Abstract
Two key genes in terpenoid indole alkaloid biosynthesis, Tdc and Str, encoding tryptophan decarboxylase and strictosidine synthase, respectively, are coordinately induced by fungal elicitors in suspension-cultured Catharanthus roseus cells. We have studied the roles of the jasmonate biosynthetic pathway and of protein phosphorylation in signal transduction initiated by a partially purified elicitor from yeast extract. In addition to activating Tdc and Str gene expression, the elicitor also induced the biosynthesis of jasmonic acid. The jasmonate precursor α-linolenic acid or methyl jasmonate (MeJA) itself induced Tdc and Str gene expression when added exogenously . Diethyldithiocarbamic acid, an inhibitor of jasmonate biosynthesis, blocked both the elicitor-induced formation of jasmonic acid and the activation of terpenoid indole alkaloid biosynthetic genes. The protein kinase inhibitor K-252a abolished both elicitor-induced jasmonate biosynthesis and MeJA-induced Tdc and Str gene expression. Analysis of the expression of Str promoter/gusA fusions in transgenic C. roseus cells showed that the elicitor and MeJA act at the transcriptional level. These results demonstrate that the jasmonate biosynthetic pathway is an integral part of the elicitor-triggered signal transduction pathway that results in the coordinate expression of the Tdc and Str genes and that protein kinases act both upstream and downstream of jasmonates.
The initiation of a plant defense response requires the perception of pathogen-derived (exogenous) or plant-derived (endogenous) signal molecules, collectively referred to as elicitors (for review, see Boller, 1995; Yang et al., 1997). Elicitor-induced defense responses include the biosynthesis of secondary metabolites (Darvill and Albersheim, 1984; Côté and Hahn, 1994) and proteinase inhibitors (Pearce et al., 1991). Protein phosphorylation is an essential component of elicitor-induced signal transduction (Chandra and Low, 1995; Suzuki et al., 1995). The lipid-based octadecanoid pathway leading to JA has also been implicated as an integral part of the signal transduction pathway leading to the activation of defense responses. The octadecanoid pathway was first implicated in wounding-induced biosynthesis of proteinase inhibitors (Farmer and Ryan, 1992). Upon wounding, the octadecanoid pathway is activated by the polypeptide systemin and by oligouronides, resulting in elevated levels of JA (Doares et al., 1995b). JA and its octadecanoid precursors activate the synthesis of wounding-inducible proteinase inhibitors (Farmer and Ryan, 1992). Induction of the synthesis of proteinase inhibitors by wounding, systemin, and oligouronides is blocked by several inhibitors of the jasmonate biosynthetic pathway (Farmer et al., 1994; Doares et al., 1995b). Furthermore, Kim et al. (1992) identified a MeJA-responsive element in the promoter of a proteinase inhibitor II gene, indicating that this gene is transcriptionally regulated in response to MeJA.
How elicitors affect JA biosynthesis and how the JA signal is transduced to effect gene expression is largely unknown. Much more is known about the JA biosynthetic pathway itself. Farmer and Ryan (1992) have proposed that a lipase generates α-linolenic acid, the first precursor in the octadecanoid pathway. α-Linolenic acid is then converted by a lipoxygenase, an allene oxide synthase, and an allene oxide cyclase into the intermediate 12-oxo-phytodienoic acid. This compound is converted into JA through the action of a reductase and three rounds of β-oxidation (Vick and Zimmerman, 1984; Mueller, 1997).
JA and its octadecanoid precursors have also been implicated as intermediate signals in elicitor-induced secondary metabolite accumulation (Gundlach et al., 1992; Mueller et al., 1993; Ellard-Ivey and Douglas, 1996; Nojiri et al., 1996). A correlation between elicitor-induced accumulation of endogenous JA and secondary metabolite accumulation was shown in cells of California poppy (Mueller et al., 1993) and rice (Nojiri et al., 1996). In parsley cells phenylpropanoid biosynthetic genes were induced by octadecanoids, and elicitor-induced gene expression was blocked by a lipoxygenase inhibitor (Ellard-Ivey and Douglas, 1996). These reports indicate that in elicitor-induced secondary metabolism JA plays a role that is similar to its role in the accumulation of wound-induced proteinase inhibitors, for which it has been elegantly demonstrated that jasmonates are intermediate signals that transcriptionally activate proteinase inhibitor genes. However, the studies that have been done on various metabolic pathways in different plant species using diverse elicitors lack the integrated approach of measuring jasmonate biosynthesis and studying the effect of JA and octadecanoid pathway inhibitors on gene expression. In general, metabolite accumulation has been studied instead of gene expression. Therefore, in most cases it remains unclear on what regulatory level jasmonates exert their effect on secondary metabolism.
