Summary
Storage and release of classical and amino acid neurotransmitters requires vesicular transporters. Some neurons lack known vesicular transporters, suggesting additional neurotransmitter systems remain unidentified. Insect mushroom bodies (MBs) are critical for several behaviors, including learning, but the neurotransmitters released by the intrinsic Kenyon cells (KCs) remain unknown. Likewise, KCs do not express a known vesicular transporter. We report the identification of a novel Drosophila gene portabella (prt) that is structurally similar to known vesicular transporters. Both larval and adult brains express PRT in the KCs of the MBs. Additional PRT cells project to the central complex and optic ganglia. prt mutation causes an olfactory learning deficit and an unusual defect in the male’s position during copulation that is rescued by expression in KCs. Since prt is expressed in neurons that lack other known vesicular transporters or neurotransmitters, it may define a previously unknown neurotransmitter system responsible for sexual behavior and a component of olfactory learning.
Keywords: learning and memory, synaptic transmission, neurotransmitter, sexual behavior, monoamines
Introduction
Synaptic transmission with classical neurotransmitters depends on two classes of structurally and pharmacologically distinct neurotransmitter transporters. These include 1: vesicular transporters that localize to synaptic vesicles, actively driving transmitter into the vesicular lumen and 2: plasma membrane transporters that terminate neurotransmission by the uptake of neurotransmitter into either the presynaptic site of release or adjacent cells. The number of known vesicular transporters is surprisingly small, and includes four distinct families for the transport of 1: monoamines such as dopamine and serotonin (VMAT1 and 2), 2: GABA and glycine (VGAT or VIAAT), 3: acetylcholine (VAChT), and 4: glutamate (VGLUT1–3) (Chaudhry et al., 2008). Additional transporters for purine nucleotides (Sawada et al., 2008) and aspartate (Miyaji et al., 2008) have recently been identified as part of the SLC17 family, related to VGLUTs. Any other novel neurotransmitters used by invertebrates and/or mammals would similarly require a distinct vesicular transporter for storage and exocytotic release.
In Drosophila and other insects, the mushroom bodies (MBs) play a critical role in olfactory learning, as well as integrating information from other sensory modalities (Davis, 2011; Keene and Waddell, 2007; Strausfeld et al., 1998; Wessnitzer and Webb, 2006). Kenyon cells (KCs) form all of the intrinsic fiber tracts of the MBs whereas several extrinsic neurons project into the MBs, providing input and output of information and/or regulating KC function (Tanaka et al., 2008). To date, neither the neurotransmitter released from the intrinsic neurons or the vesicular transporter responsible for its storage have been identified. Of the known neurotransmitter systems, the vesicular transporters for amines (DVMAT), GABA (DVGAT), and glutamate (DVGLUT) are absent from KCs (Chang et al., 2006; Daniels et al., 2008; Fei et al., 2010). Although expression data for the vesicular acetylcholine transporter is not available, the biosynthetic enzyme responsible for Ach synthesis is also absent from KCs (Gorczyca and Hall, 1987; Yasuyama et al., 2002). Several classical and peptide neurotransmitters have been identified in processes that project into the MBs (Davis, 2011). In contrast, although multiple candidates have been suggested (Schafer et al., 1988; Schurmann, 2000; Sinakevitch et al., 2001), the neurotransmitter(s) released from the KCs is not known, and could possibly constitute a previously undescribed neurotransmitter system.
The MBs have been implicated in other behaviors, including sleep (Joiner et al., 2006), aggression (Baier et al., 2002), and motor activity (Serway et al., 2009). Furthermore, both the MBs and central complex (CCX) have been linked to aspects of sexual behavior (O’Dell et al., 1995; Popov et al., 2003; Sakai and Kitamoto, 2006). Sexual behavior has been studied extensively in the fly with a particular focus on courtship, although a handful of mutations affecting copulation have been described (Yamamoto et al., 1997). The neurocircuitry underlying all of these behaviors remains poorly understood.
We report here the molecular cloning of a novel, putative vesicular transporter (CG10251) that localizes to the MBs and processes that innervate the CCX. Mutation of CG10251 inhibits learning and causes a dramatic sexual phenotype in which the male fly is unable to correctly position himself during copulation. The copulation deficit was rescued by expression of CG10251 in the MBs, suggesting a previously unknown function for this structure. We speculate that the CG10251 protein may be responsible for the storage of a previously unknown type of neurotransmitter in a subset of KCs and several other neurons in the insect nervous system. We have named the CG10251 gene portabella (prt).
Results
Identification of a novel gene similar to both vesicular monoamine and acetylcholine transporters
The D. melanogaster genome contains orthologs of all known vesicular neurotransmitter transporters, including genes similar to VGLUT, VMAT, VAChT and VGAT (Daniels et al., 2004; Fei et al., 2010; Greer et al., 2005; Kitamoto et al., 1998). We searched the genomic database for genes similar to Drosophila VMAT (DVMAT) to identify additional, potentially novel vesicular transporters. We identified a gene similar to both DVMAT and DVAChT that localizes to cytogenetic region 95A on chromosomal arm 3R. DVMAT and DVAChT localize to cytogenetic regions 50B (2R) and 91C (3R), respectively. We found that CG10251 shows 35.8% similarity to DVMAT and 30.2% similarity to DVAChT (Fig S1). In comparison, DVMAT and DVAChT share 35.5% similarity. The long open reading frame of CG10251 contains 12 predicted transmembrane domains similar to both mammalian and Drosophila VMAT and VAChT.
RNA and protein expression
To confirm that CG10251 RNA is expressed in vivo, we probed Northern blots of adult fly heads and bodies (Fig 1A). We detected a major band migrating at just above the 2 kb marker and a minor species at 5 kb. We also detected the ~2 kb species in bodies, but at low levels relative to heads. We observe similar enrichment in heads for DVMAT and other neurotransmitter transporters (Greer et al., 2005; Romero-Calderon et al., 2007). The size of the major CG10251 mRNA species was similar to the cDNA we obtained with RT-PCR (2.2 kB), suggesting that we identified the full extent of the major CG10251 transcript. Repeated trials of 5′ and 3′ RACE did not reveal additional exons (not shown); thus, the minor 5 kB species likely represents an mRNA precursor, although we cannot rule out the possibility of a low-abundance splice variant.
Fig 1.
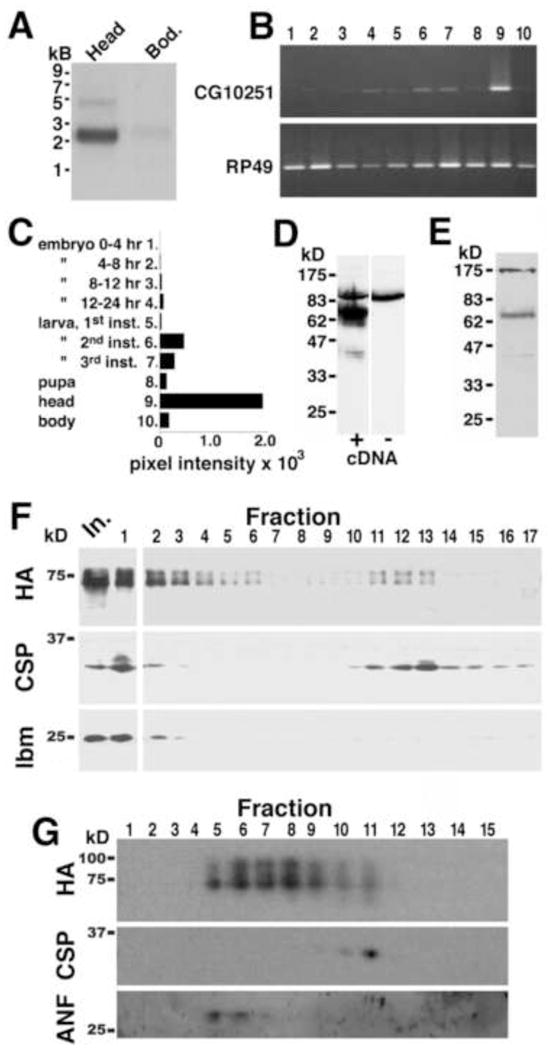
Expression of CG10251 mRNA, protein, and subcellular localization. A) Northern blots show expression of CG10251 mRNA in heads and, to a lesser extent, in bodies. B) PCR using a panel of cDNAs from various developmental stages (see labels in panel C) confirmed high expression of CG10251/prt in adult heads, relative to the body, as well as some expression in larva, and minimal expression during pupal and embryonic stages (B, top panel). Identical samples were amplified with a probe to the widely expressed gene RP49 (B, bottom panel). Normalized levels of CG10251 expression are shown graphically in C. D, E: Expression of the CG10251 protein. D) Homogenates from wild-type S2 cells (−) and cells expressing the CG10251/prt cDNA (+) were probed on Western blots using the CG10251 antiserum. We detected a band at ~70 kD in cells expressing CG10251 but not wild-type cells. E) Homogenates from wild-type adult heads showed a similarly migrating band at ~70 kD. F, G: CG10251/PRT protein localizes to both SV and dense fractions. F) Homogenates from flies expressing an HA-tagged version of PRT were fractionated on a linear glycerol velocity gradient followed by Western blotting with primary antibodies to HA (top panel), cysteine string protein (CSP, a marker for SVs, middle panel), and late bloomer (lbm, a marker for the plasma membrane, bottom panel). For all three antibodies, the input lane (In.) and cushion (fraction 1) were processed separately to prevent over-exposure, allowing better visualization of the relevant bands. G) Homogenates from flies expressing an HA-tagged version of PRT were fractionated on a linear sucrose density gradient followed by Western blotting with primary antibodies to HA (top panel), CSP (middle panel), and the fusion protein containing Atrial Natriuretic Factor (ANF) and GFP (bottom panel, a marker for LDCVs). Molecular weight markers (kD) are shown on the left side of the figure.
