Abstract
The Keap1-Nrf2 system serves as a defense mechanism against oxidative stress and electrophilic toxicants by inducing more than one hundred cytoprotective proteins, including antioxidants and phase 2 detoxifying enzymes. Since induction profiles of Nrf2 target genes have been studied exclusively in cultured cells, and not in animal models, their tissue-specificity has not been well characterized. In this paper, we examined and compared the tissue-specific expression of several Nrf2 target genes in zebrafish larvae by whole-mount in situ hybridization (WISH). Seven zebrafish genes (gstp1, mgst3b, prdx1, frrs1c, fthl, gclc and hmox1a) suitable for WISH analysis were selected from candidates for Nrf2 targets identified by microarray analysis. Tissue-restricted induction was observed in the nose, gill, and/or liver for all seven genes in response to Nrf2-activating compounds, diethylmaleate (DEM) and sulforaphane. The Nrf2 gene itself was dominantly expressed in these three tissues, implying that tissue-restricted induction of Nrf2 target genes is defined by tissue-specific expression of Nrf2. Interestingly, the induction of frrs1c and gclc in liver and nose, respectively, was quite low and that of hmox1a was restricted in the liver. These results indicate the existence of gene-specific variations in the tissue specificity, which can be controlled by factors other than Nrf2.
Introduction
Nrf2 is a transcription factor that binds to the antioxidant response element (ARE) and transactivates cytoprotective genes [1], [2]. In basal conditions, Nrf2 is degraded via the Keap1-dependent proteasome pathway, while it is stabilized after cells are exposed to electrophilic or oxidative stress, which transactivates its target genes. Many studies have identified the Keap1-Nrf2 system to have multiple sensor sites to a variety of stresses [3], [4] and more than one hundred target genes [5], [6]. Conservation of the Keap1-Nrf2 system has been demonstrated in vertebrates including zebrafish [7], [8], which is a well-established research model.
Through previous studies, we noticed that the expression of gstp1, a major target gene for zebrafish Nrf2, is not ubiquitous as expected, but is rather restricted in the nose, gill, and liver [8]–[13]. It is unclear whether this tissue-restricted induction is specific for gstp1 or a common feature for Nrf2 target genes, since gstp1 was the only gene available in our study that was suitable for WISH analysis. In addition, most studies related to Nrf2 target genes have been performed on cultured cells or a specific murine tissue, and therefore the tissue specificity of Nrf2 target genes has not yet been well characterized, even in other animals. In this paper, we identified seven zebrafish genes appropriate for WISH using microarray analysis and examined their tissue-specific expression in zebrafish larvae. Induced expression of all these genes was restricted to the nose, gill, and/or liver with gene-specific variations in tissue specificity. These results indicate tissue-restricted induction to therefore be a common feature of Nrf2 target genes, which can be a critical issue for both the pharmacological and clinical applications of Nrf2-activating compounds.
Materials and Methods
RT-PCR and WISH analyses
Zebrafish embryos and larvae were obtained by natural mating. All experiments were carried out using a wild-type AB strain. For induction studies, embryos or larvae were placed in culture dishes containing 100 µM DEM (Wako, Osaka, Japan) or 40 µM sulforaphane (LKT laboratories, St. Paul, MN). RT-PCR analysis was performed as described previously [8] using PCR primers listed in Table S1. WISH analysis was carried out as described previously [14] with slight modifications in the fixation step. Briefly, the larvae were fixed with 4% paraformaldehyde (PFA) in PBS overnight at 4°C, and washed twice in PBS, once in 50% methanol, and twice in 100% methanol, and cooled to –20°C for at least 3 hours. Fixed larvae were then brought back to room temperature (RT), washed twice in PBT (0.1% Tween 20/PBS) for 5 minutes, and immersed for 2 hours in 9% hydrogen peroxide in PBT. After immersion, the larvae were washed twice in PBTw (0.2% bovine serum albumin in PBT), treated for 20 minutes with 50 µg/ml proteinase K (Sigma-Aldrich, St. Louis, MO), and fixed with 4% PFA/PBS for 20 minutes at RT.