In Catharanthus roseus cell suspensions, the expression of two TIA biosynthetic genes, Tdc and Str, encoding Trp decarboxylase and strictosidine synthase, respectively, is coordinately induced by fungal elicitors such as YE (Pasquali et al., 1992). The enzymes encoded by these genes are important in the TIA biosynthetic pathway (for reviews, see Meijer et al., 1993; Kutchan, 1995). Both genes are present as single copies in the haploid C. roseus genome (Pasquali et al., 1992; Goddijn et al., 1994).
Using the integrated approach of measuring JA biosynthesis and using an inhibitor of the octadecanoid pathway, we show conclusively that elicitor-induced expression of the Tdc and Str genes in C. roseus cells is mediated by the octadecanoid pathway. Furthermore, we provide evidence for the existence of protein phosphorylation steps both upstream and downstream of the octadecanoid pathway. We also show that both elicitor-induced and MeJA-induced gene expression are conferred by the Str promoter, demonstrating that elicitor and MeJA act at the transcriptional level.
MATERIALS AND METHODS
Cell Culture
Cell-suspension cultures of the Catharanthus roseus line MP183L were grown as described by Pasquali et al. (1992). A BH fragment of 396 bp (−339 to +52 relative to the transcriptional start site) was excised from the Str promoter (accession no. Y10182) and fused to the gusA gene in the vector GusSH digested with BamHI and HincII (Pasquali et al., 1994). The BH derivative of GusSH was used to make transgenic C. roseus MP183L cell lines by particle bombardment according to the method of van der Fits and Memelink (1997).
Elicitor and Jasmonate Treatment
YE (DIFCO Laboratories, Detroit, MI) was dissolved in water, autoclaved, and used as a crude extract at a concentration of 400 g mL−1 to elicit C. roseus cells. PE was prepared through ultrafiltration and a number of chromatographic steps, including size-exclusion chromatography, anion-exchange chromatography, and reversed-phase HPLC (F.L.H. Menke, M. Harleveld, and J. Memelink, unpublished data). This resulted in a partially purified elicitor preparation of unknown absolute quality. We estimated the active component in PE to be purified at least 1000-fold. Crude YE/PE and induced the alkalinization of the cell culture medium within 5 to 10 min. The kinetics and the amplitude of the alkalinization response were proportional to the amount of elicitor added. Using this semiquantitative alkalinization assay, the amount of PE used for induction experiments was calibrated to give an alkalinization response that was equivalent to the effect of 400 g mL−1 crude YE. MeJA (Bedoukian Research, Danbury, CT) and α-linolenic acid and linoleic acid (both from Sigma) were diluted in DMSO.
Inhibitor Treatment
Ibuprofen (Sigma), K-252a (Calbiochem), staurosporine (Sigma), and OA and calyculin A (both from Calbiochem) were dissolved in DMSO. DIECA and acetyl-salicylic acid (both from Sigma) were dissolved in 15 mm KPO4, pH 6.5. Each inhibitor was added 10 min before the addition of elicitor or MeJA. Cells were incubated in the presence of the elicitor or MeJA for 6 h unless indicated otherwise.
RNA Extraction and Northern Analysis
We extracted RNA and performed northern analysis as described previously by Menke et al. (1996) Unless indicated otherwise, 10-μg RNA samples were loaded onto the gels. All northern blots used 32P-labeled cDNA probes. For Tdc we used a cDNA clone that was full length and otherwise homologous to the cDNA cloned by De Luca et al. (1989). The full-length cDNA clone for Str (Pasquali et al., 1992) and a cDNA corresponding to Rps9 (40S ribosomal protein S9) were isolated from C. roseus (L. van der Fits and J. Memelink, unpublished data).
JA Extraction and Quantification
Three- or four-day-old C. roseus cell suspensions (MP183L) were incubated with YE or PE for 0 to 6 h. The cells were harvested by vacuum filtration using an 80-μm mesh. We divided the samples into portions for RNA extraction and portions for JA extraction. The cells were snap frozen in liquid nitrogen and stored at −80°C. We used 6 to 12 g (fresh weight) of the cells to extract jasmonates and extracted and quantified the jasmonates according to the method described by Mueller and Brodschelm (1994).
RESULTS
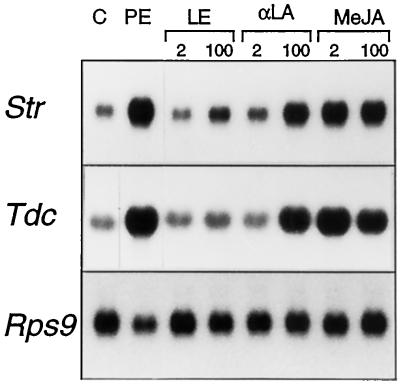
Tdc and Str gene expression in suspension-cultured C. roseus cells is coordinately induced by the addition of fungal elicitors (Pasquali et al., 1992). The crude elicitor preparations used by Pasquali et al. (1992) are less suitable for signal transduction studies because they may consist of multiple components that exert different effects on C. roseus cells. Therefore, the purification and characterization of a YE elicitor were undertaken (F.L.H. Menke, M. Harleveld, and J. Memelink, unpublished data), and a PE that induces Tdc and Str gene expression was obtained (Fig. 1).
