Abstract
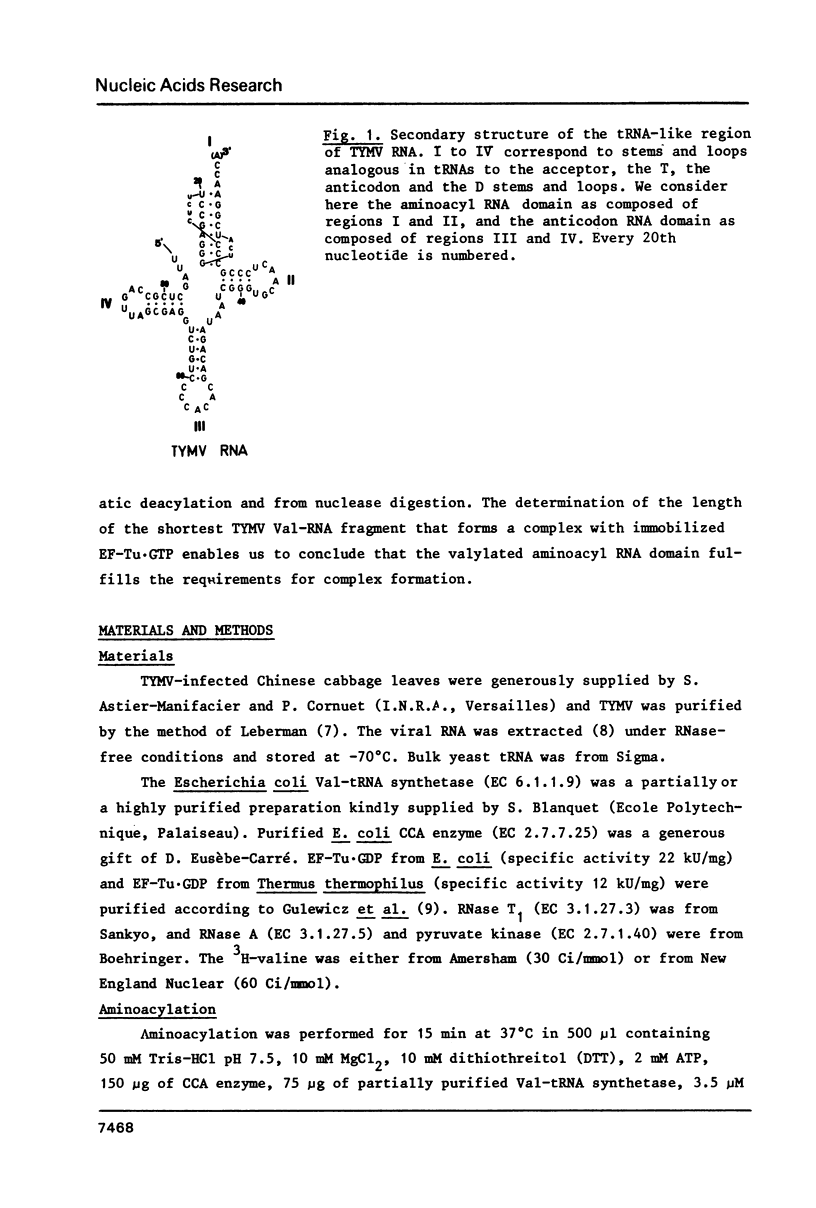
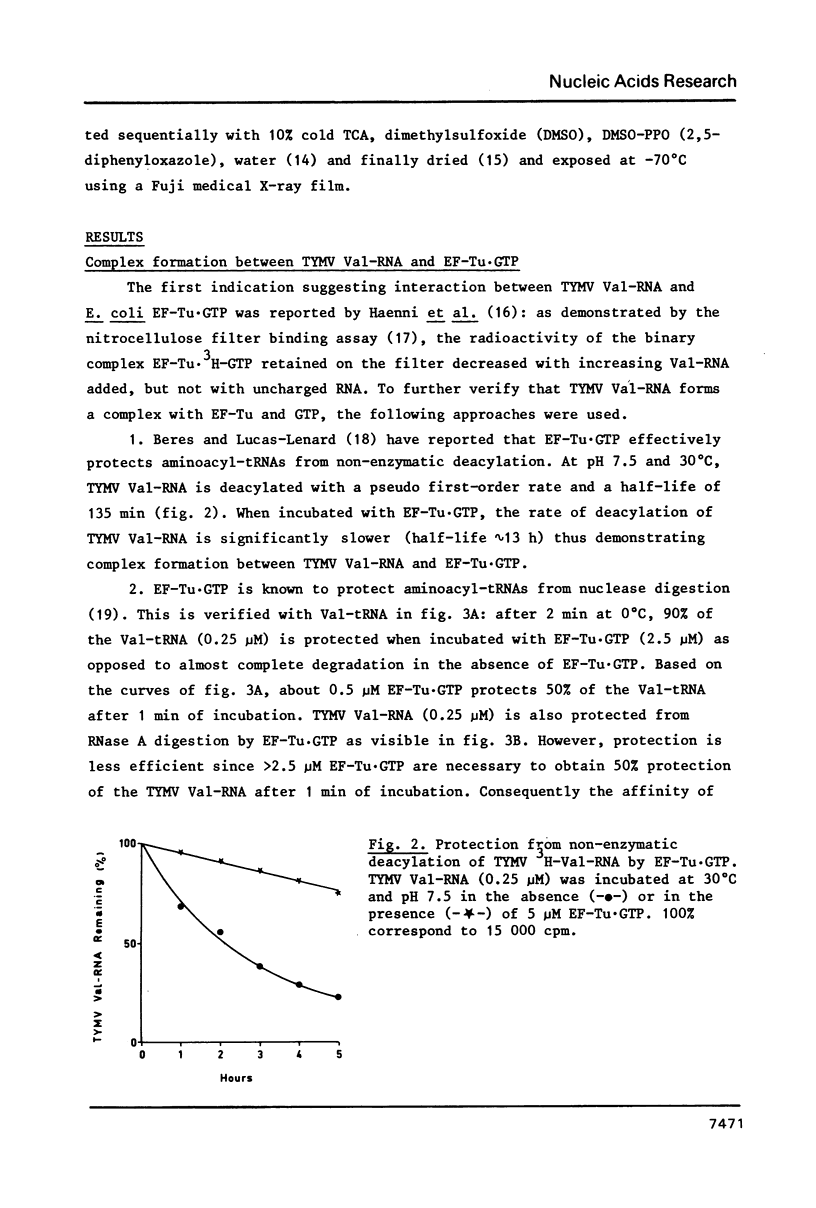
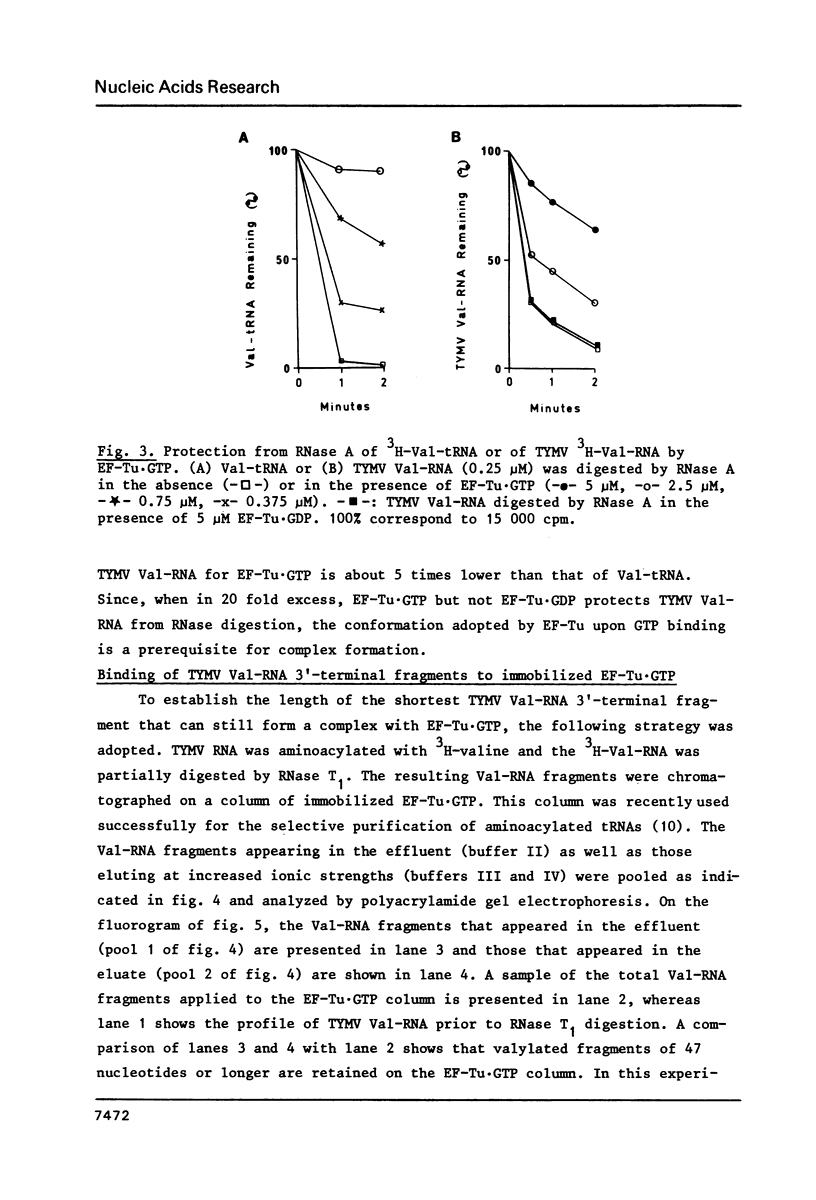
Turnip yellow mosaic virus (TYMV) Val-RNA forms a complex with the peptide elongation factor Tu (EF-Tu) in the presence of GTP: the Val-RNA is protected by EF-Tu·GTP from non-enzymatic deacylation and nuclease digestion. The determination of the length of the shortest TYMV Val-RNA fragment that binds EF-Tu·GTP leads us to conclude that the valylated aminoacyl RNA domain equivalent in tRNAs to the continuous helix formed by the acceptor stem and the T arm is sufficient for complex formation. Since the aminoacyl RNA domain is also sufficient for adenylation by the ATP(CTP):tRNA nucleotidyltransferase, an analogy can be drawn between these two tRNA-specific proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Derwenskus K. H., Fischer W., Sprinzl M. Isolation of tRNA isoacceptors by affinity chromatography on immobilized bacterial elongation factor Tu. Anal Biochem. 