We performed PCR with a commercially available cDNA panel representing various developmental stages and a CG10251-specific primer set (Fig 1B, C). Our data suggest that CG10251 is primarily expressed during adulthood and late larval stages rather than during embryonic development. We have attempted in situ hybridization of embryos for CG10251 expression, but have been unable to detect a signal (not shown), possibly due to the low levels of CG10251 mRNA at this stage.
We next generated an antibody against the CG10251 carboxy terminus and probed homogenates of S2 cells transfected with CG10251 cDNA to test its activity (Fig 1D). Cells expressing CG10251 (+) showed a broad 70 kD signal whereas untransfected S2 cells (−) did not. We probed homogenates of adult heads and detected a band at 70 kD, as well as additional bands at the top of the gel that may represent non-specific cross reactivity (Fig 1E). A faint band immediately above the major band suggests that a portion of CG10251 may undergo post-translational modification. This species was more visible in biochemically fractionated samples (see below). Another faint band at 40 kD may represent a degradation product. We confirmed the specificity of the antiserum with the CG10251 mutant (see below). We thus demonstrated the in vivo expression of both CG10251 mRNA and protein.
Biochemical fractionation
The similarity of CG10251 to DVMAT and DVAChT suggests that it, too, might encode a vesicular transporter. CG10251 localized to intracellular membranes at steady-state when expressed in S2 cells, and in vitro endocytosis assays revealed that CG10251 internalized from the cell surface as we have observed for DVMAT and DVGLUT (not shown). We therefore tested whether the protein would also localize to synaptic vesicles (SVs) in vivo. Relatively low expression of endogenous CG10251 made it difficult to detect in initial biochemical fractionation experiments (not shown). To facilitate these analyses we created a fly transgene expressing an HA-tagged version of the protein and used the pan-neuronal elav-Gal4 driver. To determine whether CG10251 localizes to SVs, we applied homogenates from flies expressing CG10251 to a glycerol velocity gradient. A portion of CG10251 peaked in fractions containing the peak for SV marker cysteine string protein (CSP, fractions 11–13, Fig 1F). These data suggest that at least a fraction of the protein localizes to SVs, consistent with the prediction from sequence analysis that CG10251 is a vesicular transporter.
We also performed sucrose density fractionation to determine whether CG10251 might localize to other types of secretory vesicles, in particular large dense core vesicles (LDCVs). We found that while some CG10251 co-localized with CSP in light fractions, most of the immunoreactivity was found in heavier fractions, some coincident with a fusion protein containing mammalian Atrial Natriuretic Factor (ANF, Fig 1G), a marker for LDCVs (Rao et al., 2001). These data suggest that CG10251 likely localizes to LDCVs as well as SVs, similar to mammalian VMAT2, which preferentially localizes to LDCVs in cultured cells and in vivo (Nirenberg et al., 1995).
Localization in the larval nervous system
To localize CG10251 in vivo, we labeled whole mounts of 3rd instar larval brain and ventral ganglia. A small subset of cells in the ventral ganglia expressed CG10251 (Fig 2A, N). We did not detect significant co-localization with 5HT, TH, or Ddc (not shown). Thus, CG10251 is unlikely to store either dopamine or serotonin, in contrast to DVMAT, which localizes to these cell types (Greer et al., 2005). Other aminergic transmitters in the fly include octopamine and tyramine; however, DVMAT is likely responsible for their transport as well (Greer et al., 2005) and both localize to large midline cells (Monastirioti et al., 1995; Nagaya et al., 2002). We did not detect cells expressing CG10251 at the midline.
Fig 2.
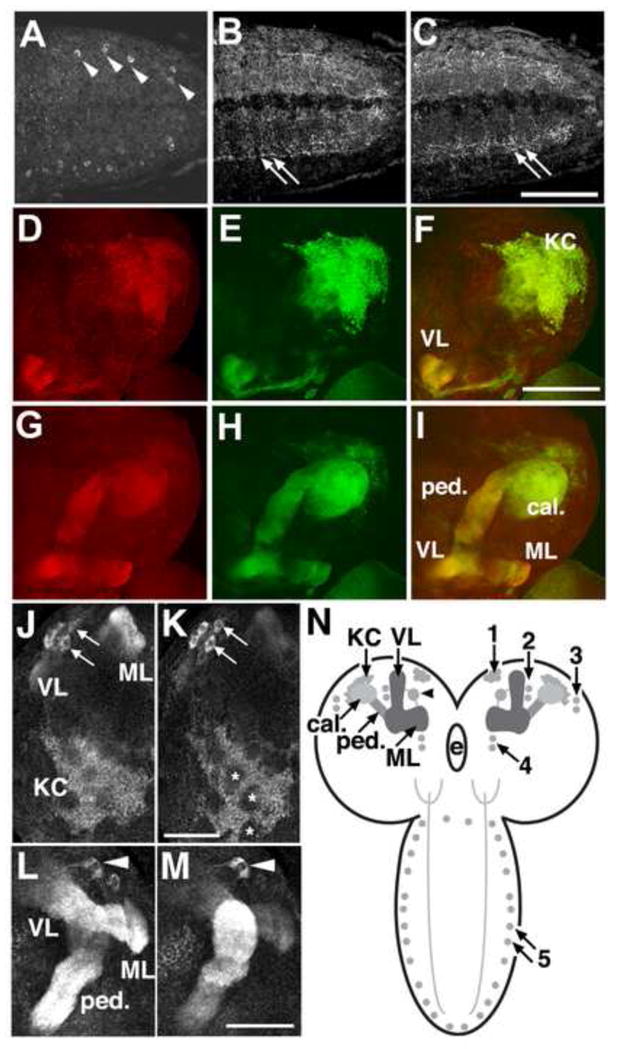
Localization of PRT in the larval ventral ganglion. A–C: One to two cell bodies per hemisegment express PRT and localize to lateral aspects of the ventral nerve cord. Processes project medially into the neuropil in a complex pattern that runs throughout the ventral ganglion. Confocal images show selected optical slices though the caudal aspect of the ventral ganglion to show the labeled cell bodies (A, arrowheads) and neuropil (B, C, arrows). Scale bar: 50 μm. D–I: PRT expression in larval mushroom bodies. Larval brains expressing the membrane bound form of GFP (mCD8-GFP) with the MB driver OK107-Gal4 were labeled for PRT followed by a Cy3 conjugated secondary antibody. Confocal images show a dorsalateral view of the brain (D–F) and more ventromedial sections (G–I). GFP expression (green), PRT labeling (red) and the merged images (F, I) are shown as horizontal sections with dorsal/posterior regions at the top of each image. We detect PRT in all aspects of the MBs, including the Kenyon cells (KC), calyx (ca), the penduncle (ped), and the vertical and medial lobes (VL and ML). Scale bar: 100 μm. J–M: Expression in larval Kenyon cells, a ventromedial cluster of cells, and a large extrinsic neuron. J, K) Two optical sections show expression of PRT in the Kenyon cells (KC) of the larval MBs. Patches of unlabeled areas in the center of the KC cluster are indicated (K, asterisks). A small portion of the medial lobes (ML) and vertical lobes (VL) are visible in J. An additional group of four to five cells (J, K, arrows) at the ventral-medial aspect of each optic lobe are also labeled. L, M) Two optical sections show a large extrinsic neuron (arrowhead) sending processes to both the medial (ML) and vertical lobes (VL). The peduncle of the MB is also indicated (ped). J–M show horizontal sections with the posterior side down. Scale bars: 50 μm. See also Fig S2. N) In the frontal perspective cartoon, the KC bodies are shown as light gray circles. Processes from these cells project ventrally and rostrally to form the peduncle (ped), then branch into medial (ML) and vertically (VL) projecting lobes in the central brain. The dendrites of the KCs form the calyx (ca). A relatively large, bilaterally symmetric extrinsic neuron expressing PRT projects into each ipsilateral MB. Additional cells expressing PRT in the brain and ventral ganglia are indicated as darker gray circles, and prominently labeled processes indicated with stippling. The numbers represent arbitrary designations of cell clusters in the larva. e=esophageal foramen.
In the larval brain, we observed robust expression of CG10251 in the MBs (Fig 2D–M). To confirm localization to cells in the MBs, we expressed mCD8-GFP with the MB driver 0K107-Gal4 (Connolly et al., 1996) (Fig 2E, H), and co-labeled larval brains for CG10251 (Fig 2D, G). We observed overlap in the medial and vertical lobes that make up the axonal projections of the KCs, the calyces or dendritic bundles, as well as the KC bodies (Fig 2D-I). These data indicate that CG10251 is expressed by at least a subset of the KCs intrinsic to the MBs and therefore may be responsible for storage of neurotransmitter in these cells. In light of this expression pattern and the proposed transport function of CG10251 we have renamed the gene portabella (prt) and refer to the CG10251 protein as PRT.