Figure 1.
PE and octadecanoids induce the TIA biosynthetic genes Tdc and Str. C. roseus cells were exposed to DMSO as a control (C) or PE, linoleic acid (LE), α-linolenic acid (α-LA), or MeJA for 6 h, after which time cells were harvested and total RNA was isolated. Northern blots were hybridized with Tdc, Str, and Rps9 cDNAs. The micromolar concentrations of the octadecanoids are given at the top.
Octadecanoids Induce TIA Biosynthetic Gene Expression
The octadecanoid pathway has been implicated in wounding- and elicitor-induced signaling leading to defense gene expression in plants (Farmer and Ryan, 1992; Gundlach et al., 1992). We tested the ability of octadecanoids to induce TIA biosynthetic gene expression in suspension-cultured C. roseus cells. Cultures were treated for 6 h with MeJA, the jasmonate precursor α-linolenic acid, and a related compound, linoleic acid, which cannot function directly as a precursor in the octadecanoid pathway at concentrations between 2 and 200 μm. Figure 1 shows that 2 μm MeJA induced TIA biosynthetic gene expression to a high level. At 2 μm, α-linolenic acid did not induce gene expression over the control level. At 100 μm α-linolenic acid, the levels of Tdc and Str transcripts were comparable to those induced by PE. Higher concentrations of α-linolenic acid did not further increase the transcript levels (data not shown). The octadecanoid linoleic acid did not significantly induce Str or Tdc gene expression levels in the concentration range tested (Fig. 1; data for 200 μm not shown). Figure 1 also shows that PE, MeJA, and the two octadecanoids had little effect on the level of Rps9 mRNA, encoding the 40S ribosomal protein S9. These results indicate that the expression of Tdc and Str genes in suspension-cultured C. roseus cells is induced by MeJA and its precursor, but not by a related octadecanoid that does not form part of the octadecanoid pathway.
PE Induces JA Biosynthesis
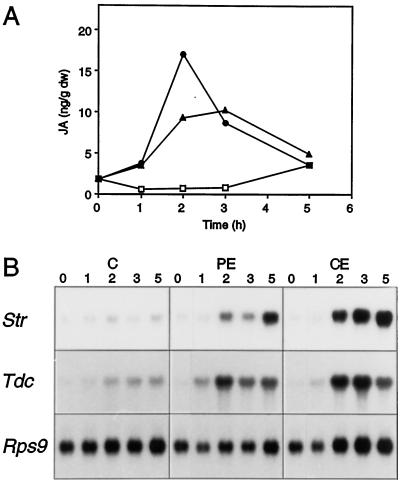
The fact that both PE and octadecanoids induced TIA biosynthetic gene expression suggests that the octadecanoid pathway mediated elicitor-induced signaling. If so, then involvement of the octadecanoid pathway should be reflected by an increase in endogenous levels of jasmonates in C. roseus cells in response to PE. To test this hypothesis, suspension cultures were exposed for different lengths of time to PE and crude YE. Figure 2A shows a representative experiment. PE induced the accumulation of endogenous JA within 1 h, reached a maximum at 3 h, and then decreased to background level within 5 h. The addition of crude YE induced the accumulation of JA with similar kinetics (Fig. 2A). The corresponding northern blot (Fig. 2B) shows a steady increase in the levels of Tdc and Str mRNAs, starting at 2 h and continuing up to 5 h for both PE and crude YE, whereas no significant changes were observed in the levels of Rps9 mRNA. In the noninduced control cultures, neither endogenous JA nor Tdc or Str transcripts accumulated this time. Four independent experiments within were done and all showed the same pattern, although the maximum amount of JA that was induced upon elicitor treatment varied from 20 to 120 ng g−1 (dry weight). These results indicate that the PE induces a transient accumulation of endogenous JA and a concomitant increase in TIA biosynthetic gene expression.
Figure 2.
YE elicitor induces JA biosynthesis. A, Amount of JA in C. roseus cells at different times after the addition of water as a control (□) or PE (▴) or crude (•) YE. DW, Dry weight. B, Str, Tdc, and Rps9 mRNA levels at different times after the addition of water as a control (C) or of PE or crude (CE) YE. RNA was extracted from the same sample of tissue used to extract JA. The times in hours are indicated (top).