1984 Jan;136(1):161–167. doi: 10.1016/0003-2697(84)90318-x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentz C., Briand J. P., Romby P., Hirth L., Ebel J. P., Glegé R. The tRNA-like structure of turnip yellow mosaic virus RNA: structural organization of the last 159 nucleotides from the 3' OH terminus. EMBO J. 1982;1(2):269–276. doi: 10.1002/j.1460-2075.1982.tb01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewicz K., Faulhammer H. G., Sprinzl M. Properties of native and nicked elongation factor Tu from Thermus thermophilus HB 8. Eur J Biochem. 1981 Dec;121(1):155–162. doi: 10.1111/j.1432-1033.1981.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Joshi S., Chapeville F. tRNA-like structures in the genomes of RNA viruses. Prog Nucleic Acid Res Mol Biol. 1982;27:85–104. doi: 10.1016/s0079-6603(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Jekowsky E., Schimmel P. R., Miller D. L. Isolation, characterization and structural implications of a nuclease-digested complex of aminoacyl transfer RNA and Escherichia coli elongation factor Tu. J Mol Biol. 1977 Aug 15;114(3):451–458. doi: 10.1016/0022-2836(77)90262-5. [DOI] [PubMed] [Google Scholar]
- Jerez C., Sandoval A., Allende J., Henes C., Ofengand J. Specificity of the interaction of aminoacyl ribonucleic acid with a protein-guanosine triphosphate complex from wheat embryo. Biochemistry. 1969 Jul;8(7):3006–3014. doi: 10.1021/bi00835a049. [DOI] [PubMed] [Google Scholar]
- Jonák J., Rychlík I., Smrt J., Holý A. The binding site for the 3'-terminus of aminoacyl-tRNA in the molecule of elongation factor Tu from Escherichia coli. FEBS Lett. 1979 Feb 15;98(2):329–332. doi: 10.1016/0014-5793(79)80210-0. [DOI] [PubMed] [Google Scholar]