Microarray analysis
A microarray analysis was performed using custom-made 16 K MZH chips. The MZH chips contained in a total of 16399 probes of a 65 oligonucleotides in length purchased from Sigma-Aldrich. The collected zebrafish embryos were quickly homogenized with 1 ml of QIAzol reagent (Qiagen, Hilden, Germany), and subsequently stored at -80°C. Total RNA was extracted according to the manufacturer’s instructions. Isolated total RNA was then further purified using the RNAeasy mini kit (Qiagen). Amino-allyl-modified amplified RNA was synthesized in one amplification round from 1 µg of purified total RNA using the amino-allyl RNA amplification kit (Sigma-Aldrich). Subsequently, 5 µg of amino-allyl-modified amplified RNA was used for coupling of monoreactive Cy3 and Cy5dyes (GE Healthcare, Little Chalfont, UK) and column purified. Dual-color hybridization of the MZH chips was performed according to the manufacturer’s instructions for the AceGene DNA microarray (Hitachi Solutions, Tokyo, Japan). Each experiment was repeated in triplicate. After hybridization, MZH chips were scanned using the Affymetrix 428 array scanner (Affymetrix, Santa Clara, CA). The microarray data were processed from raw data image files with Affymetrix Jaguar (Affymetrix) and normalized. The processed data were subsequently imported into Excel (Microsoft, Redmond, WA) to compare expression profiles of DEM-treated samples to control samples. The cut-offs for significance regarding the ratios of DEM-treated samples vs control samples were set at a 2-fold change.
We have deposited the raw data at GEO under accession numbers (GSM799460, GSM799461, GSM799462, GSM799463), and we can confirm all details are MIAME compliant.
Plasmid construction
cDNA clones were prepared of the following transcripts by RT-PCR using total RNA from 5 days post-fertilization (dpf) zebrafish larvae: gstal, zgc:158387 (mgst3b), sepw2b, bcat1, zgc:110343 (prdx1), zgc:163022 (frrs1c), zgc:92066 (fthl), gclc, gclm, and hmox1 (hmox1a). Specific primers were designed based on cDNA information (http://zfin.org), and cDNA products were subcloned into the pBluescript II SK vector (Table S2). Plasmids pCS2nrf2, pCS2FLmKeap1, and pKSgstp1N have been described previously [8], [9], [12].
Knockdown and overexpression analyses of Nrf2
Synthetic capped nrf2 RNA was made with an SP6 mMESSAGE mMACHINE in vitro transcription kit (Ambion, Austin, TX) using pCS2nrf2. The Nrf2-morpholino oligonucleotide (nrf2MO) has been described previously [9]. mRNA or morpholino oligonucleotides were injected into yolk of the zebrafish at the one-cell stage using an IM300 microinjector (Narishige, Tokyo, Japan).
Sectioning of zebrafish larvae
After carrying out WISH analysis, larvae were fixed with 4% PFA/PBS, embedded in 1.5% SeaPlaque GTG agarose (Takara Bio, Osaka, Japan), and dehydrated through graded ethanol series (30%, 50%, 90% and twice 100%) in PBS for 15 minutes each. Glycol methacrylate (Technovit 8100; Heraeus Kulzer, Wehrheim, Germany), with low-temperature polymerization, was used according to the manufacturer's instructions. After embedding, 6-µm serial sections were made from whole bodies of zebrafish larvae with an RM 2045 microtome (Leica, Wetzler, Germany).
Results
Identification of Nrf2 target genes in zebrafish
In order to investigate tissue-specific expression of Nrf2 target genes, we searched for zebrafish Nrf2 targets other than gstp1 that were able to be used for WISH analysis. Microarray analysis was carried out using cDNA prepared from 4-dpf larvae treated with or without 100 µM DEM for 12 hours. In total, 16,000 zebrafish cDNAs were screened and 42 genes were identified that showed more than a 2-fold induction compared with DEM-treated larvae and untreated larvae (Table S3). The reliability of this screen was demonstrated by the fact that gstp1 produced a top ranking score in this analysis. In microarray analysis, gclm and nqo1 were induced by DEM at levels of only 1.97- and 1.93-fold, respectively. Since they have generally been used as Nrf2 target genes in mammalian cells, we selected them together with gclc and hmox1, for further analysis, in addition to 42 identified genes (Table S4).