The Octadecanoid Pathway Mediates Elicitor-Induced TIA Biosynthetic Gene Expression
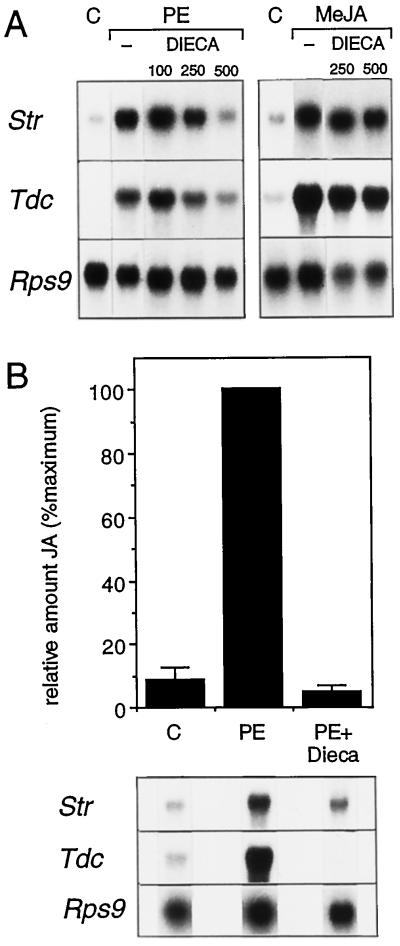
If jasmonates act as intermediates in elicitor-induced signaling, then inhibitors of the octadecanoid pathway should block PE-induced TIA biosynthetic gene expression. To test this hypothesis, we incubated cells with the octadecanoid pathway inhibitor DIECA and then studied the effect on PE-induced responses. DIECA inhibits the octadecanoid pathway by reducing the intermediate 13-S-hydroperoxylinolenic acid to 13-hydroxylinolenic acid, which is not an intermediate in the octadecanoid pathway (Farmer et al., 1994). DIECA completely blocked the elicitation of Str and Tdc gene expression in C. roseus cells at 500 μm, but the level of Rps9 mRNA was not affected (Fig. 3A). At 100 μm, DIECA had no effect (Fig. 3A). The two highest concentrations of DIECA tested did not significantly affect MeJA-induced Str and Tdc gene expression (Fig. 3A). This indicates that DIECA acts on the octadecanoid pathway upstream of (Me)JA, which is in agreement with the results of Farmer et al. (1994).
Figure 3.
A, Effect of DIECA on TIA biosynthetic gene expression. C. roseus cell suspensions were incubated with DIECA for 10 min prior to the addition of water as a control (C), PE, or 50 μm MeJA, and cells were harvested 6 h later. The micromolar concentrations of inhibitors are indicated at the top. B, Effect of octadecanoid inhibitor on PE-induced JA accumulation. C. roseus cells were incubated with water or PE for 3 h. DIECA was added 10 min before the addition of PE. Bars represent se (n = 3).
In a separate experiment to determine the effect of DIECA (500 μm) on JA biosynthesis, we found an inhibition of 95% relative to the PE-induced accumulation of JA (Fig. 3B). The corresponding northern blot showed that DIECA had a clear inhibitory effect on Tdc and Str mRNA accumulation in this experiment but did not affect the level of Rps9 mRNA. From Figure 3B, it is clear that strong inhibition of JA accumulation by DIECA resulted in a comparably strong inhibition of Tdc and Str mRNA accumulation. The results obtained with DIECA show that elicitor-induced Str and Tdc gene expression in C. roseus was mediated through the octadecanoid pathway.
