell–like cells from adult human bone marrow stromal cells. J Cell Sci. 117:4411–4422 [DOI] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Prinjaba RK, Campbell G, Anderson PN. 2003. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci. 24:1083–1102 [DOI] [PubMed] [Google Scholar]
- Imura T, Tane K, Toyoda N, Fushiki S. 2008. Endothelial cell-derived bone morphogenic proteins regulate glial differentiation of cortical progenitors. Eur J Neurosci. 27:1596–1606 [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. 2006. Evidence for stroke-induced neurogenesis in the human brain. PNAS. 103:13198–13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. 1999. Neural stem cells in the adult human brain. Exp Cell Res. 253:733–736 [DOI] [PubMed] [Google Scholar]
- Jurga M, Markiewicz I, Sarnowska A, Habich A, Kozłowska H, Lukomska B, Bużanska L, Domańska-Janik K. 2006. Neurogenic potential of human umbilical cord blood-neural-like stem cells depends on their previous long-term culture conditions. J Neurosci Res. 83:627–637 [DOI] [PubMed] [Google Scholar]
- Kinrade EF, Hidalgo A. 2004. Lateral neuron-glia interactions steer the response of axons to the Robo code. Neuron Glia Biol. 1:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. 2004. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 200:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Környei Z, Szlávik V, Szabó B, Gócza E, Czirók A, Madarász E. 2005. Humoral and contact interactions in astroglia/stem cell co-cultures in the course of glia-induced neurogenesis. Glia. 49:430–444 [DOI] [PubMed] [Google Scholar]
- Kozlowska H, Jablonka J, Janowski M, Jurga M, Kossut M, Domanska-Janik K. 2007. Transplantation of a novel human cord blood-derived neural stem-like cell line in rat model of cortical infarct. Stem Cells Dev. 16:481–488 [DOI] [PubMed] [Google Scholar]
- Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. 2005. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir. 147:985–992 [DOI] [PubMed] [Google Scholar]
- Lai B, Mao XO, Greenberg DA, Jin K. 2008. Endothelium-induced proliferation and electrophysiological differentiation of human embryonic stem cell–derived neuronal precursors. Stem Cells Dev. 17:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. 1999. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 13:450–464 [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. 1999. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 22:7526–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. 2005. Stem cell therapy for human brain disorders. Kidney Int. 68:1937–1939 [DOI] [PubMed] [Google Scholar]
- Liu HY, Zhang QJ, Li HJ, Han ZC. 2006. Effect of intracranial transplantation of CD34+ cells derived from human umbilical cord blood in rats with cerebral ischemia. Chin Med J. 119:1744–1748 [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. 2007. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 38:146–152 [DOI] [PubMed] [Google Scholar]
- Lyck L, Dalmau I, Chemnitz J, Finsen B, Daa Schrøder H. 2008. Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex. J Histochem Cytochem. 56:201–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ. 2008. Adult glial precursor proliferation in mutant SOD1 (G93A) mice. Glia. 56:200–2008 [DOI] [PubMed] [Google Scholar]
- Manly BFJ. 1997. Randomization, bootstrap and Monte Carlo methods in biology. London: Chapman & Hall [Google Scholar]
- Markiewicz I, Lukomska B. 2006. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp. 66:343–358 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Fouchet P, Gautthier LR, Lassalle B, Boussin FD, Mouthon MA. 2006. Coculture with endothelial cells reduces the population of cycling LeX neural precursors but increases that of quiescent cells with a side population phenotype. Exp Cell Res. 312:707–718 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Sii-Felice K, Fouhcet P, Etienne O, Haton C, Mabondzo A, Boussin FD, Mouthon MA. 2008. Endothelial cell–derived bone morphogenic proteins control proliferation of neural stem/progenitor cells. Mol Cell Neurosci. 38:569–577 [DOI] [PubMed] [Google Scholar]
- McCarthy K, de Vellis J. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 85:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szaloyova I, Lange GD, Crain B. 2003. Transplanted bone marrow generates new neurons in human brains. PNAS. 100:1364–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. 2003. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 302:1760–1765 [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. 2005. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. PNAS. 102:18171–18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Momoki-Soga T, Inoue N. 2003. Astrocyte-derived factors instruct differentiation of embryonic stem cells into neurons. Neurosci Res. 46:241–249 [DOI] [PubMed] [Google Scholar]