- Jonák J., Smrt J., Holý A., Rychlík I. Interaction of Escherichia coli EF-Tu.GTP and EF-Tu.GDP with analogues of the 3' terminus of aminoacyl-tRNA. Eur J Biochem. 1980 Apr;105(2):315–320. doi: 10.1111/j.1432-1033.1980.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Joshi S., Chapeville F., Haenni A. L. tRNA-like structures of plant viral RNAs: conformational requirements for adenylation and aminoacylation. EMBO J. 1983;2(7):1123–1127. doi: 10.1002/j.1460-2075.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
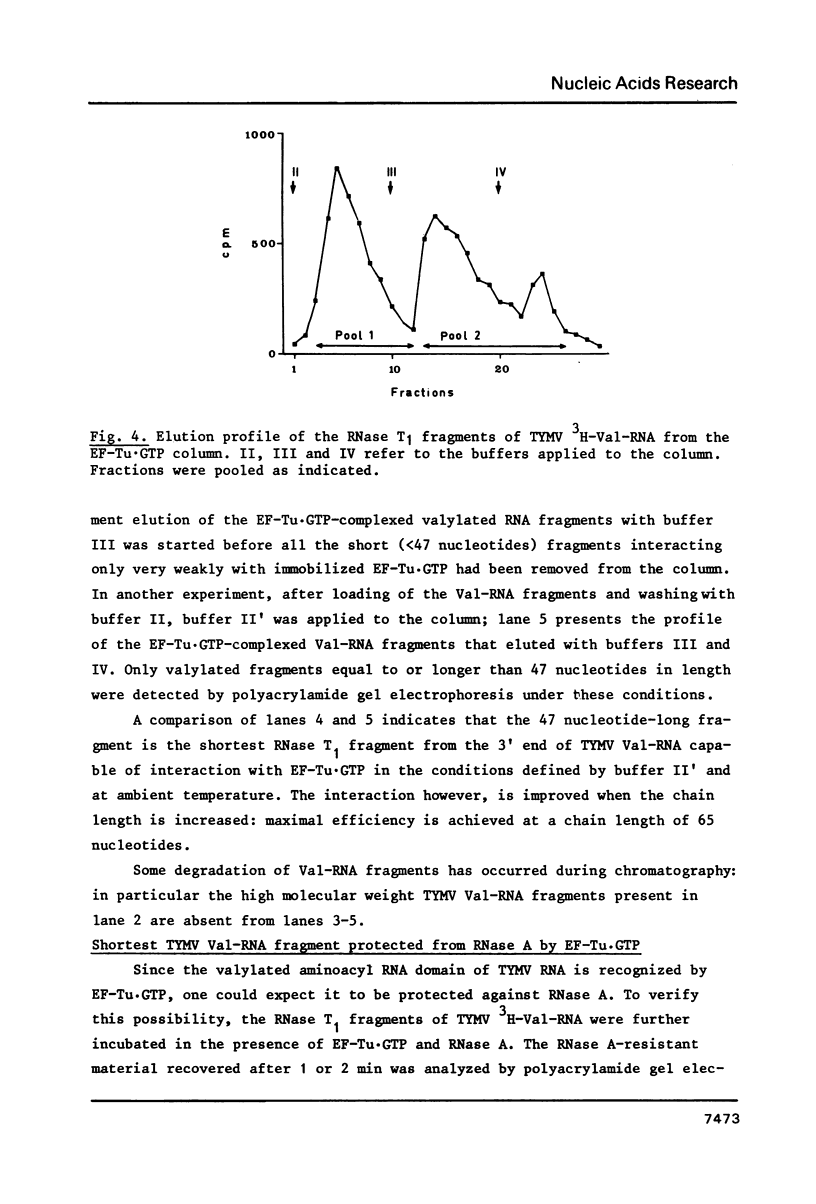
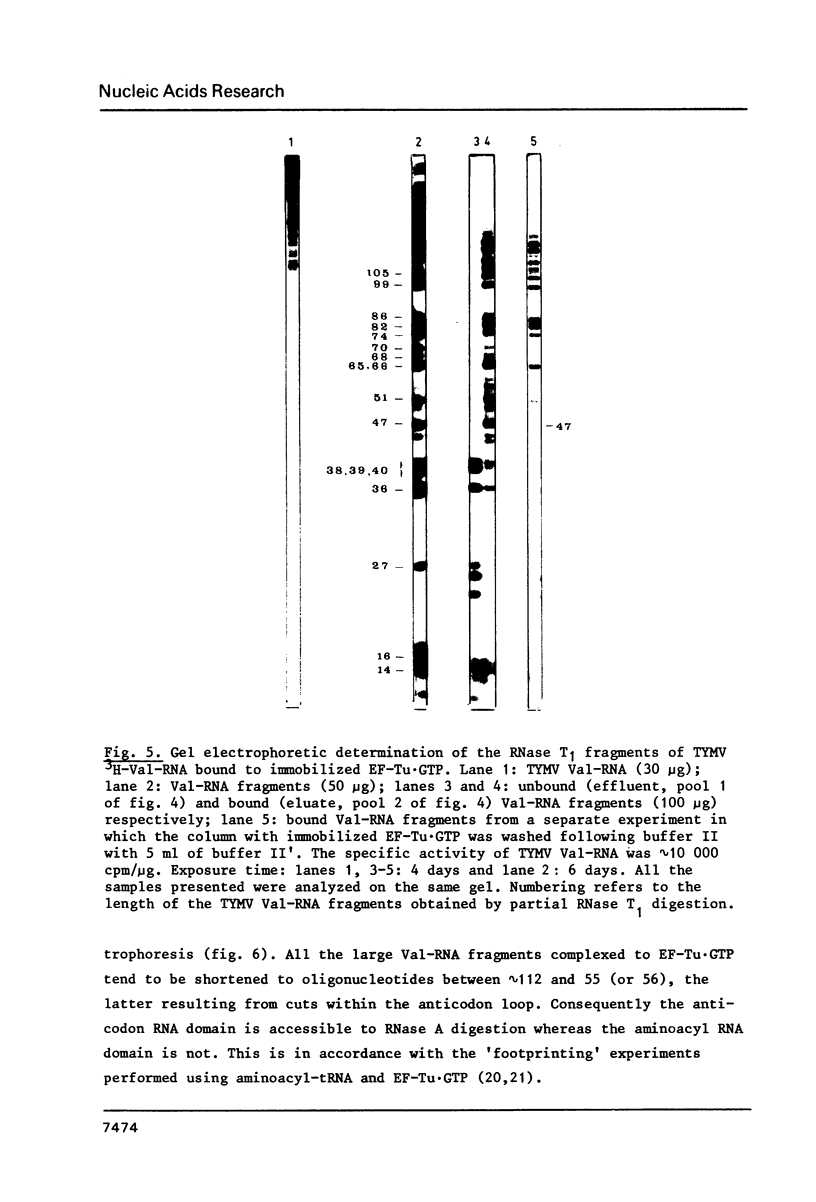
- Joshi S., Chapeville F., Haenni A. L. Length requirements for tRNA-specific enzymes and cleavage specificity at the 3' end of turnip yellow mosaic virus RNA. Nucleic Acids Res. 1982 Mar 25;10(6):1947–1962. doi: 10.1093/nar/10.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Haenni A. L. Fluorographic detection of nucleic acids labelled with weak beta-emitters in gels containing high acrylamide concentrations. FEBS Lett. 1980 Aug 25;118(1):43–46. doi: 10.1016/0014-5793(80)81214-2. [DOI] [PubMed] [Google Scholar]