Involvement of Protein Phosphorylation/Dephosphorylation
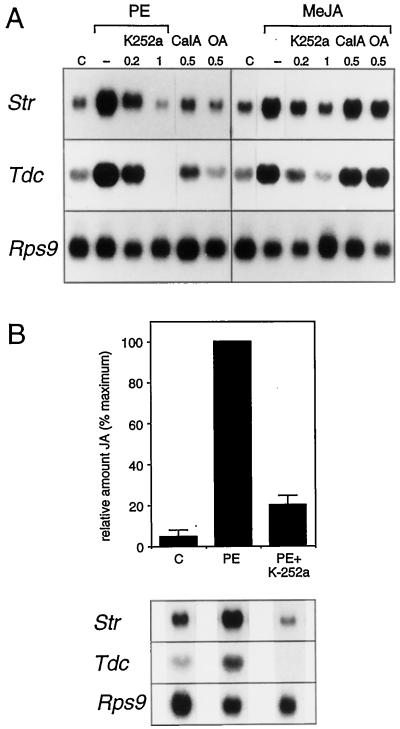
The mechanisms by which the octadecanoid signal is generated and subsequently transduced to affect gene expression are not known. To begin to address this question, we studied the effect of inhibitors of protein kinases and protein phosphatases on elicitor- and MeJA-induced gene expression. Treatment of C. roseus cells with different concentrations of the protein kinase inhibitor K-252a resulted in the inhibition of elicitor-induced TIA biosynthetic gene expression (Fig. 4A). Treatment of the cells with 200 nm K-252a resulted in a slight reduction of the amounts of Str and Tdc mRNA that accumulated 6 h after the addition of PE. At 1 μm, the mRNA accumulation in response to the elicitor was completely blocked for both Str and Tdc. The level of Rps9 mRNA was not affected by incubation with this protein kinase inhibitor, indicating that at these concentrations K-252a does not cause a general down-regulation of gene expression. We obtained similar results using 1 μm of the protein kinase inhibitor staurosporine (data not shown). MeJA-induced TIA biosynthetic gene expression was also sensitive to the protein kinase inhibitors K-252a (Fig. 4A) and staurosporine (data not shown). We found complete inhibition of MeJA-induced expression at 1 μm K-252a for both the Str and the Tdc genes (Fig. 4A). The inhibitory effect of the protein kinase inhibitors K-252a and staurosporine on PE- and MeJA-induced Tdc and Str gene expression shows that downstream of MeJA one or more protein kinases mediate the signaling that leads to TIA biosynthetic gene expression.
Figure 4.
Protein kinases act upstream and downstream of JA. A, Effect of inhibitors on TIA biosynthetic gene expression. C. roseus cell suspensions were incubated with DSMO as a control (C), K-252a, calyculin A (CalA), or OA for 10 min before the addition of either PE or 50 μm MeJA, and cells were harvested 6 h later. The micromolar concentrations of the inhibitors are indicated at the top. B, Inhibition of PE-induced JA accumulation by a protein kinase inhibitor. C. roseus cells were incubated with 1 μm K-252a for 10 min before the addition of PE, and cells were harvested 3 h later. Bars represent the se (n = 3).
To investigate whether a protein kinase is also active upstream of the octadecanoid pathway, we measured the effect of PE on JA levels in the presence of K-252a. In cells that were treated with 1 μm K-252a, the amount of JA that accumulated 3 h after the addition of PE was reduced to 15%, relative to the elicitor-induced level (Fig. 4B). In the same sample the amounts of Tdc and Str mRNAs were also reduced compared with the PE-induced sample, whereas the level of Rps9 mRNA was not affected. These results indicate that upstream of the octadecanoid pathway one or more protein kinases mediate the signaling triggered by PE, leading to jasmonate biosynthesis. The fact that K-252a and staurosporine both inhibited a broad range of protein kinases precludes any speculation about their identity.
We tested the effect of the protein phosphatase inhibitors calyculin A and OA on the expression of TIA biosynthetic genes. Addition of the protein phosphatase inhibitor calyculin A at 500 nm induced the accumulation of Tdc and Str mRNA in the absence of elicitor. The protein phosphatase inhibitor OA did not induce TIA biosynthetic gene expression in the absence of elicitor at the concentrations tested (Fig. 4A). We also tested the effect of the protein phosphatase inhibitors in the presence of MeJA. Treatment with either calyculin A or OA at 500 nm had no effect on the amounts of Tdc and Str transcripts induced by MeJA. Calyculin A and OA had no effect on the level of Rps9 mRNA. The induction of gene expression by calyculin A indicates that a protein phosphatase may be involved in the attenuation of TIA biosynthetic gene expression. The results show that protein kinase(s) mediated the elicitor-induced signal both upstream and downstream of the octadecanoid pathway and that protein phosphatase(s) was involved in attenuating the signal.
The Str Promoter Responds to Elicitor and MeJA
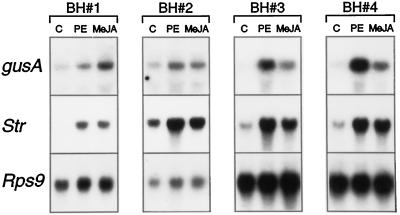
To test whether elicitor- and MeJA-induced mRNA accumulation is due to transcriptional activation, an Str promoter fragment fused to the gusA reporter gene was introduced in suspension-cultured C. roseus cells through particle bombardment. Each transgenic cell line was a mixed population, estimated to consist of thousands of independent transformants. The transgene expression level therefore reflected an average and can be considered to be independent of the chromosomal position or copy number. Four independent mixed cell populations were generated. Figure 5 shows the expression of the gusA gene driven by 396 bp of the Str promoter upstream of the translational start codon (fragment BH). In transgenic cells incubated with PE or MeJA, gusA mRNA accumulated as compared with the noninduced control (Fig. 5). The responses of the transgene and the endogenous Str gene were qualitatively similar in each independent cell line. It can be concluded that elicitor and MeJA induce transcription directed by the BH fragment of the Str promoter.