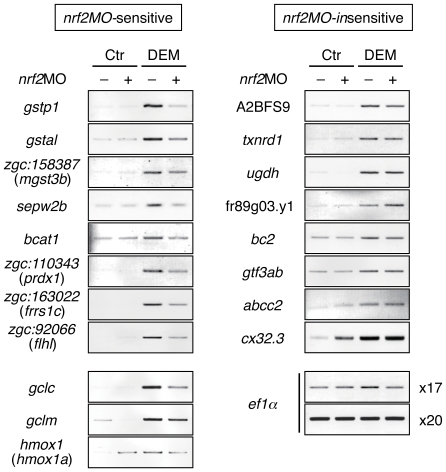
We next carried out RT-PCR analysis to confirm the results of microarray analysis. RT-PCR analysis was performed using RNA isolated from 4-dpf larvae treated with DEM and isolated RNA (Figure 1 and Table S3). Thirty-three genes were analyzed out of 46 selected genes, and 19 genes were identified to be induced by DEM. To clarify whether these inductions were directed by Nrf2, we carried out RT-PCR analysis using Nrf2-knockdown larvae using nrf2-specific morpholino oligonucleotide. As a result, the induction of eleven genes was clearly demonstrated to be Nrf2-dependent (Figure 1).
Figure 1. Screening of Nrf2 target genes in zebrafish.
DEM-induced expression of candidate genes for Nrf2 target analyzed by RT-PCR. Embryos were injected with or without nrf2MO and treated with or without 100 µM DEM for six hours at 2 hours post-fertilization (hpf) using total RNA from the whole bodies.
We constructed phylogenetic trees of the 11 identified genes, with the exception of gstp1, using the zebrafish genome and cDNA information; we also renamed some genes (Figures S1–S10). Finally, the genes were identified as gstal (glutathione S-transferase alpha like), mgst3b (microsomal glutathione S-transferase 3b), sepw2b (selenoprotein W2b), bcat1 (branched chain aminotransferase 1), prdx1 (peroxiredoxin 1), frrs1c (ferric-chelate reductase 1c), fthl (ferritin heavy chain like), gclc (glutamate-cysteine ligase catalytic subunit), gclm (glutamate-cysteine ligase modifier subunit) and hmox1a (heme oxygenase 1a). Gsta, Prdx1, Fth, Gclc, Gclm and Hmox1 have been identified as typical Nrf2 target genes in mammalian cells [15]–[19]. Mgst3 has also been suggested to be an Nrf2 target from several microarray studies [20]–[22]. These results indicated that target genes of Nrf2 are conserved among vertebrates. More interestingly, SepW2, Bcat1, and Frrs1 have never been indentified as Nrf2 targets, including from microarray analyses. Among these three, two are redox-regulating proteins which are possible candidates for Nrf2 targets: SepW2 is a member of the selenoprotein family, which has been shown to possess anti-oxidative stress activity [23], and Frrs1 is an iron-metabolizing enzyme that reduces ferric ion [24]. Bcat1 is a metabolizing enzyme for branched-chain amino acids and plays important roles in ammonia metabolism [25].
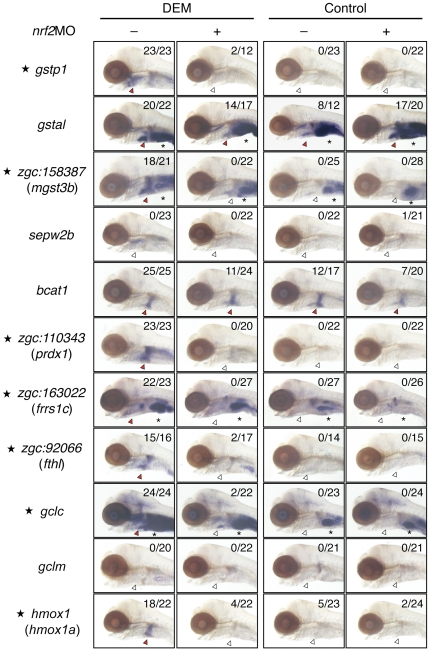
A WISH analysis was carried out using eleven genes as probes (Figure 2). Among them, seven genes (gstp1, mgst3b, prdx1, frrs1c, fthl, gclc and hmox1a) showed clear and strong induction in response to DEM, suggesting they will be useful for studying tissue specificity of Nrf2 target genes. The remaining four genes showed either weak induction (sepw2b and gclm) or strong constitutive expression (gstal and bcat1), thus indicating that they were unsuited for gene expression studies. We, therefore, used the seven genes showing a strong induction for further analyses.
