Abstract
We present the complete nucleotide sequence of the larval beta I-globin gene of Xenopus laevis including 240 nucleotides of the 5' flanking region and 594 nucleotides beyond the polyadenylation site. The site of transcription initiation was mapped by S1 nuclease, and the site of polyadenylation was determined by comparison with corresponding cDNA clones. The larval Xenopus beta I-gene shows the same internal structure as the beta-globin genes of higher vertebrates, viz. 3 exons interrupted by 2 intervening sequences. The first intervening sequence, which is of exceptional length, spans over 564 nucleotides and interrupts the coding sequence at amino acid 30, whereas the second one comprises 968 nucleotides and is located between the amino acids 104 and 105. The second intervening sequence contains a long inverted repeat of almost perfect homology. The 5' flanking region contains a TATA- and a CAAT-box at positions -33 and -58, respectively. An additional TATA-box is located at -197 and two more CAAT-boxes occur at positions -105 and -237.
Full text
PDF


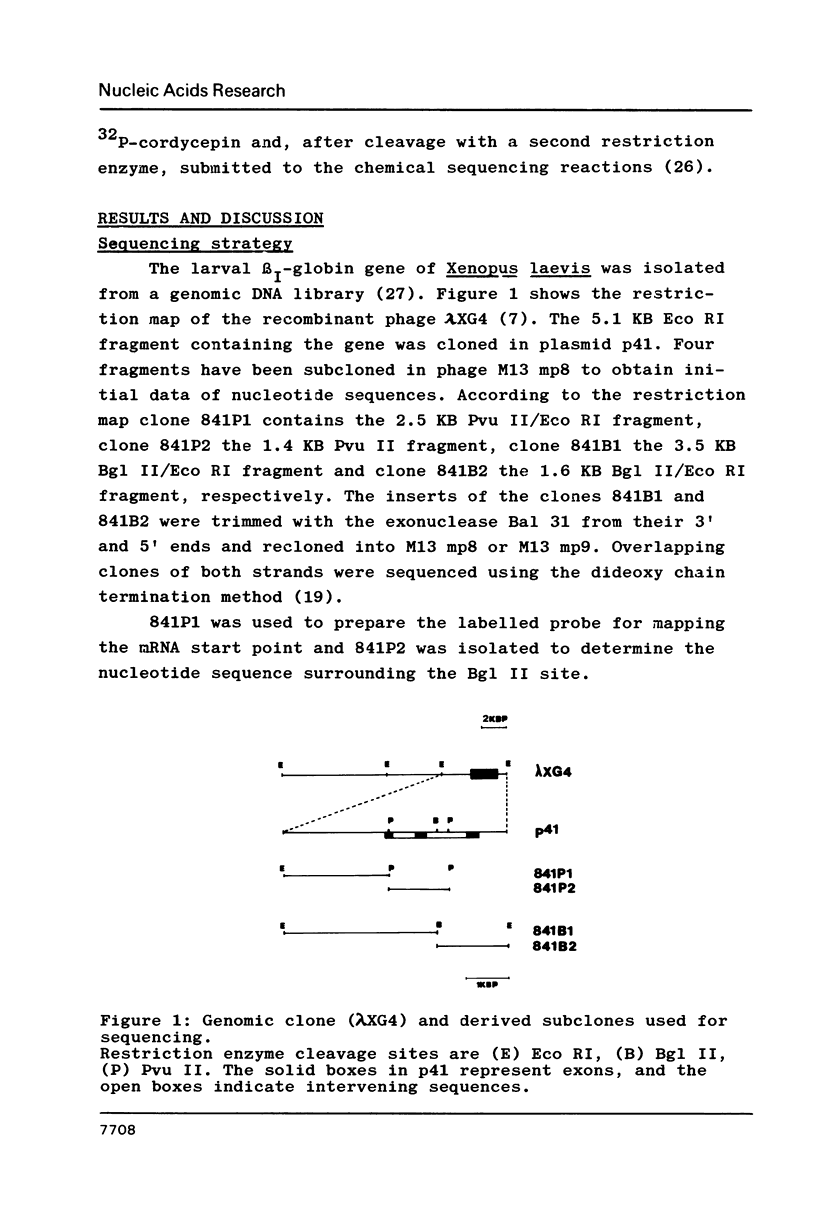










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman E. J. Molecular cloning and sequencing of OAX DNA: an abundant gene family transcribed and activated in Xenopus oocytes. EMBO J. 1983;2(8):1417–1422. doi: 10.1002/j.1460-2075.1983.tb01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alan M., Grindlay G. J., Stefani L., Paul J. Epsilon globin gene transcripts originating upstream of the mRNA cap site in K562 cells and normal human embryos. Nucleic Acids Res. 1982 Sep 11;10(17):5133–5147. doi: 10.1093/nar/10.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Andres A. C., Hosbach H. A., Weber R. Comparative analysis of the cDNA sequences derived from the larval and the adult alpha 1-globin mRNAs of Xenopus laevis. Biochim Biophys Acta. 1984 Apr 5;781(3):294–301. doi: 10.1016/0167-4781(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banville D., Kay R. M., Harris R., Williams J. G. The nucleotide sequence of the mRNA encoding a tadpole beta-globin polypeptide of Xenopus laevis. J Biol Chem. 1983 Jul 10;258(13):7924–7927. [PubMed] [Google Scholar]
- Baralle F. E., Brownlee G. G. AUG is the only recognisable signal sequence in the 5' non-coding regions of eukaryotic mRNA. Nature. 1978 Jul 6;274(5666):84–87. doi: 10.1038/274084a0. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Degrave W., Derynck R., Tavernier J., Haegeman G., Fiers W. Nucleotide sequence of the chromosomal gene for human fibroblast (beta 1) interferon and of the flanking regions. Gene. 1981 Aug;14(3):137–143. doi: 10.1016/0378-1119(81)90109-8. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