Figure 5.
The Str promoter responds to elicitor and MeJA mRNA levels of gusA, Str and Rps9 in independent transgenic cell lines containing the BH construct (BH#1, BH#2, BH#3, and BH#4). Cells were incubated for 6 h with DMSO as a control (C) or with PE or MeJA. RNA (20 μg) was loaded per lane.
DISCUSSION
For wound-inducible proteinase inhibitor synthesis it has been well established that the octadecanoid pathway is involved in signal transduction, leading to transcriptional activation of proteinase inhibitor genes. The involvement of the octadecanoid pathway in elicitor-inducible expression of genes active in secondary metabolism, however, has not been as well characterized. In this study we established the involvement of the octadecanoid pathway in elicitor-induced transcriptional activation of genes of the TIA biosynthetic pathway in C. roseus cells.
Both Str and Tdc were up-regulated in response to low levels of exogenous MeJA and higher levels of the jasmonate precursor α-linolenic acid. These results are in agreement with the observation that C. roseus seedlings (Aerts et al., 1994) and suspension-cultured cells (Gantet et al., 1998) can accumulate TIAs in response to MeJA and imply that octadecanoids stimulate TIA metabolism via induction of the biosynthetic genes. Furthermore, we observed a transient stimulation of endogenous jasmonate accumulation by the PE, which corresponded with the induction of the TIA biosynthetic genes Tdc and Str. A similar transient increase in endogenous JA was reported for the Rauvolfia, Agrostis, Eschscholtzia, Phaseolus, and Taxus species, as well as in rice and tobacco cell suspensions in response to elicitors (Gundlach et al., 1992; Mueller et al., 1993; Nojiri et al., 1996; Rickauer et al., 1997). However, it is not just elicitors that can trigger a transient increase in endogenous JA in plants; wounding (Peña-Cortés et al., 1995; O'Donnell et al., 1996) and UV radiation (Conconi et al., 1996) can also cause this increase. Together with our results, these data suggest a general role for the octadecanoid pathway in induced defense responses. Elevation of the level of JA in the plant cell seems to be sufficient to induce defense responses. Alternatively, elevated jasmonate levels could serve to lower the threshold for induction of a specific defense response by a pathogenic signal (Wasternack and Parthier, 1997). According to the latter scenario, a defined signal leads to a distinct response output due to a specific signal transduction cascade in combination with general, obligatory signaling. In both cases, inhibition of the octadecanoid pathway would leave plant cells unresponsive to the stress signal, thus inhibiting the induction of defense responses.
Inhibitors of jasmonate biosynthesis are useful for determining a direct involvement of the octadecanoid pathway in response to external stress. The induction of proteinase inhibitor genes in tomato by wounding and systemin can be blocked by DIECA (Farmer et al., 1994) and by acetyl-salicylic acid (Doares et al., 1995a). Nojiri et al. (1996) showed ibuprofen to be a potent inhibitor of elicitor-induced phytoalexin production in rice suspension cultures. In the current study in which suspension-cultured C. roseus cells were used, DIECA inhibited elicitor-induced TIA biosynthetic gene expression. This octadecanoid pathway inhibitor acts on the octadecanoid intermediate 13-S-hydroperoxylinolenic acid, and inhibited only elicitor-induced Tdc and Str gene expression; it did not affect the levels of Rsp9 mRNA (which encodes the 40S ribosomal protein S9). The results obtained using JA biosynthesis inhibitors should be interpreted with caution because these inhibitors may not be very specific. DIECA, for example, is also used as a free radical scavenger (Jabs et al., 1997). When we used other known inhibitors of the octadecanoid pathway, we found that they were not specific in our system. The lipoxygenase inhibitors ibuprofen and acetyl-salicylic acid inhibited elicitor- and MeJA-induced Tdc and Str mRNA accumulation but also had a strong negative effect on the level of Rps9 transcript. This indicates that these substances may be phytotoxic or that they may down-regulate transcription in general. These observations point out that it is important to evaluate the effect of these inhibitors on so-called housekeeping genes such as Rsp9.