Figure 2. WISH analysis of Nrf2 target genes.
Expression of eleven Nrf2 target genes was analyzed by WISH. Embryos were injected with or without nrf2MO and treated with or without 100 µM DEM for six hours (three hours only for hmox1a) at 5 dpf. Lateral views. Numbers indicate the induction positive embryos/tested embryos. Red and white arrowheads indicate positive and negative expression, respectively. Asterisks denote basal expression in the intestine.
Tissue-restricted induction of Nrf2 target genes
Tissue-restricted induction of seven Nrf2 target genes was examined by WISH (Figures 3 and S11), using 5-dpf larvae, since the induction was much clearer compared with that in 4-dpf larvae. As a result, the induction of mgst3b, prdx1, frrs1c, fthl, and gclc was observed in nose, gill, and liver, similar to gstp1, although the expression of frrs1c and gclc in the liver and nose, respectively, was relatively weak. Induction in the intestine was observed in the case of mgst3b, prdx1, frrs1c and gclc, but we did not take them into account since a considerable level of basal expression was detected. Interestingly, hmox1a was only induced in the liver, suggesting the existence of gene-specific differences in tissue specificity. Expression profiles of hmox1a and frrs1c in the liver were confirmed by section analysis in comparison with a liver-specific marker fabp10 (Figure S11) [26]. These results indicate that tissue-restricted induction is a common characteristic among Nrf2 target genes, not only for gstp1.
Figure 3. Tissue-restricted induction of Nrf2 target genes.
5-dpf larvae were treated with or without 100 µM DEM for six hours (three hours only for hmox1a) and expression of indicated genes was analyzed by WISH. Lateral and ventral views. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the nose, gill and liver. Asterisks denote basal expression in the intestine.
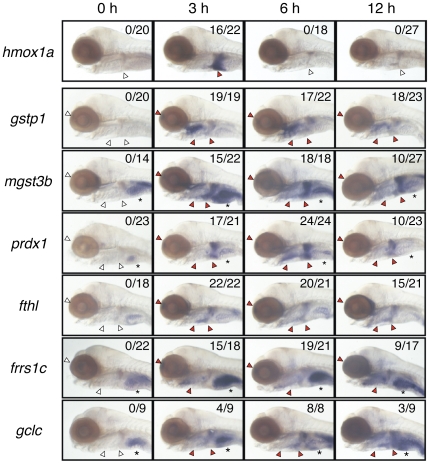
The induction time profiles of seven genes were also analyzed in detail (Figure 4). All genes showed induction beginning three hours after DEM treatment. Interestingly, expression of hmox1a was rapidly reduced beginning six hours after DEM treatment to almost the same level as the non-induced condition, whereas expression levels of the remaining six genes were maintained until twelve hours after treatment. A negative feedback regulation may exist in the case of hmox1a.
Figure 4. Induction time profiles of Nrf2 target genes.
5-dpf larvae were treated with 100 µM DEM for indicated hours and expression of seven genes was analyzed by WISH. Lateral views. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the nose, gill and liver. Asterisks denote the basal expression in the intestine.
To determine whether tissue specificity and induction time profiles of Nrf2 target genes vary according to differences of Nrf2-activating compounds, expression profiles of seven genes by sulforaphane were analyzed (Figures S12). Induction profiles of all seven genes were basically identical to those in the case of DEM, suggesting that tissue-restricted induction of Nrf2 target genes is intrinsic properties of each gene.
Gene regulation by Nrf2
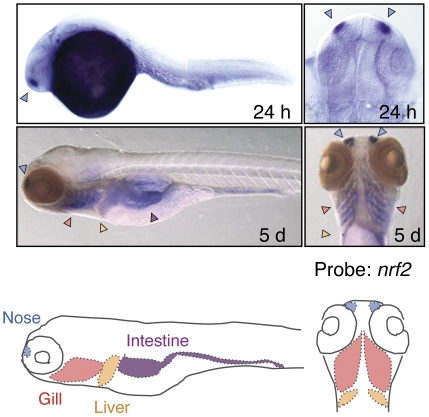
Considering that all genes tested showed restricted-expression in the nose, gill, liver, and intestine, gene expression in these four tissues seemed to be a default state for Nrf2 target genes. This may suggest that Nrf2 or its activator dominantly exists in these tissues and directly transactivates the target genes. To test this hypothesis, we analyzed the expression of nrf2 in 5-dpf larvae by WISH (Figure 5). As expected, nrf2 is specifically expressed in the nose, gill, liver, and intestine, suggesting that tissue-restricted induction of Nrf2 target genes is based on the tissue-specific expression of nrf2 mRNA.