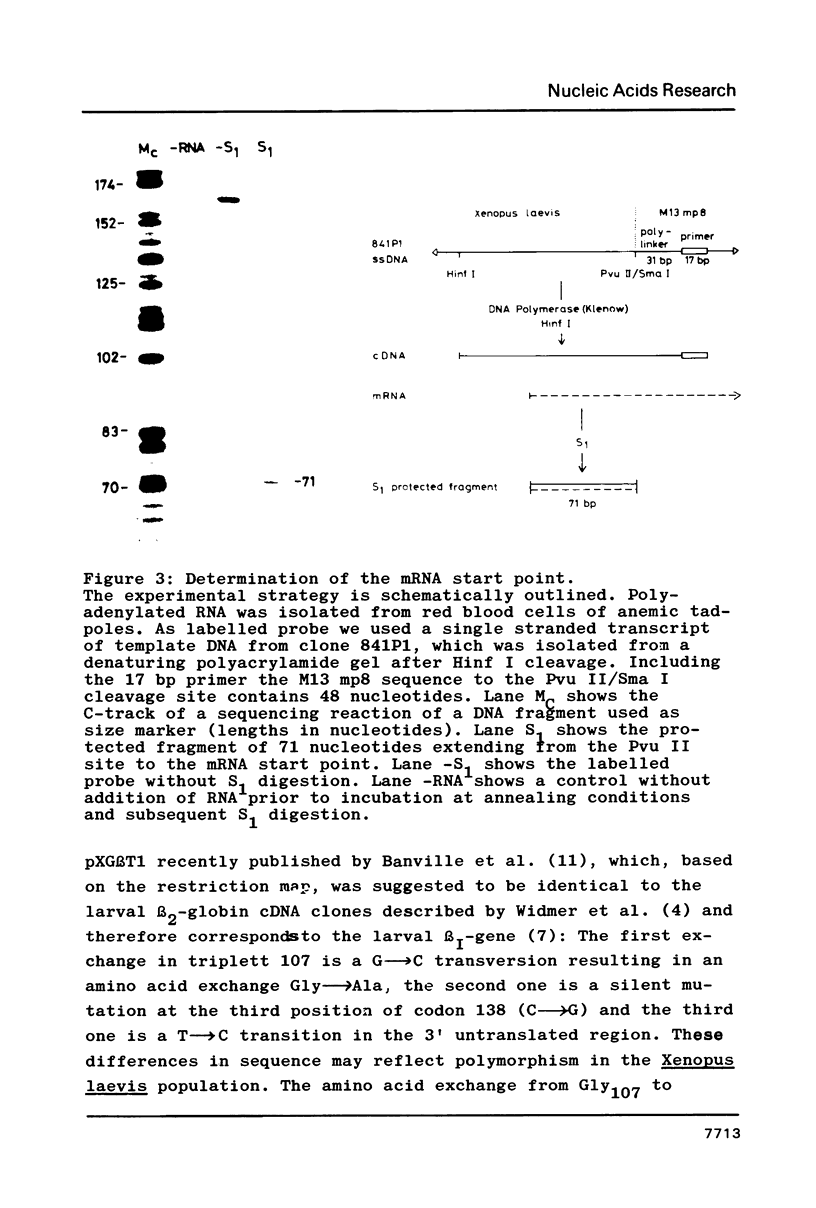
- Dodgson J. B., Stadt S. J., Choi O. R., Dolan M., Fischer H. D., Engel J. D. The nucleotide sequence of the embryonic chicken beta-type globin genes. J Biol Chem. 1983 Oct 25;258(20):12685–12692. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Rusling D. J., McCune K. C., Dodgson J. B. Unusual structure of the chicken embryonic alpha-globin gene, pi'. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1392–1396. doi: 10.1073/pnas.80.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Grindlay G. J., Lanyon W. G., Allan M., Paul J. Alternative sites of transcription initiation upstream of the canonical cap site in human gamma-globin and beta-globin genes. Nucleic Acids Res. 1984 Feb 24;12(4):1811–1820. doi: 10.1093/nar/12.4.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hardison R. C. The nucleotide sequence of the rabbit embryonic globin gene beta 4. J Biol Chem. 1983 Jul 25;258(14):8739–8744. [PubMed] [Google Scholar]
- Hentschel C. C., Kay R. M., Williams J. G. Analysis of Xenopus laevis globins during development and erythroid cell maturation and the construction of recombinant plasmids containing sequences derived from adult globin mRNA. Dev Biol. 1979 Oct;72(2):350–363. doi: 10.1016/0012-1606(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Hosbach H. A., Wyler T., Weber R. The Xenopus laevis globin gene family: chromosomal arrangement and gene structure. Cell. 1983 Jan;32(1):45–53. doi: 10.1016/0092-8674(83)90495-6. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Barrie P. A., Harris S., Fawcett D. H., Nugent Z. J., Boyd A. C. Isolation and sequence analysis of a hybrid delta-globin pseudogene from the brown lemur. J Mol Biol. 1982 Apr 15;156(3):487–503. doi: 10.1016/0022-2836(82)90262-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Wood D., Simons J. P., Kay R. M., Williams J. G. Linkage of adult alpha- and beta-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980 Sep;21(2):555–564. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kay R. M., Harris R., Patient R. K., Williams J. G. Complete nucleotide sequence of a cloned cDNA derived from the major adult alpha-globin mRNA of X. laevis. Nucleic Acids Res. 1983 Mar 11;11(5):1537–1542. doi: 10.1093/nar/11.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel W., Meyerhof W., Hummel S., Grundmann U. Molecular cloning and sequencing of mRNAs coding for minor adult globin polypeptides of Xenopus laevis. Nucleic Acids Res. 1983 Mar 11;11(5):1543–1553. doi: 10.1093/nar/11.