Relatively little is known about the activation of jasmonate biosynthesis in response to stress. There is some evidence for protein kinases acting upstream of the octadecanoid pathway in elicitor- and wounding-induced responses (Blechert et al., 1995; Seo et al., 1995). The inhibition of PE-induced accumulation of endogenous JA in C. roseus cells by the protein kinase inhibitor K-252a suggests the need for protein phosphorylation in elicitor-induced signaling leading to stimulation of JA biosynthesis. Because K-252a inhibits a broad range of protein kinases, no conclusion can be made about the nature of the kinase(s) involved. However, Seo et al. (1995) suggested that a mitogen-activated protein kinase in tobacco may be a mediator of wound signal transduction upstream of the octadecanoid pathway. More recently, Stratmann and Ryan, (1997) showed that wounding and systemin in tomato plants can activate a mitogen-activated protein kinase-related kinase upstream of the octadecanoid pathway. Based on these results one might speculate that the kinase(s) acting upstream of the octadecanoid pathway in C. roseus is a member of the mitogen-activated protein kinase family. Downstream of the octadecanoid pathway one or more protein kinases are involved in transducing the JA signal. A protein kinase (cascade) ultimately changes the activity of transcription factors that regulate the expression of genes through the recognition of specific sequences in the promoter regions.
A number of cis-acting elements in the promoters of defense-related genes in diverse plant species that mediate responses to elicitors (Raventós et al., 1995; Rushton et al., 1996) or to MeJA (Kim et al., 1992; Xiang et al., 1996; Rouster et al., 1997) have been identified. Using transgenic C. roseus suspensions harboring Str promoter/gusA fusions we have demonstrated that elicitor- and MeJA-induced activation of Str gene expression occurs at the transcriptional level. In the Str promoter a region of 396 bp directly upstream of the translational start codon (BH) was sufficient to confer elicitor- and MeJA-responsive expression. This region of the Str promoter contains a G-box, an element that Kim et al. (1992) implicated in MeJA-responsive proteinase inhibitor II gene expression in potato. Detailed analysis of the Str promoter using 5′ and internal deletion mutants is required to reveal whether the G-box is an important element in the Str promoter.
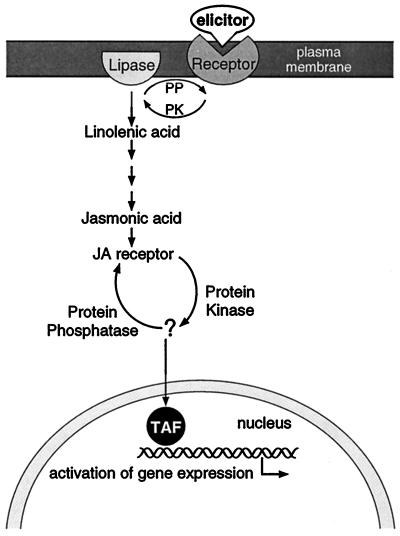
Figure 6 shows a hypothetical model that is consistent with the results described in this article. Elicitation of TIA biosynthetic gene expression is mediated through protein phosphorylation and the octadecanoid pathway leading to jasmonate. We have shown here that in C. roseus cells upstream of the octadecanoid pathway and downstream of jasmonate, one or more protein kinases are involved in mediating the signal toward Tdc and Str gene expression.
Figure 6.
Model for elicitor signal transduction leading to TIA biosynthetic gene expression. The model shows the involvement of protein phosphorylation and the octadecanoid pathway in elicitor-induced TIA biosynthetic gene expression. Figure was adapted from Farmer and Ryan (1992). PK, Protein kinase; PP, protein phosphatase; TAF, transcription-activating factor.
ACKNOWLEDGMENTS
The authors acknowledge Marga Harteveld for excellent assistance with purification of the PE and Leslie van der Fits for help with the transformation of C. roseus suspension-cultured cells.