We note that subsets of KCs did not appear to be labeled (asterisks, Fig 2K) by the PRT antibody. A similar pattern has been reported for several developmental markers expressed in KCs (Noveen et al., 2000) suggesting that PRT may be expressed at a relatively late stage during differentiation and perhaps only in a subpopulation of KCs.
We observed PRT expression in at least one bilateral extrinsic neuron projecting ipsilaterally to the vertical and medial lobes of the larval MBs (arrowheads, Fig 2L, M). The location and projections from this cell appear similar to that described for a neuron expressing the amnesiac peptide, which is critical for memory formation in Drosophila (Waddell et al., 2000). However, co-labeling experiments suggest the extrinsic neurons expressing PRT are distinct from those expressing amnesiac (Fig S2). Expression of PRT in these cells and four other small clusters in the larval brain is shown schematically in Fig 2N.
Localization in the adult nervous system
The Drosophila nervous system undergoes extensive remodeling during metamorphosis, resulting in adult MBs that are morphologically distinct from the larval structures. In the adult, each vertical lobe of the MB can be recognized as distinct α and α′ lobes and the medial lobes include distinct β, β′ and γ lobes (Crittenden et al., 1998). We observed strong PRT expression in the adult MBs, including labeling of all 5 lobes (Fig 3A–C). Relative to the lobes, labeling of the calyx and KC bodies was less intense in the adult than the larva (Fig 3E). This pattern likely reflects the localization of the protein to secretory vesicles that are concentrated in the axons and less abundant in mature dendrites and cell bodies.
Fig 3.
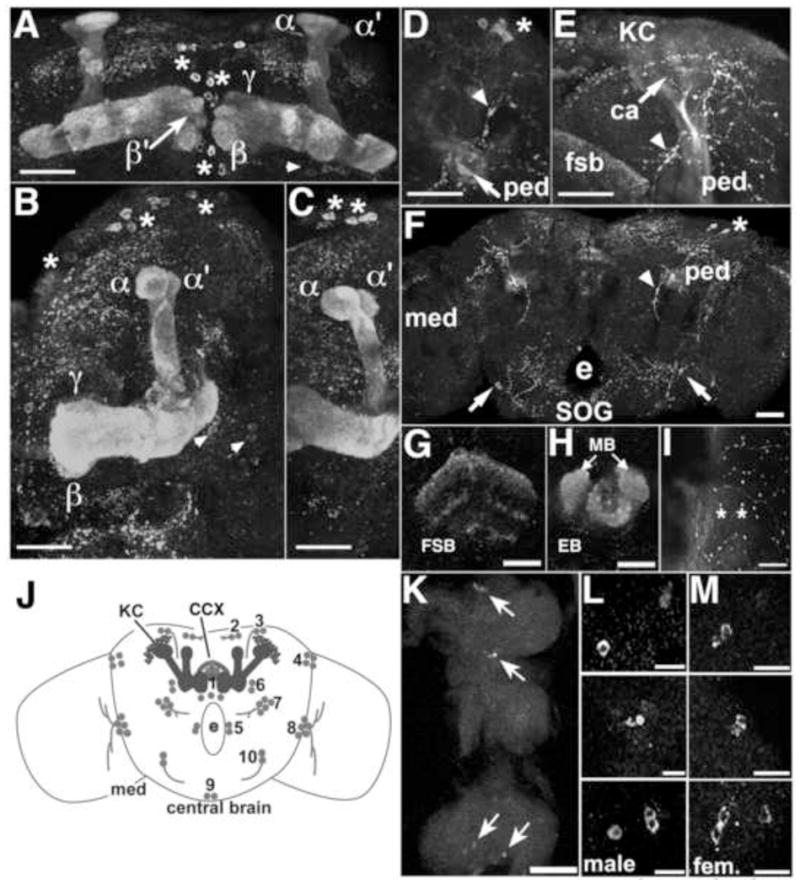
PRT expression in adult mushroom bodies, central complex and thoracic ganglion. PRT is expressed in the medial lobes β, γ (A, B), and β′ (A), the peduncle (ped, D–F) and the vertical lobes α and α′ (A–C). D–F) Optical sections through the peduncle shows expression in a subset of fibers on the lateral edge and in the center. Labeling of the KCs and calyx (E) is low intensity relative to the lobes, consistent with our expectation that a vesicular transporter localizes primarily to the axons and terminals in a mature neuron, not the cell bodies and dendrites. Additional labeled cell bodies are indicated with asterisks (A–D, F), arrowheads (A, B), and arrows (F). Arrowheads also indicate labeled projections (D–F). Scale bars: A–F, 25 μm. e = esophageal foramen, SOG = subesophageal ganglion. G–I: Projections to the central complex and medulla in the adult brain. PRT is expressed in both the fan shaped body (G, FSB) and the ellipsoid body (H, EB) of the central complex. Additional labeling (H) represents the tips of the MB medial lobes. Cell bodies at the border between the central brain and the medulla arborize in the medulla and show prominent varicosities (I, asterisks). Scale bars: G, H, 25 μm; I, 10 μm. J) The schematic shows a frontal view of the central brain that summarizes expression of PRT in the adult, including expression in the MBs and the central complex, shown in solid gray. Additional neurons and processes expressing PRT are shown as gray circles or stippling, respectively. The numbers represent arbitrary designations of cell clusters in the adult. K) Arrows indicate clusters of 2–4 labeled cell bodies in the thoracic ganglion. Anterior is up, posterior down. Scale bar: 50 μm. L, M) Higher magnification of the 3 cell clusters with the anterior cluster at the top, posterior cluster at the bottom, showing no sexual dimorphism between the male (L) and female (M). Scale bars: 10 μm.
We also detected PRT expression in the peduncle, formed by KC axons before they branch into the lobes (Fig 3D–F). PRT was not distributed uniformly throughout the peduncle and a portion of the core was weakly labeled (Fig 3D–F and data not shown). This pattern suggests that PRT may not be expressed in all KCs although further experiments will be needed to confirm this. Several additional cell bodies near the MBs express PRT (Fig 3A–C, F) as well as 1 cluster of 2–3 cells in the subesophageal ganglion that project medially toward the esophogeal foramen (arrows, Fig 3F).
During metamorphosis there is also extensive development of the central complex (CCX), a midline structure just posterior to the MB medial lobes involved in motor activity (Strauss, 2002) and visual memory (Liu et al., 2006). PRT labeling of the adult brain revealed that it is expressed in components of the CCX including the neuropil of the ellipsoid and fan shaped bodies (Fig 3G, H).
We also detected PRT expression in two bilaterally symmetric clusters of two and three cells each near the medial aspect of the optic lobe that project outward toward the medulla (asterisks, Fig 3I). The cartoon in Fig 3J summarizes the PRT expressing cells in the adult. Other than the KCs there are approximately 56 labeled cell bodies. For comparison, the adult brain contains approximately 300 dopaminergic and 106 serotonergic cells (Monastirioti, 1999).
To complete our survey of the adult central nervous system, we also labeled the thoracic ganglion and found 3 clusters with 2–4 cells each that lie along the ventral midline (Fig 3K–M). This expression pattern was not sexually dimorphic (Fig 3L, M).
Generation of a prt mutant
To investigate the function of PRT, we generated a mutant fly. A survey of the public database revealed a previously generated line with a SUPor-P element inserted into the 5′ UTR of prt (Fig 4A). Line KG07780 was obtained from the Bloomington Drosophila Stock Center (Indiana University) and we confirmed that the SUPor-P element was located 118 bp upstream of the predicted initiating methionine (not shown). We used imprecise excision to generate a prt mutation. Lines were screened by PCR with primers flanking the P element insertion. In CantonS (CS) we detected a major product that migrated at 1.2 kb, consistent with the size predicted by the primary sequence (Fig 4B). In one line, the major band migrated at 400 bp, consistent with an 850 bp deletion (Fig 4B). We designated this allele prt1. We immunolabeled adult brains to determine whether prt1 mutants produce any residual protein and failed to detect any labeling of the MBs or elsewhere (Fig 4C). These data confirm the specificity of the antiserum to PRT. In addition, the size of the deletion and the absence of residual protein suggest that prt1 is either a severe hypomorph or null mutation (see also deficiency analysis below).
Fig 4.