Figure 5. Tissue-specific expression of the Nrf2 gene.
Expression of Nrf2 gene in 24-hpf embryos and 5-dpf larvae were analyzed by WISH. Lateral (upper left, lower left), dorsal (upper right), and ventral (lower right) views. Arrowheads in light blue, red and orange indicate expression in the nose, gill and liver.
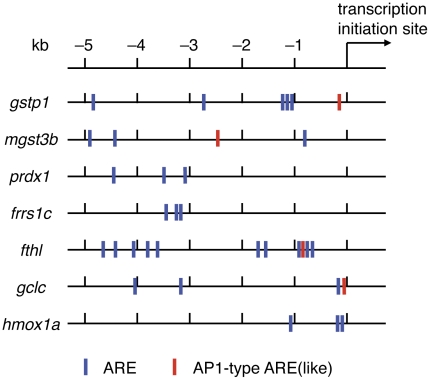
We previously demonstrated that the expression of gstp1 and gclc (γgcsh) to be strongly induced when Nrf2 is overexpressed in zebrafish embryos [9]. We next investigated whether the other five Nrf2 targets can also be induced by Nrf2 overexpression. Nrf2 was overexpressed by injecting nrf2 mRNA into one-cell-stage embryos, and the expression of Nrf2 target genes was analyzed by RT-PCR seven hours after injection (Figure 6). The results indicate that all seven genes were induced by Nrf2 overexpression. Using the zebrafish genome database (http://www.ensembl.org/Danio_rerio/Info/Index), we searched the ARE sequences in the 5-kb upstream regions of the deduced transcription initiation sites in these genes (Figure 7). All genes were found to have more than three ARE sites in the 5-kb regions, to which Nrf2 may bind and regulate. All of these results suggest that induction of these seven genes is directly regulated by Nrf2.
Figure 6. Target gene induction in zebrafish embryos by Nrf2 overexpression.
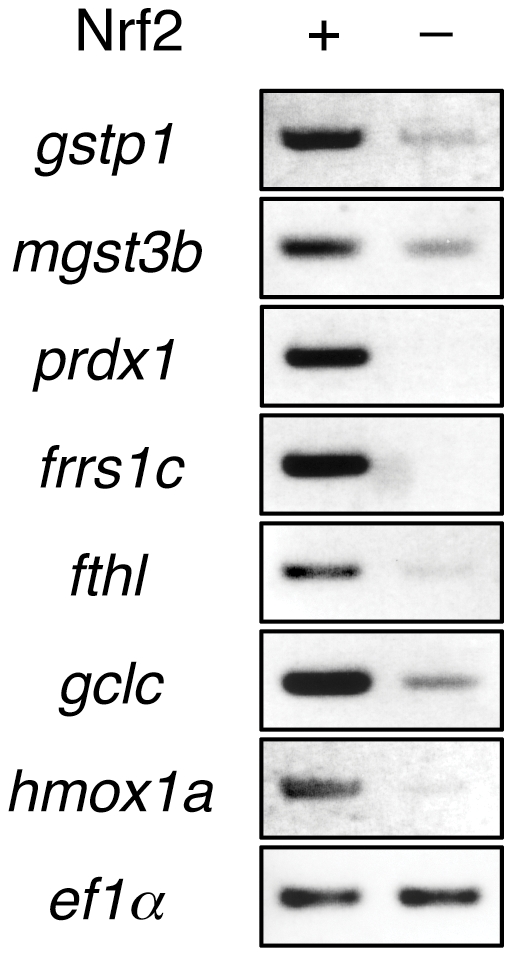
RT-PCR analysis using total RNA from the whole bodies of 30 embryos and specific primers of indicated genes.
Figure 7. ARE sequences in upstream regions of Nrf2 target genes.