Abbreviations:
- BH
BglII/HincII
- DIECA
diethyldithiocarbamic acid
- JA
jasmonic acid
- MeJA
methyl jasmonate
- OA
okadaic acid
- PE
partially purified YE elicitor
- TIA
terpenoid indole alkaloid
- YE
yeast extract
LITERATURE CITED
- Aerts RJ, Gisi D, De Carolis E, De Luca V, Bauman TW. Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J. 1994;5:635–643. [Google Scholar]
- Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Mueller MJ, Xia Z-Q, Zenk MH. The octadecanoid pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Chandra S, Low PS. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA. 1995;92:4120–4123. doi: 10.1073/pnas.92.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA. The octadecanoid signaling pathway in plants mediates a response to ultraviolet radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Darvill AG, Albersheim P. Phytoalexins and their elicitors: a defense against microbial infection in plants. Annu Rev Plant Physiol. 1984;35:243–275. [Google Scholar]
- De Luca V, Marineau C, Brisson N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci USA. 1989;86:2582–2586. doi: 10.1073/pnas.86.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Narváez-Vásques J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995a;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995b;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard-Ivey M, Douglas CJ. Role of jasmonates in the elicitor- and wound-inducible expression of defense genes in parsley and transgenic tobacco. Plant Physiol. 1996;112:183–192. doi: 10.1104/pp.112.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Caldelari D, Pearce G, Walker-Simmons MK, Ryan CA. Diethyldithiocarbamic acid inhibits the octadecanoid signaling pathway for the wound induction of proteinase inhibitors in tomato leaves. Plant Physiol. 1994;106:337–342. [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantet P, Imbault N, Thiersault M, Doireau P. Necessity of a functional octadecanoid pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol. 1998;39:220–225. [Google Scholar]
- Goddijn OJM, Lohman FP, De Kam RJ, Schilperoort RA, Hoge JHC (1994) Nucleotide sequence of the tryptophan decarboxylase gene of Catharanthus roseus and expression of tdc-gusA gene fusions in Nicotiana tabacum. Mol Gen Genet 242: 217–225 [DOI] [PubMed]
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-R, Choi J-L, Costa MA, An G. Identification of G-box sequence as an essential element for methyl-jasmonate response of potato proteinase inhibitor II promoter. Plant Physiol. 1992;99:627–631. doi: 10.1104/pp.99.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan TM. Alkaloid biosynthesis—the basis for metabolic engineering of medicinal plants. Plant Cell. 1995;7:1059–1070. doi: 10.1105/tpc.7.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AH, Verpoorte R, Hoge JHC. Regulation of enzymes and genes involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Plant Res. 1993;3:145–164. [Google Scholar]
- Menke FLH, Kijne JW, Memelink J (1996) Digging for gene expression levels in Catharanthus roseus: nonradioactive detection of plant mRNA levels. In M Leous, K Matter, C Schröder, B Ziebolz, eds, Biochemica 2. Boehringer-Mannheim, Mannheim, Germany, pp 16–18
- Mueller MJ. Enzymes involved in jasmonic acid biosynthesis. Physiol Plant. 1997;100:653–663. [Google Scholar]
- Mueller MJ, Brodschelm W. Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry. Anal Biochem. 1994;218:425–435. doi: 10.1006/abio.1994.1202. [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Brodschelm W, Spannagl E, Zenk MH. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA. 1993;90:7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojiri H, Sugimori M, Yamane H, Nishimura Y, Yamada A, Shibuya N, Kodama O, Murofushi N, Omori T. Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol. 1996;110:387–392. doi: 10.1104/pp.110.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Pasquali G, Goddijn OJM, de Waal A, Verpoorte R, Schilperoort RA, Hoge JHC, Memelink J. Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol. 1992;18:1121–1131. doi: 10.1007/BF00047715. [DOI] [PubMed] [Google Scholar]
- Pasquali G, Ouwerkerk PBF, Memelink J. Versatile transformation vectors to assay the promoter activity of DNA elements in plants. Gene. 1994;149:373–374. doi: 10.1016/0378-1119(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Fisahn J, Willmitzer L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc Natl Acad Sci USA. 1995;92:4106–4113. doi: 10.1073/pnas.92.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raventós D, Jensen AB, Rask M-B, Casacuberta JM, Mundy J, San Segundo B. A 20 bp cis-acting element is both necessary and sufficient to mediate elicitor response of a maize PRms gene. Plant J. 1995;7:147–155. doi: 10.1046/j.1365-313x.1995.07010147.x. [DOI] [PubMed] [Google Scholar]
- Rickauer M, Brodschelm W, Bottin A, Véronési C, Grimal H, Esquerré-Tugayé MT. The jasmonate pathway is involved differentially in the regulation of different defence responses in tobacco cells. Planta. 1997;202:155–162. [Google Scholar]
- Rouster J, Leah R, Mundy J, Cameron-Mills V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997;11:513–523. doi: 10.1046/j.1365-313x.1997.11030513.x. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Tovar Torres J, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoter of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1991. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Huang J-F, Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991;96:130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Ryan CA. Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA. 1997;94:11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Fukuda Y, Shinshi H. Studies on elicitor-signal transduction leading to differential expression of defense genes in cultured tobacco cells. Plant Cell Physiol. 1995;36:281–289. [Google Scholar]
- van der Fits L, Memelink J. Comparison of the activities of CaMV 35S and FMV 34S promoter derivatives in Catharanthus roseus cells transiently and stably transformed by particle bombardment. Plant Mol Biol. 1997;33:943–946. doi: 10.1023/a:1005763605355. [DOI] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC. Biosynthesis of jasmonic acid by several plant species. Plant Physiol. 1984;75:458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signalled plant gene expression. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- Xiang C, Miao Z-H, Lam E. Coordinated activation of as-1 type elements and tobacco glutathion S-transferase gene by auxin, salicylic acid, methyl-jasmonate, and hydrogen peroxide. Plant Mol Biol. 1996;32:415–426. doi: 10.1007/BF00019093. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shah J, Klessig DF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]