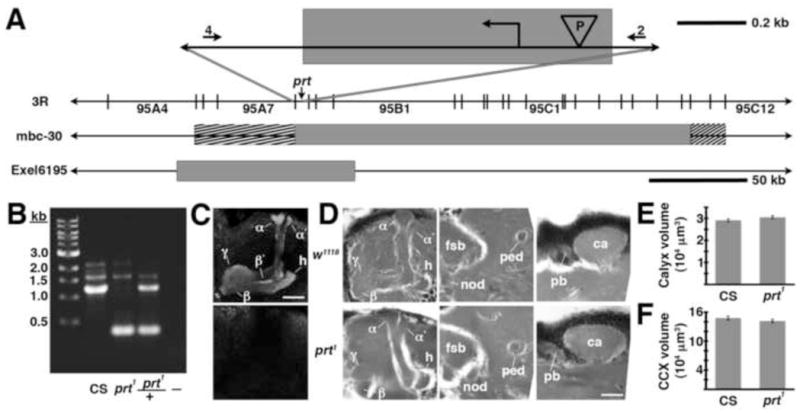
The prt1 mutation. A) The relative position of the prt gene is shown in cytological region 95A8 on the 3rd chromosome. The shaded box at the top of the figure represents an approximately 850 bp deletion created with imprecise excision of a P-element (“P” in the inverted triangle), including the initiating methionine (
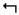 ). The numbers 4 and 2 indicate primers used to detect the deletion. The lines below show deficiencies that uncover prt (Df(3R)mbc-30, Df(3R)Exel6195). The shaded boxes represent the deletion. The cross-hatched boxes indicate breakpoints approximated by polytene chromosome squash. Scale bars: 50 kb for deficiencies and 0.2 kb for inset. B) PCR was used to detect the prt1 mutation. Wild-type CS flies show a major band at 1.2 kb, prt1 flies show a major band at 400 bp, and prt1/+ heterozygotes show both. (−) indicates a no-template control. A 1 kb standard is shown on the left. C) Single confocal slices through the mushroom body lobes of whole-mount brains labeled with anti-PRT. A w1118 brain is on top with labeling of the MB lobes (α, α′, β, β′, and γ) and heel (h) and a prt1 brain is on bottom, showing no detectable anti-PRT labeling. Scale bar: 20 μm. D) H&E stained paraffin sections show that prt1 mutants have grossly intact MB morphology (α, α′, β, and γ lobes and heel (h), peduncle (ped), and calyx (ca)) and central complex (fan-shaped body (fsb), noduli (nod) and protocerebral bridge (pb)). The β′ lobe is also grossly intact in prt1 (not shown). Scale bar: 20 μm. E, F) Volumetric analyses of the MB calyx (E) and CCX (F) in CS and prt1 did not significantly differ (Student’s t-test, p = 0.1934 (E) and p = 0.2496 (F)). Bars represent means ± SEMs, n = 10 for all groups.
). The numbers 4 and 2 indicate primers used to detect the deletion. The lines below show deficiencies that uncover prt (Df(3R)mbc-30, Df(3R)Exel6195). The shaded boxes represent the deletion. The cross-hatched boxes indicate breakpoints approximated by polytene chromosome squash. Scale bars: 50 kb for deficiencies and 0.2 kb for inset. B) PCR was used to detect the prt1 mutation. Wild-type CS flies show a major band at 1.2 kb, prt1 flies show a major band at 400 bp, and prt1/+ heterozygotes show both. (−) indicates a no-template control. A 1 kb standard is shown on the left. C) Single confocal slices through the mushroom body lobes of whole-mount brains labeled with anti-PRT. A w1118 brain is on top with labeling of the MB lobes (α, α′, β, β′, and γ) and heel (h) and a prt1 brain is on bottom, showing no detectable anti-PRT labeling. Scale bar: 20 μm. D) H&E stained paraffin sections show that prt1 mutants have grossly intact MB morphology (α, α′, β, and γ lobes and heel (h), peduncle (ped), and calyx (ca)) and central complex (fan-shaped body (fsb), noduli (nod) and protocerebral bridge (pb)). The β′ lobe is also grossly intact in prt1 (not shown). Scale bar: 20 μm. E, F) Volumetric analyses of the MB calyx (E) and CCX (F) in CS and prt1 did not significantly differ (Student’s t-test, p = 0.1934 (E) and p = 0.2496 (F)). Bars represent means ± SEMs, n = 10 for all groups.
For both mammals and invertebrates, developmental perturbations of neurotransmitter metabolism can have neuroanatomical sequelae (Budnik et al., 1989; Lawal et al., 2010; Levitt et al., 1997). We therefore analyzed the morphology of the MBs and CCX in the prt1 mutant and found it grossly intact in paraffin sections of adult brains stained with H&E (Fig 4D and data not shown). To rule out more subtle neuroanatomical changes, we performed volumetric analyses of the MB calyx and CCX (ellipsoid body + fan shaped body). We detected no difference in either calyx or CCX volume between CS and prt1 (Fig 4E, F), indicating that prt1 does not result in significant anatomical defects.
Behavioral analysis
To further examine changes in the function of the MBs and other tissues expressing PRT, we investigated prt1 mutant behavior. We first outcrossed the prt1 flies for 6 generations into the wild-type strain CS. Outcrossing removed a closely linked mutation that reduced viability and fertility (not shown), and all behavioral experiments were performed using the outcrossed lines. The outcrossed prt1 flies were viable, fertile, and showed no obvious external morphological defects.
The relatively high level of PRT expression in the MBs as compared to other structures suggests it may play a role in olfactory classical conditioning, known to require the MBs (Davis, 2011). We used a modified T-maze to test olfactory classical conditioning as previously described (de Belle and Heisenberg, 1994). As controls for these experiments, we first established that prt1 flies had normal avoidance of both electric shock and the odors used to test learning (Fig 5A–C). We next tested olfactory learning. We found that prt1 mutants have a learning defect, evidenced by a decreased performance index immediately following training (Fig 5D). The performance indices for prt1 were also reduced at 30 minutes and 6 hours after training (Fig 5D). The difference between CS and prt1 was consistent over short-term (30 minutes) and middle-term (6 hours) phases of memory, suggesting normal memory decay in prt1 flies.
Fig 5.
prt1 mutants show a learning deficit. A) prt1 mutants show normal avoidance of electric shock (90V) and the odor concentrations used for olfactory learning assays, B) octanol (10−4) and C) benzaldhyde (2 × 10−4). Bars represent means ± SEMs, n = 5 for CS in (A) and n = 6 for all other groups. D) Performance indices indicate a learning defect for prt1, seen immediately following training (0), decreased short-term memory (0.5), and reduced middle-term memory (6). * = p < 0.05, ** = p < 0.01. Statistical analyses included Student’s t-test for A-C and 2-way ANOVA with Bonferroni’s post test for D. 2-way ANOVA reveals no significant interaction of time and genotype (p = 0.3976) suggesting normal memory decay in prt1. Symbols represent means ± SEMs, n = 12 for learning, n = 6 for memory.
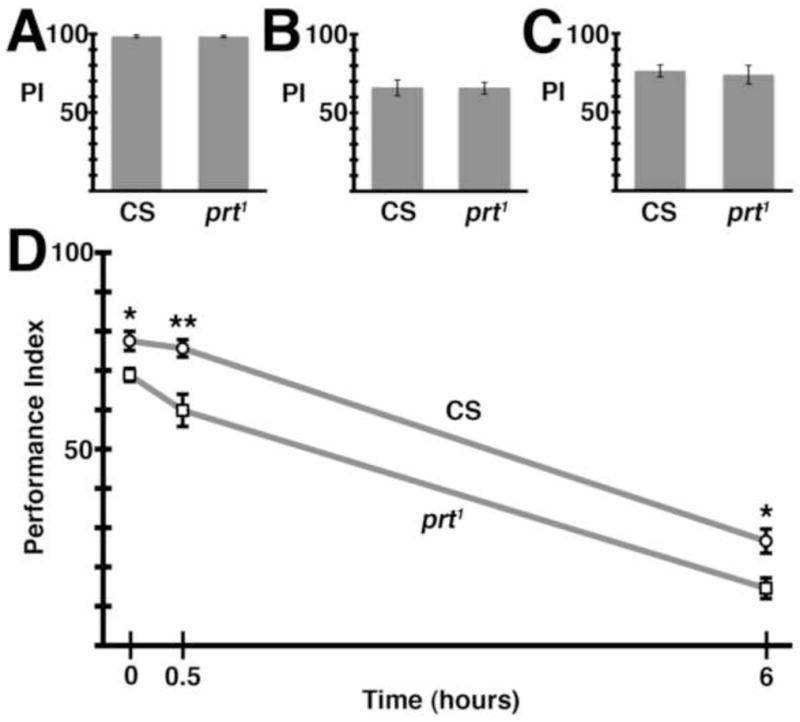
We next tested prt1 behavior using several other well-established assays. Performance indices for negative geotaxis and fast phototaxis were equivalent to those of wild-type flies (Fig S3A, B) indicating that gross locomotor activity and the response to both mechanical stimuli and visible light are intact in prt1. We did find a modest impairment in courtship behavior. While prt1 males performed all of the necessary courting rituals, they spent less time courting (Fig 6A).
Fig 6.
prt1 reproductive behavior. A) prt1 mutant males spent 26% less time courting (*, p = 0.0182). B) The top two panels show normal copulatory positions seen in wild-type flies while the bottom two panels show improper positioning exemplary of prt1. C) prt1 mutants struggle throughout copulation. Quantification of the time a male spends out of position during copulation revealed a significant difference between CS and prt1 (first two bars, p < 0.001). Inter-genotype pairings suggest the prt1 male was responsible for this phenotype (last two bars, p < 0.01). D) Two different deficiencies uncovered prt. Both prt1/Df(3R)mbc-30 and prt1/Df(3R)Exel6195 phenocopied the copulation behavior seen in prt1 (last two columns, p < 0.05 and 0.001, respectively). E) prt1 mutants were fertile but had reduced fecundity (# progeny/mating pair), 53% that of CS (*, p = 0.0167). * = p < 0.05, ** = p < 0.01, *** = p < 0.001. Statistical analyses included Student’s t-test for A & E and ANOVA with Bonferroni’s post test for multiple comparisons for all other assays. Bars represent means ± SEMs. The n for each group is shown with each column. See also Movies S1 and S2, and Fig S3.