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy E., Maniatis T. The nucleotide sequence of a rabbit beta-globin pseudogene. Cell. 1980 Sep;21(2):545–553. doi: 10.1016/0092-8674(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Gross M., Houck C. M., Franke A. E., Gray P. V., Goeddel D. V. DNA sequence of a major human leukocyte interferon gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5435–5439. doi: 10.1073/pnas.78.9.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- May F. E., Weber R., Westley B. R. Isolation and characterisation of the Xenopus laevis albumin genes: loss of 74K albumin gene sequences by library amplification. Nucleic Acids Res. 1982 May 11;10(9):2791–2807. doi: 10.1093/nar/10.9.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Partington G. A., Baralle F. E. Isolation of a Xenopus laevis alpha-globin gene. J Mol Biol. 1981 Jan 15;145(2):463–470. doi: 10.1016/0022-2836(81)90216-3. [DOI] [PubMed] [Google Scholar]
- Patient R. K., Banville D., Brewer A. C., Elkington J. A., Greaves D. R., Lloyd M. M., Williams J. G. The organization of the tadpole and adult alpha globin genes of Xenopus laevis. Nucleic Acids Res. 1982 Dec 20;10(24):7935–7945. doi: 10.1093/nar/10.24.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient R. K., Harris R., Walmsley M. E., Williams J. G. The complete nucleotide sequence of the major adult beta globin gene of Xenopus laevis. J Biol Chem. 1983 Jul 25;258(14):8521–8523. [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Cleary M. L., Haynes J. R., Lingrel J. B. Structure and evolution of goat gamma-, beta C- and beta A-globin genes: three developmentally regulated genes contain inserted elements. Cell. 1981 Dec;27(2 Pt 1):359–369. doi: 10.1016/0092-8674(81)90419-0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M. Complete nucleotide sequence of the human delta-globin gene. Cell. 1980 Oct;21(3):639–646. doi: 10.1016/0092-8674(80)90427-4. [DOI] [PubMed] [Google Scholar]
- Taya Y., Devos R., Tavernier J., Cheroutre H., Engler G., Fiers W. Cloning and structure of the human immune interferon-gamma chromosomal gene. EMBO J. 1982;1(8):953–958. doi: 10.1002/j.1460-2075.1982.tb01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H. J., Andres A. C., Niessing J., Hosbach H. A., Weber R. Comparative analysis of cloned larval and adult globin cDNA sequences of Xenopus laevis. Dev Biol. 1981 Dec;88(2):325–332. doi: 10.1016/0012-1606(81)90176-7. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kay R. M., Patient R. K. The nucleotide sequence of the major beta-globin mRNA from Xenopus laevis. Nucleic Acids Res. 1980 Sep 25;8(18):4247–4258. doi: 10.1093/nar/8.18.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]