- Ng WP, Cartel N, Roder J, Roach A, Lozano A. 1996. Human central nervous system myelin inhibits neurite outgrowth. Brain Res. 720:17–24 [DOI] [PubMed] [Google Scholar]
- Nielsen H, Ladeby R, Drojdahl N, Peterson A, Finsen B. 2006. Axonal degeneration stimulates the formation of NG2+ cells and oligodendrocytes in the mouse. Glia. 54:105–115 [DOI] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter M, Keirstead HS. 2005. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 49:385–396 [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. 2003. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 9:439–447 [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodrigez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. 2006. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 494:415–434 [DOI] [PubMed] [Google Scholar]
- Pan Y, Nastav JB, Zhang H, Bretton RH, Panneton WM, Bicknese AR. 2005. Engraftment of freshly isolated or cultured human umbilical cord blood cells and the effect of cyclosporin A on the outcome. Exp Neurol. 192:365–372 [DOI] [PubMed] [Google Scholar]
- Plane JM, Andjelkovic AV, Keep RF, Parent JM. 2010. Intact and injured endothelial cells differentially modulate postnatal murine forebrain neural stem cells. Neurobiol Dis. 37:218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Deleidi M, Martino G. 2005. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Rev. 48:211–219 [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbara NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Garcia-Verdugo JM, et al. 2004. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 427:740–744 [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patelm N, et al. 2000. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 164:247–256 [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. 2004. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 304:1338–1340 [DOI] [PubMed] [Google Scholar]
- Simons M, Trajkovic K. 2006. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci. 119:4381–4389 [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. 2002. Astroglia induce neurogenesis from adult neural stem cells. Nature. 417:39–44 [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Daley BF, Pitzer MR, McGuire SO, Sladek JR, Collier TJ. 2000. Oligodendrocyte-type 2 astrocyte-derived trophic factors increase survival of developing dopamine neurons through the inhibition of the apoptotic cell death. J Comp Neurol. 426:143–153 [PubMed] [Google Scholar]
- Su Z, Cao L, Zhu Y, Liu X, Huang Z, Huang A, He C. 2007. Nogo enhances the adhesion of olfactory ensheathing cells and inhibits their migration. J Cell Biol. 120:1877–1887 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. 2010. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 58:721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YA, Baer AS, Lubec G, Hoeger H, Widhalm G, Kotter MR. 2008. Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg Focus. 24:E5. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Katoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. 2007. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 25:3489–3498 [DOI] [PubMed] [Google Scholar]
- Walczak P, Chen N, Hudson JE, Willing AE, Garbuzova-Davis SN, Song S, Sanberg PR, Sanchez-Ramos J, Bickford PC, Zigova T. 2004. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J Neurosci Res. 76:244–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, II, Scheffler B, Steindler DA. 2006. Microglia instruct subventricular zone neurogenesis. Glia. 54:815–825 [DOI] [PubMed] [Google Scholar]
- Wang C, Popescu D, Wu C, Zhu J, Macklin W, Wang Y. 2010. In situ fluorescence imaging of myelination. J Histochem Cytochem. 58:611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. 2010. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 28:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. 2003. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line–derived neurotrophic factor. J Neurosci. 23:4967–4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. 2003. Autoregulation of neurogenesis by GDF11. Neuron. 23:197–207 [DOI] [PubMed] [Google Scholar]
- Zar JH. 1999. Biostatistical analysis. Upper Saddle River (NJ): Prentice Hall [Google Scholar]
- Zhang S, Zhang Q, Zhang JH, Qin X. 2008. NgR acts as an inhibitor to axonal regeneration in adults. Front Biosci. 1:2030–2040 [DOI] [PubMed] [Google Scholar]
- Zhang YW, Denham J, Thies RS. 2006. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 15:943–952 [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. 2006. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 9:268–275 [DOI] [PubMed] [Google Scholar]