In the course of performing courtship assays, copulation was observed. Normally, males mount the female from behind, curling their abdomen upwards to allow coupling. Once coupled, the male maintains a forward-facing orientation, in the same direction as the female (Fig 6B, top pictures, Movie S1). Copulation typically lasts ~20 minutes (Jagadeeshan and Singh, 2006). For the first several minutes of this period, wild-type pairs may move forward or adjust positions, but for the remainder of this time the flies remain essentially motionless.
Copulation in prt1 mutants differs dramatically. Similar to wild-type flies, prt1 males mount the female and curl their abdomen to begin copulation. However, after coupling, the prt1 male continuously struggles to maintain his orientation and can be seen in a variety of different positions relative to the female (Fig 6B, bottom pictures, Movie S2). We quantified the amount of time that prt1 males spent in a position distinct from that usually seen in wild-type flies as a percentage of the total copulation duration (Fig 6C). While CS flies primarily remain centered on the dorsal abdomen of the female, prt1 flies spent nearly half of copulation severely misaligned. Although the genitalia of the male and female remain in contact, the male can be positioned perpendicular to the normal axis or rotated nearly 180 degrees from horizontal. Moreover, during copulation, prt1 mating pairs move about the observation chamber, with the female dragging the male behind. In cross-genotype mating experiments, prt1 males mated to CS females showed defective copulation whereas CS males mated to prt1 females did not (Fig 6C). Thus, the prt1 males were primarily, if not exclusively, responsible for the defect in copulation.
To determine whether the change in the males’ position was due to a defect in genital morphology, we examined both the prt1 male and female genitalia using scanning electron microscopy. We found that the external genitalia of prt1 males and females were indistinguishable from wild-type (Fig S3D, E). We also examined the prt1 males’ sex combs, specialized foreleg structures used to grasp the female during copulation (Ahuja and Singh, 2008; Ng and Kopp, 2008). The morphology of prt1 sex combs was intact in scanning electron micrographs (Fig S3F) without obvious gaps between bristles, although the number of bristles in the prt1 sex combs was slightly lower than controls (Fig S3G) (Ahuja and Singh, 2008; Tokunaga, 1961).
We employed deficiency analysis to help determine the severity of the prt1 sexual phenotype (Fig 6D). Two deficiency lines that uncover the prt locus were used and the extent of their chromosomal deletions is represented in Fig 4A. The copulatory phenotype seen in the prt1 homozygote was replicated in both the prt1/Df(3R)mbc-30 and prt1/Df(3R)Exel6195 transheterozygotes (Fig 6D). It is possible that the prt1 copulation phenotype cannot get measurably worse, and further deficiency analysis using other aspects of the prt1 phenotype will be necessary to more precisely assess the severity of the prt1 allele. However, in light of our current data showing that the severity of the phenotype seen in prt1/Df was equivalent to that of the prt1 homozygotes, and our molecular analysis showing undetectable levels of PRT protein, we conclude that the prt1 allele is either a severe hypomorph or a null mutation.
prt1 mutants were surprisingly fecund given their contorted mating positions. They were able to produce approximately half the number of offspring as CS flies (Fig 6E and S3C). Either insemination occurred during periods when the male was correctly oriented, or wild-type position is not required for insemination. The total duration of copulation was also decreased in prt1 (Fig 7B). It is perhaps surprising that the decrease in copulation time and fecundity were not more severe given the tremendous struggling observed in mating pairs.
Fig 7.
Genetic rescue of copulation defect (see legends on right for genotypes). A) The combination of the Da-Gal4 driver with a UAS-prt transgene rescued the copulation phenotype (columns 6 & 7, p < 0.01 and 0.05, respectively); driver or UAS-prt transgene alone did not (columns 3–5). B) prt1 mutants had significantly shorter copulation duration (columns 1 & 2, p < 0.001), rescued with Da-Gal4 plus UAS-prt transgenes (columns 6 & 7, p < 0.001 for both) but not driver or UAS-prt alone (columns 3–5). C) PRT transgenic expression with a MB driver (OK107-Gal4) rescued the prt1 copulation phenotype (columns 13 & 14, p < 0.05 and 0.001, respectively); driver or UAS-prt transgenes alone did not (columns 10–12). D) The short duration phenotype was similarly rescued using OK107-Gal4 plus UAS-prt transgenes (columns 13 & 14, p < 0.01 and 0.001, respectively). The UAS-prt transgene on the 2nd rescued in the absence of driver, presumably due to leaky PRT expression (column 12, p < 0.05 compared to prt1). * = p < 0.05, ** = p < 0.01, *** = p < 0.001, ANOVA with Bonferroni’s post test for multiple comparisons. Bars represent means ± SEMs. The n for each group is shown with each column.
Rescue of prt1 copulation phenotype
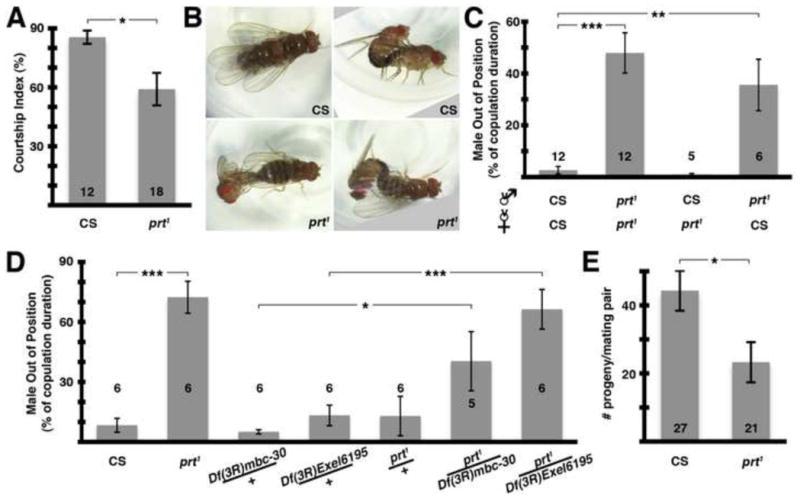
To confirm that the copulation defects we observed were due to prt1 rather than another spurious mutation, we performed genetic rescue experiments with UAS-prt transgenes. The circuitry involved in copulation is not known. Therefore, to maximize our chances of expressing prt1 in the relevant tissue we used the broadly distributed driver daughterless-Gal4 (Da-Gal4). As controls, we tested the Da-Gal4 driver alone and the UAS-prt transgenes alone, and none rescued the copulation deficit (Fig 7A). In contrast, when Da-Gal4 was used in combination with a UAS-prt transgene on the 3rd chromosome we saw rescue of the copulation phenotype (Fig 7A). We replicated these results using a UAS-prt transgene on the 2nd chromosome (Fig 7A).
Similarly, we found that the decrease in copulation duration was rescued using Da-Gal4 and either of these UAS-prt transgenes, but not by Da-Gal4 or UAS-prt alone (Fig 7B). Taken together, these data confirm that prt plays a critical role in an important but poorly described aspect of D. melanogaster sexual behavior.
While the MBs have been previously linked to courtship behavior (O’Dell et al., 1995; Sakai and Kitamoto, 2006), we wanted to explore whether the MBs could also be involved in copulatory behavior. To this end we performed genetic rescue experiments using OK107-Gal4, a driver commonly used for expression in the MBs (e.g. Connolly et al., 1996). OK107-Gal4 combined with a UAS-prt transgene on either the 2nd or 3rd chromosome resulted in rescue of the copulation phenotype while the controls did not (Fig 7C).
We found that the decrease in copulation duration was also rescued using OK107-Gal4 and either of these UAS-prt transgenes (Fig 7D); the 2nd chromosome UAS-prt transgene alone also appeared to rescue copulation duration, presumably due to leaky expression of PRT. Although we cannot rule out the possibility that other cells expressing OK107-Gal4 are responsible for these effects, these data suggest that the MBs play a critical role in copulatory behavior.
At present we do not know the PRT substrate and suggest that it may be an unknown neurotransmitter. The absence of candidates prevents the validation of PRT’s proposed transport activity using most standard biochemical assays. We therefore employed a genetic approach to test the hypothesis that PRT might function as a vesicular transporter in vivo. For mammalian VMATs and VAChT, a wealth of data have identified specific residues required for either transport activity or substrate recognition (See (Parsons, 2000) for review and Supplementary Information). A number of these important residues are conserved in DVMAT, DVAChT and PRT. These include aspartates (D) in the first and tenth transmembrane domains (TM1 and TM10) of DVMAT, DVAChT and PRT (Fig S1). For both rat VMAT2 and VAChT, mutation of the aspartate in TM10 abolishes transport activity, while mutation of the aspartate in TM1 of VMAT2, but not VAChT, inhibits transport (Kim et al., 1999; Merickel et al., 1997; Merickel et al., 1995).
We used site-directed mutagenesis to convert these homologous sites to alanine (D59A or D483A) and expressed each in vivo as a UAS transgene. Both UAS-prtD59A and UAS-prtD483A showed robust expression on Western blots (not shown); however, neither rescued the prt1 phenotype (Fig 8). Thus, residues conserved in VMAT2, DVMAT and PRT, and required for VMAT2 activity, are also required for PRT function. Furthermore, the aspartate in TM1 required for both PRT and VMAT2 transport activity is not required for VAChT. These data support the idea that PRT functions as a vesicular transporter more similar to VMATs than VAChT.