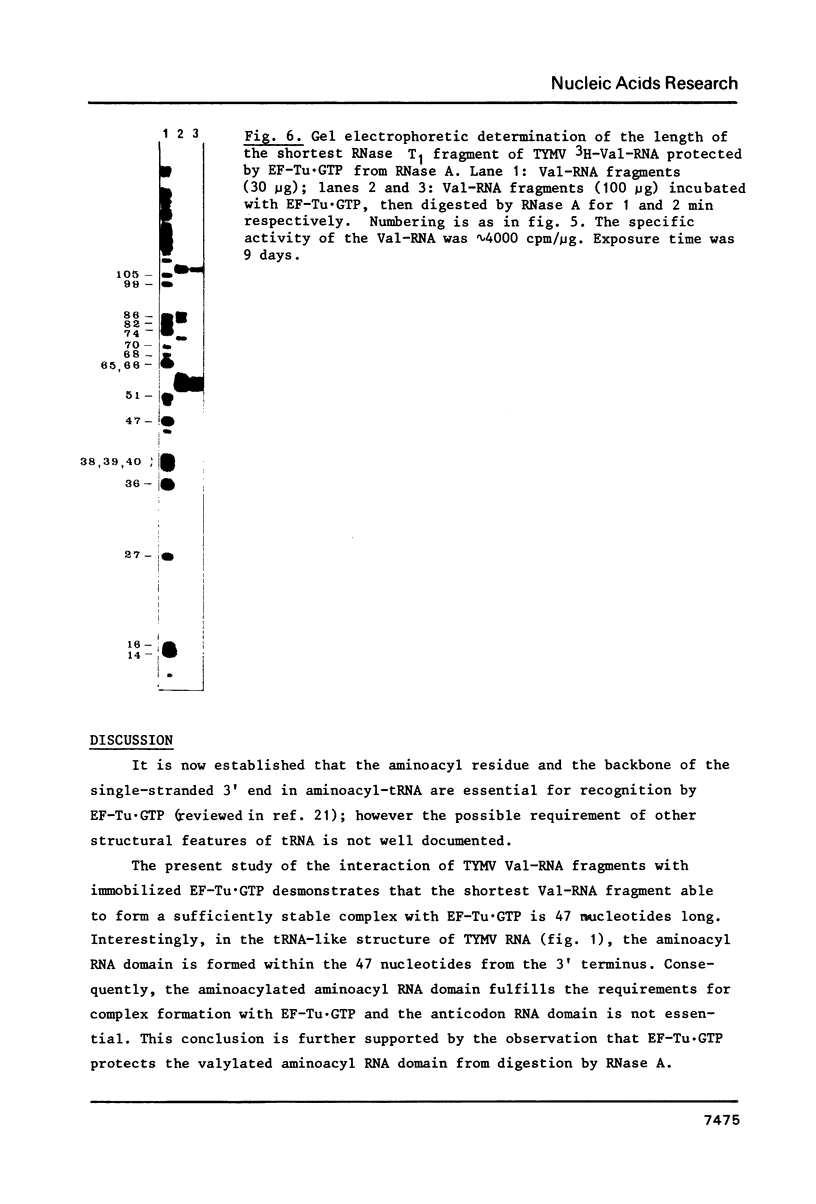
- Joshi S., Haenni A. L., Hubert E., Huez G., Marbaix G. In vivo aminoacylation and 'processing' of turnip yellow mosaic virus RNA in Xenopus laevis oocytes. Nature. 1978 Sep 28;275(5678):339–341. doi: 10.1038/275339a0. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Tanada S., Takemura S. Properties of alanyl-oligonucleotide, puromycin, and Staphylococcus epidermidis glycyl-tRNA in interaction with elongation factor Tu:GTP complex. FEBS Lett. 1975 Mar 1;51(1):321–324. doi: 10.1016/0014-5793(75)80917-3. [DOI] [PubMed] [Google Scholar]
- Krauskopf M., Chen C. M., Ofengand J. Interaction of fragmented and cross-linked Escherichia coli valine transfer ribonucleic acid with T u factor-guanosine triphosphate complex. J Biol Chem. 1972 Feb 10;247(3):842–850. [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Deutscher M. P. Separation of functionally distinct regions of a macromolecular substrate. Stimulation of tRNA nucleotidyltransferase by a nonreacting fragment of tRNA. J Biol Chem. 1979 Apr 25;254(8):2585–2587. [PubMed] [Google Scholar]
- Ofengand J. Assay for AA-tRNA recognition by the EFTu-GTP complex of Escherichia coli. Methods Enzymol. 1974;29:661–667. doi: 10.1016/0076-6879(74)29057-8. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone D., Parmeggiani A. Transfer ribonucleic acid deprived of the C-C-A 3'-extremity can interact with elongation factor Tu. Biochemistry. 1983 Sep 13;22(19):4400–4405. doi: 10.1021/bi00288a009. [DOI] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Rietveld K., Van Poelgeest R., Pleij C. W., Van Boom J. H., Bosch L. The tRNA-like structure at the 3' terminus of turnip yellow mosaic virus RNA. Differences and similarities with canonical tRNA. Nucleic Acids Res. 1982 Mar 25;10(6):1929–1946. doi: 10.1093/nar/10.6.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]