Fig 8.
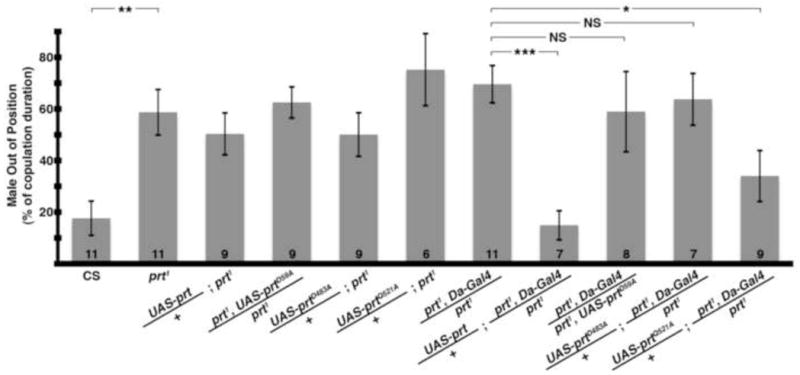
Point mutants of conserved aspartates fail to rescue copulation defect. Transgenic expression with a ubiquitous driver (Da-Gal4) rescued the prt1 copulation phenotype with wild-type PRT (8th column, p < 0.001) but not with the D59A (9th column) or D483A mutations (10th column). In contrast, transgenic expression of the Q521A mutation did rescue the prt1 copulation phenotype (final column, p < 0.05). The driver or UAS-prt transgenes alone did not rescue the behavior (columns 3–7, p > 0.05 for all compared to prt1). * = p < 0.05, ** = p < 0.01, *** = p < 0.001, ANOVA with Bonferroni’s post test for multiple comparisons. Bars represent means ± SEMs. The n for each group is shown with each column. NS: not significant.
To obtain additional insight into the structural requirements for PRT activity, we turned our attention to another, more ambiguous site. Mutation of a conserved aspartate in TM11 of either VMAT2 or VAChT blocks transport activity (Kim et al., 1999; Merickel et al., 1997); however, PRT contains an uncharged glutamine in TM11 (Q521, star, Fig S1B). The presence of a nonconserved glutamine at this site in PRT suggested that it might not be essential for its activity. Indeed, in contrast to PRT mutants D59A and D483A, the Q521A mutant partially rescued the prt1 mutant phenotype (Fig 8).
Together our data suggest that PRT likely functions as a vesicular transporter similar to the VMATs. However, PRT did not display appreciable affinity for known substrates such as dopamine or serotonin in in vitro transport assays with DVMAT as a positive control (not shown). Moreover, PRT localizes to cells that do not express any of the enzymes required for the synthesis of known monoamines (see below). These data along with the differential structural requirements for activity support the possibility that PRT recognizes a substrate distinct from either VMATs or VAChT.
Discussion
We have identified a novel gene portabella (prt) that appears structurally similar to vesicular monoamine and acetylcholine transporters. This gene corresponds to predicted gene CG10251 and localizes to chromosomal region 3R:95A. PRT is expressed in a subset of cells in both larval and adult nervous systems, including the KCs of the MBs. The prt1 mutant phenotype includes a reduction in learning and an unusual sexual phenotype, characterized primarily by the inability of males to stay in position during copulation. PRT expression in the KCs and other cells that lack known neurotransmitter systems suggests that prt represents the first component of a previously unknown neurotransmitter system in insects and perhaps other species.
At present it is difficult to perform biochemical assays to conclusively demonstrate PRT transport activity since we do not know its substrate(s) and believe that it recognizes a previously unknown neurotransmitter. We have attempted to circumvent this limitation using a genetic approach to test whether sites required for transport in VMAT and VAChT are also required for PRT function in vivo. We focused on two sites conserved in PRT, VMAT, and VAChT: aspartates in TM1 and TM10. Both are required for VMAT transport activity and the TM10 aspartate is required for transport activity in VAChT. We found that mutation of either site in PRT (D59A & D483A) abrogates its ability to rescue the prt1 mutant phenotype. Thus, given that 1) PRT is the only member of the vesicular transporter family present in KCs, 2) the likelihood that KCs, like all other neurons, exocytotically release at least one type of classical or amino acid neurotransmitter and 3) the fact that sites required for transport activity in VMAT are also required for the function of PRT in vivo, we propose that PRT functions to store an unknown neurotransmitter in secretory vesicles of at least a subset of KCs, as well as other neurons, and is an “orphan” vesicular transporter.
It is possible that the PRT substrate is a previously identified small molecule. This substrate would presumably be similar to monoamines since PRT is similar to VMAT and mutation of a site that abolishes transport in VMAT but not VAChT (D59 in TM1) blocks PRT activity in vivo. However, PRT’s primary structure differs from VMAT at several key sites ((Parsons, 2000) and Supplementary Information). Most notably, a charged amino acid conserved in VMATs (as well as VAChT) in TM11 is an uncharged glutamine in PRT. Whereas mutation of the analogous aspartate in VMAT and VAChT completely blocks transport activity, a mutant form of PRT containing an alanine at this site (Q521A) is able to partially rescue the prt1 mutant behavioral phenotype. We therefore speculate that PRT transports, and some KCs employ, a novel compound not previously recognized as a neurotransmitter. Importantly, this would explain why the enzymes required for the synthesis of all known monoamine neurotransmitters (and acetylcholine) are not expressed in the MBs (Bao et al., 2010; Burg et al., 1993; Cole et al., 2005; Gorczyca and Hall, 1987; Konrad and Marsh, 1987; Monastirioti et al., 1996; Neckameyer and White, 1993). It would also explain why the neurotransmitter released from KCs has remained frustratingly obscure for so many years, despite extensive study of the MBs.
How could a novel neurotransmitter remain unknown for so long? First, it may be insect- or invertebrate-specific and, generally, the neurochemistry of the mammalian brain has received more attention than that of invertebrates. Additionally, subtle phenotypes caused by disrupting this unique neurotransmitter system may have escaped previous genetic screens. Despite the copulation defect, prt1 mutants are fertile and males court successfully, albeit less avidly. Similarly, the learning phenotype of prt1 is relatively subtle compared to some other mutants and could easily have been overlooked. More generally, it is important to recall that the identification of most neurotransmitters has lagged far behind the physiological and behavioral characterization of their attendant cells and circuits. Decades elapsed between the characterization of dopamine as a precursor for noradrenaline and the determination that it functions as a bona fide transmitter. We also note that serotonin, histamine, GABA, and glutamate were not fully acknowledged to be neurotransmitters until the 1970’s. Although the chemical released from KCs may also be familiar to biologists as an intermediate metabolite, there are multiple precedents for known molecules having eluded identification as neurotransmitters.
The prt1 phenotype
We find that prt1 mutants show behavioral deficits in learning and sexual behavior. Another mutation that influences both the MBs and sexual behavior is the icebox allele of neuroglian, an L1-type cell adhesion molecule, which results in reduced female receptivity, subtle changes in male courtship, and dramatic structural abnormalities of the MBs (Carhan et al., 2005). In addition, classical olfactory learning mutants, such as dunce, amnesiac, and rutabaga, similarly affect experience-dependent modification of sexual behavior (Ackerman and Siegel, 1986; Gailey et al., 1984).
The localization of PRT to the MBs is consistent with the prt1 learning phenotype, but given the well-established importance of the MBs for learning, we were initially surprised that prt1 showed a relatively mild learning deficit. There are several possible explanations for this apparent discrepancy. For example, PRT may reside mainly in subsets of KCs required for MB functions other than learning. Despite the robust MB labeling, it is difficult to say whether PRT expresses in only a fraction of adult KCs. Alternatively, prt1 mutants may have undergone an adaptive response that minimizes the effects of the mutation on some circuits, such as those required for learning, while other PRT circuits may be less able to adapt, such as those required for copulatory behavior.
The prt1 copulation phenotype is dramatic and unusual. Previously described mutants with defects in copulation include: dissatisfaction, in which males have difficulty curling their abdomens and females are unreceptive during both courtship and copulation (Finley et al., 1997); celibate (Hall et al., 1980), nerd (Ferveur and Jallon, 1993), and platonic (Yamamoto et al., 1997), all of whom court normally but fail to initiate copulation; coitus interuptus (Hall et al., 1980) and okina (Yamamoto et al., 1997) both of which shorten copulation. In addition, certain combinations of fruitless alleles lengthen copulation (Lee et al., 2001) and lingerer (Kuniyoshi et al., 2002) mutants cannot terminate copulation.
None of these previously described mutants phenocopy the positioning defect of prt1. Rather, the most similar deficit reported is in flies in which selected sensilla have been manually removed (Acebes et al., 2003). Male flies use mechanosensory sensilla on their claspers and lateral plates for proprioception during copulation, and ablation of these sensilla results in asymmetrical mating postures. Although the terminalia of prt1 males are indistinguishable from wild-type, it remains possible that other peripheral deficits contribute to the observed defect in copulation. However, our data thus far suggest that the prt1 behavioral phenotype is due to deficits in the function of the nervous system, since expression of PRT in the MBs using the OK107-Gal4 driver completely rescues the behavioral phenotype. We speculate that male prt1 flies may have difficulty in either receiving or processing sensory information during copulation. This proposal is consistent with the previously described role of the MBs as centers of sensory integration (Strausfeld et al., 1998; Wessnitzer and Webb, 2006) in addition to their established importance for learning and memory.
Further study of prt1 may help determine the mechanism by which neurotransmission in the MBs integrates information required for memory and sexual behavior. Furthermore, if PRT indeed functions as a vesicular transporter, the determination of its substrate will identify the elusive, and most likely novel, neurotransmitter that is stored in Kenyon cells.
Experimental Procedures
Animals
D. melanogaster strains were obtained from the Bloomington Drosophila Stock Center (Indiana University). The wild-type Canton-S strain was used for all studies except as indicated in the text. Flies were maintained on standard molasses-agar media at 25°C under a 12-hour light-dark cycle.
cDNA cloning and expression constructs
(See Supplementary Information for further details) RT-PCR was performed using head RNA isolated as described (Greer et al., 2005) followed by amplification using the SuperScript One Step RT-PCR System (Invitrogen). We subcloned the predicted coding region of CG10251 into the pCRII TOPO, pcDNAI Amp, and pMT vectors (Invitrogen) for in vitro expression, and into pExp-UAS (Exelixis) and pUASTattB (Bischof et al., 2007) for expression in vivo. A fragment of the CG10251 cDNA representing the predicted carboxy terminus was subcloned into the pGEX KG vector provided by Greg Payne (UCLA) for antibody production.
mRNA analysis
Amplification of stage-specific cDNAs was performed using Drosophila Rapid-Scan Panels (OriGene) and probes generated using the primers DVX1 and DVX6 for CG10251. The expression of CG10251 was normalized to RP49 by arbitrarily defining the pixel intensity of the RP49 band in lane 9 as 1.0. The normalized value for CG10251 for lane n was calculated as the observed pixel intensity for CG10251 x (RP49 lane n/RP49 lane 9). Northern blots were performed as described (Greer et al., 2005) using a probe generated with the primers unk19A2 and unk19B2. See Supplementary Information for primer sequences.
Antibody production
The GST fusion protein encoding the C-terminus of CG10251 was used by CoCalico Inc (Reamstown, PA) to generate an antiserum in rabbits. The antiserum was affinity purified using the fusion protein immobilized on nitrocellulose as described previously (Greer et al., 2005).
Protein expression and detection
S2 cells were transfected and expression induced using the metallothionein promoter in pMT vector, and Western blots were performed as described previously (Chang et al., 2006; Greer et al., 2005) with the antiserum to CG10251/PRT used at a concentration of 1/1000. For Western blot analysis of glycerol velocity and sucrose density gradients (see below), primary antibodies included mouse anti-HA.11 (1:1000, Covance Research Products, Denver, CO) to detect CG10251/PRT, mouse mAb to Drosophila cysteine string protein (DCSP, 1:1000, Developmental Studies Hybridoma Bank, Iowa City, IA) (Zinsmaier et al., 1990), rabbit anti-late bloomer (lbm, 1:250), a gift of Aaron DiAntonio (Washington University) as marker for the plasma membrane, and rabbit anti-ANF antibody (1:4000, Peninsula Laboratories/Bachem, San Carlos, CA) as a marker for LDCVs. Either anti-mouse or anti-rabbit HRP conjugated secondary antibodies were incubated (1:2000, Amersham Biosciences, Piscataway, NJ) for 45 minutes at ambient temperature, followed by SuperSignal West Pico Luminol/Peroxide (Pierce, Rockford, IL), and exposure to Kodak Biomax Light Film (Rochester, NY).
Glycerol velocity gradient fractionation
Flies containing UAS-prt-HA driven by a pan-neuronal driver elav-Gal4 were used. Glycerol gradient fractionation was performed as described (Daniels et al., 2004).
Sucrose density gradient fractionation
Frozen adult fly heads were homogenized in 10 mM K Hepes, pH 7.4, 1 mM Na EGTA, 0.1 mM MgCl2, proteinase inhibitor cocktail (Roche, Indianopolis, IN), and 2mM dithiothreitol (DTT) and were centrifuged for 1 min at 10,000×g, 4°C to obtain the postnuclear supernatant. After addition of EDTA to 10 mM, the supernatant was loaded onto a 20–55% linear w/v sucrose gradient in 10mM HEPES, pH 7.4, 1 mM EGTA, 1mM MgCl2, and 2 mM DTT. After centrifugation at 30,000 rpm (~111,000×g) for 12–16 hours, 4°C in a Beckman SW 41 Ti rotor, 15 fractions were collected from the bottom of the tube and analyzed by Western blot.
Immunohistochemistry
Wandering 3rd instar larvae and adult flies were dissected in 4% paraformaldehyde and immunofluorescently labeled as described (Greer et al., 2005) with anti-PRT (1:300) and 1:400 goat anti-rabbit Cy3 (Jackson Immuno) or 1:1000 goat anti-rabbit Alexa Fluor 488 (Invitrogen) as secondary antibodies.
P-element excision
To excise a SUPor-P element from the 5′ UTR of prt in the line KG07780, KG07780 homozygotes were crossed to flies containing the Δ2–3 transposase marked with Sb in a yw background using standard genetic techniques. The progeny were screened for loss of the y+ body color phenotype, rather than loss of the w+ eye color, since SUPor-P in KG07780 rescues y− but not w−. To screen for loss of the 5′ end of the P and/or the 5′ end of prt, genomic DNA from heterozygotes was amplified using the primers DVX8 and Pele5R, representing the 5′ end of the P. Lines showing a loss of the 850 bp amplicon seen in the parent were rescreened, using the primer pair DVXCG4 and DVX8 to detect small deletions and the primer pair DVXCG2 and DVX6 to detect larger deletions. The deletion in DVXΔ50 line was confirmed and further characterized using the 3′ primers DVX4 and DVX6 with the 5′ primers DVXCG1, DVXCG2, and DVXCG3. See Supplementary Information for primer sequences.
Histology and volumetric measurement
For gross anatomical visualization, flies were prepared for mass histology as described (Heisenberg and Bohl, 1979) and processed with Hematoxylin and Eosin stain. For volumetric analysis, cold anesthesia was used and sections were not stained. Anatomy was imaged under fluorescence microscopy double-blind with respect to genotype. Calyx volume was derived from planimetric measurements (de Belle and Heisenberg, 1994). CCX measurements summated volumes for the ellipsoid and fan shaped bodies.
Fly husbandry for behavioral assays
Flies were housed on standard molasses media at 25°C on a 12 hour light-dark cycle and were passed into fresh tubes both the night before and the morning of behavioral testing. Unless otherwise noted, all fly lines used for behavioral analysis were outcrossed into a CS background for at least 6 generations to minimize any effects of genetic background on behavior (de Belle and Heisenberg, 1996). Cold-anesthesia and gentle aspiration were used to manipulate flies prior to all behavioral experiments. Flies were allowed to recover from anesthesia for a minimum of 48 hours before analysis.
Olfactory associative conditioning
Learning and memory experiments were performed as described (de Belle and Heisenberg, 1994). Octanol (10−4) and benzaldehyde (2×10−4) diluted in heavy mineral oil (Sigma) were used as the training odors and 90V for the associated shock. Flies were tested immediately after training to measure learning, 30 minutes after training for short-term memory, and 6 hours after training for middle-term memory.
Courtship
Individual male-virgin female pairs (3–7 days post-eclosion) were placed in 8 mm (inner diameter) by 4 mm (height) polypropylene chambers via aspiration and digitally recorded for 30 minutes or until copulation. Assays were performed at 23°C in a dedicated test area maintained at 80% humidity to maximize courtship activity (K. Han, personal communication). The recordings were scored for selected male courtship behaviors including following and wing song. A courtship index was calculated as the total time the male spent actively courting as a percentage of the total observation time (Villella et al., 1997).
Copulation
Virgin males and females were collected over ice and housed in same-sex groups of 10 for 2–13 days. One naïve male and one naïve female were gently aspirated into a small chamber (9 mm diameter, 3 mm height), covered with a glass coverslip and allowed to copulate. The entire copulation event was recorded and scored later by an observer blind to genotype. A male was considered out of position if his midline, as viewed from above, deviated more than 45° laterally or 90° vertically, relative to the female’s midline. The time a male spent out of position was measured and reported as a percentage of the total copulation duration.
Fecundity (progeny/mating pair)
CS and prt1 virgin females and young (< 1 day old) males were collected with cold anesthesia and stored overnight in groups of 7. The following day, individual mating pairs, including a male and a virgin female of the same genotype, were introduced into a vial with gentle aspiration. The parents were kept together for 4 days and then removed. Vials with 1 or 2 dead parents were discarded. All progeny eclosing within 20 days of the parents’ introduction were counted for each mating pair.
Site-directed mutagenesis
The QuikChange II XL Site-Directed Mutagenesis Kit (Agilent) was used to introduce the base pair substitutions encoding the D59A, D483A, and Q521A point mutations in PRT. See Table S1 for the primer sequences. The mutants were subcloned into both the pExp-UAS (Exelixis) and pUASTattB (Bischof et al., 2007) vectors to generate P-element based and phiC31 integrase based transgenic fly lines.
Genetic rescue of copulation behavior
UAS-prt transgenes were driven by either Da-Gal4 or OK107-Gal4. For rescue with PRT point mutants, Da-Gal4 was used with UAS-prtD483A and wild-type UAS-prt inserted on the 2nd chromosome (BDSC stock #24484) via phiC31 recombination (Bischof et al., 2007) as well as UAS-prtD59A on the 3rd and UAS-prtQ521A on the 2nd chromosome, generated with P-element mediated transformation (Spradling and Rubin, 1982).
Supplementary Material
Acknowledgments
We thank Volker Hartenstein for his critical reading of the manuscript, and acknowledge David Patton (deceased) for his important contributions to early phases of this work. We would also like to thank Marianne Cilluffo and colleagues at the UCLA Microscopic Techniques Laboratory for their help with paraffin embedding, sectioning, and mounting of histological samples, Alicia Thompson at the USC Center for Electron Microscopy and Microanalysis (CEMMA) for her help in performing scanning electron microscopy, and the anonymous reviewers for their excellent suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acebes A, Cobb M, Ferveur JF. Species-specific effects of single sensillum ablation on mating position in Drosophila. J Exp Biol. 2003;206:3095–3100. doi: 10.1242/jeb.00522. [DOI] [PubMed] [Google Scholar]
- Ackerman SL, Siegel RW. Chemically reinforced conditioned courtship in Drosophila: responses of wild-type and the dunce, amnesiac and don giovanni mutants. J Neurogenet. 1986;3:111–123. doi: 10.3109/01677068609106898. [DOI] [PubMed] [Google Scholar]
- Ahuja A, Singh RS. Variation and evolution of male sex combs in Drosophila: nature of selection response and theories of genetic variation for sexual traits. Genetics. 2008;179:503–509. doi: 10.1534/genetics.107.086363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Bao X, Wang B, Zhang J, Yan T, Yang W, Jiao F, Liu J, Wang S. Localization of serotonin/tryptophan-hydroxylase-immunoreactive cells in the brain and suboesophageal ganglion of Drosophila melanogaster. Cell Tissue Res. 2010;340:51–59. doi: 10.1007/s00441-010-0932-5. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Wu CF, White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989;9:2866–2877. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MG, Sarthy PV, Koliantz G, Pak WL. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J. 1993;12:911–919. doi: 10.1002/j.1460-2075.1993.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhan A, Allen F, Armstrong JD, Hortsch M, Goodwin SF, O’Dell KM. Female receptivity phenotype of icebox mutants caused by a mutation in the L1-type cell adhesion molecule neuroglian. Genes Brain Behav. 2005;4:449–465. doi: 10.1111/j.1601-183X.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Edwards RH, Fonnum F. Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annu Rev Pharmacol Toxicol. 2008;48:277–301. doi: 10.1146/annurev.pharmtox.46.120604.141146. [DOI] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O’Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 2008;508:131–152. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: a case study of the mushroom body miniature gene (mbm) Proc Natl Acad Sci U S A. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderon R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol. 2010;213:1717–1730. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF, Jallon JM. Nerd, a locus on chromosome III, affects male reproductive behavior in Drosophila melanogaster. Naturwissenschaften. 1993;80:474–475. doi: 10.1007/BF01136042. [DOI] [PubMed] [Google Scholar]
- Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailey DA, Jackson FR, Siegel RW. Conditioning Mutations in DROSOPHILA MELANOGASTER Affect an Experience-Dependent Behavioral Modification in Courting Males. Genetics. 1984;106:613–623. doi: 10.1093/genetics/106.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca MG, Hall JC. Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. J Neurosci. 1987;7:1361–1369. doi: 10.1523/JNEUROSCI.07-05-01361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang HY, Houshyar R, Bainton RJ, Diantonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Hall JC, Siegel RW, Tomkins L, Kyriacou CP. Neurogenetics of courtship in Drosophila. Stadley Genet Symp. 1980;12:43–82. [Google Scholar]
- Heisenberg M, Bohl K. Isolation of anatomical brain mutants of Drosophila by histological means. Z Naturforsch. 1979;34c:143–147. [Google Scholar]
- Jagadeeshan S, Singh RS. A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: role of cryptic female choice and male sex-drive in the evolution of male genitalia. J Evol Biol. 2006;19:1058–1070. doi: 10.1111/j.1420-9101.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kim MH, Lu M, Lim EJ, Chai YG, Hersh LB. Mutational analysis of aspartate residues in the transmembrane regions and cytoplasmic loops of rat vesicular acetylcholine transporter. J Biol Chem. 1999;274:673–680. doi: 10.1074/jbc.274.2.673. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Wang W, Salvaterra PM. Structure and organization of the Drosophila cholinergic locus. J Biol Chem. 1998;273:2706–2713. doi: 10.1074/jbc.273.5.2706. [DOI] [PubMed] [Google Scholar]
- Konrad KD, Marsh JL. Developmental expression and spatial distribution of dopa decarboxylase in Drosophila. Dev Biol. 1987;122:172–185. doi: 10.1016/0012-1606(87)90343-5. [DOI] [PubMed] [Google Scholar]
- Kuniyoshi H, Baba K, Ueda R, Kondo S, Awano W, Juni N, Yamamoto D. lingerer, a Drosophila gene involved in initiation and termination of copulation, encodes a set of novel cytoplasmic proteins. Genetics. 2002;162:1775–1789. doi: 10.1093/genetics/162.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Villella A, Taylor BJ, Hall JC. New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol. 2001;47:121–149. doi: 10.1002/neu.1021. [DOI] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Merickel A, Kaback HR, Edwards RH. Charged residues in transmembrane domains II and XI of a vesicular monoamine transporter form a charge pair that promotes high affinity substrate recognition. J Biol Chem. 1997;272:5403–5408. doi: 10.1074/jbc.272.9.5403. [DOI] [PubMed] [Google Scholar]
- Merickel A, Rosandich P, Peter D, Edwards RH. Identification of residues involved in substrate recognition by a vesicular monoamine transporter. J Biol Chem. 1995;270:25798–25804. doi: 10.1074/jbc.270.43.25798. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Echigo N, Hiasa M, Senoh S, Omote H, Moriyama Y. Identification of a vesicular aspartate transporter. Proc Natl Acad Sci U S A. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. J Neurogenet. 1993;8:189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- Ng CS, Kopp A. Sex combs are important for male mating success in Drosophila melanogaster. Behav Genet. 2008;38:195–201. doi: 10.1007/s10519-008-9190-7. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Liu Y, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci U S A. 1995;92:8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveen A, Daniel A, Hartenstein V. Early development of the Drosophila mushroom body: the roles of eyeless and dachshund. Development. 2000;127:3475–3488. doi: 10.1242/dev.127.16.3475. [DOI] [PubMed] [Google Scholar]
- O’Dell KM, Armstrong JD, Yang MY, Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Parsons SM. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- Popov AV, Sitnik NA, Savvateeva-Popova EV, Wolf R, Heisenberg M. The role of central parts of the brain in the control of sound production during courtship in Drosophila melanogaster. Neurosci Behav Physiol. 2003;33:53–65. doi: 10.1023/a:1021179331583. [DOI] [PubMed] [Google Scholar]
- Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Romero-Calderon R, Shome RM, Simon AF, Daniels RW, DiAntonio A, Krantz DE. A screen for neurotransmitter transporters expressed in the visual system of Drosophila melanogaster identifies three novel genes. Dev Neurobiol. 2007;67:550–569. doi: 10.1002/dneu.20342. [DOI] [PubMed] [Google Scholar]
- Sakai T, Kitamoto T. Differential roles of two major brain structures, mushroom bodies and central complex, for Drosophila male courtship behavior. J Neurobiol. 2006;66:821–834. doi: 10.1002/neu.20262. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S, Bicker G, Ottersen OP, Storm-Mathisen J. Taurine-like immunoreactivity in the brain of the honeybee. J Comp Neurol. 1988;268:60–70. doi: 10.1002/cne.902680107. [DOI] [PubMed] [Google Scholar]
- Schurmann FW. Acetylcholine, GABA, glutamate and NO as putative transmitters indicated by immunocytochemistry in the olfactory mushroom body system of the insect brain. Acta Biol Hung. 2000;51:355–362. [PubMed] [Google Scholar]
- Serway CN, Kaufman RR, Strauss R, de Belle JS. Mushroom bodies enhance initial motor activity in Drosophila. J Neurogenet. 2009;23:173–184. doi: 10.1080/01677060802572895. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Farris SM, Strausfeld NJ. Taurine-, aspartate- and glutamate-like immunoreactivity identifies chemically distinct subdivisions of Kenyon cells in the cockroach mushroom body. J Comp Neurol. 2001;439:352–367. doi: 10.1002/cne.1355. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Tokunaga C. The differentiation of a secondary sex comb under the influence of the gene engrailed in Drosophila melanogaster. Genetics. 1961;46:157–176. doi: 10.1093/genetics/46.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Wessnitzer J, Webb B. Multimodal sensory integration in insects--towards insect brain control architectures. Bioinspir Biomim. 2006;1:63–75. doi: 10.1088/1748-3182/1/3/001. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Jallon JM, Komatsu A. Genetic dissection of sexual behavior in Drosophila melanogaster. Annu Rev Entomol. 1997;42:551–585. doi: 10.1146/annurev.ento.42.1.551. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- Zinsmaier KE, Hofbauer A, Heimbeck G, Pflugfelder GO, Buchner S, Buchner E. A cysteine-string protein is expressed in retina and brain of Drosophila. J Neurogenet. 1990;7:15–29. doi: 10.3109/01677069009084150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