ARE sequences (TGAG/CNNNGC) and AP1-type ARE (like) (TGAG/CTCAGN or TGAG/CTCANC) in the 5-kb region of deduced transcription initiation sites of indicated genes were searched using the zebrafish genome database.
Discussion
The zebrafish is a good system to observe the tissue-specific expression of many genes, including gene induction, as described in this study. Indeed, tissue-specific induction in systems other than Keap1-Nrf2, such as the heat-shock genes, has been reported from studies in zebrafish [27], [28]. These observations would be difficult to analyze using cultured cell lines, demonstrating a significant advantage for zebrafish system. Zebrafish is also good for drug toxicity screening and testing environmental toxicants [29], [30]. Since Nrf2 is activated by a variety of toxic compounds and oxidative stress, it would be worthwhile for these screens and tests to analyze tissue-restricted induction of Nrf2 target genes. The seven target genes of Nrf2 selected in this paper would be useful for such studies.
In our analysis, all tested genes showed tissue-restricted induction. Furthermore, we found gene-specific variation in tissue specificity, e.g., a weak induction of frrs1c and gclc in the liver and nose, respectively, and no hmox1a induction in the nose and gill. The requirements of metabolizing enzymes encoded by each target gene may be different among tissues and the level of enzymes are controlled at a transcriptional level. An important finding in this study is that the expression level of Nrf2 itself is different among tissues, which will be the critical point to exert tissue-restricted induction of its target genes. We hypothesized that the gene expression profiles of the Nrf2 gene defines a default state of tissue specificity of target genes. In cases of frrs1c, gclc and hmox1a, some tissue- and gene-specific transcriptional repressors may be involved to exert their tissue-specific variations. Nrf2-dependent ARE gene regulation has been shown to be negatively regulated by other ARE-binding proteins such as Nrf1, Nrf3, Bach1, small Maf proteins, and c-Maf [31]–[35]. Since we have previously demonstrated that the target genes of Nrf-Maf proteins tend to differ somewhat among family proteins [36], [37], it is possible that these ARE-acting factors may bind in a gene-specific manner and interfere with DNA binding of Nrf2. Furthermore, ATF3, c-Myc and p53 have also been reported to repress Nrf2-dependent gene activation [38]–[40]. Some of these transcription factors may bind near the ARE in a gene- and tissue-specific manner and inhibit the transcription activity of Nrf2.
In contrast to Nrf2 regulation at a post translational step, molecular mechanisms of Nrf2 gene regulation have not been extensively studied. The exception is the upregulation of the Nrf2 gene by Nrf2 itself and aryl hydrocarbon receptor in response to their chemical activators [41], [42]. We previously reported that the embryonic expression of the Nrf2 gene is quite low both in the mouse and zebrafish and it becomes elevated near birth [12], [43]. The downregulation of Nrf2 was found in prostate cancer which may be related to the initiation of cellular transformation [44]. A low Nrf2 expression in the brain has been reported in humans, mice, and chickens [45]–[47], as was also the case in our present study. For the efficient medical applications of the Keap1-Nrf2 system for the treatment of neurodegenerative diseases [48], [49], Nrf2 should be considerably expressed in the brain. However, we found the expression of Nrf2 in the brain to be low. It will thus be valuable to find new methods and procedures to elevate the Nrf2 expression in both brain and prostate cancer cells.
Supporting Information
Phylogenetic tree of Gsta family proteins. Amino acid sequences of full-length proteins were analyzed. The tree was constructed by the neighbor-joining method using the ClustalW program (http://clustalw.ddbj.nig.ac.jp/top-j.html). c, chicken; h, human; m, mouse; r, rat; xt, Xenopus tropicalis; z, zebrafish.
(TIF)
Phylogenetic tree of Mgst3 family proteins. Amino acid sequences of full-length proteins were analyzed. ci, Ciona intestinalis; x, Xenopus laevis.
(TIF)
Phylogenetic tree of SepW family proteins. Amino acid sequences of full-length proteins were analyzed. d, Drosophila melanogaster.
(TIF)
Phylogenetic tree of Bcat family proteins. Amino acid sequences of full-length proteins were analyzed. ce, Caenorhabditis elegans.
(TIF)
Phylogenetic tree of Prdx family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Frrs family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Fth family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Gclc family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Gclm family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Hmox family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Expression of frrs1c and hmox1c in the liver. Transverse sections of 5-dpf larvae through the trunk at the level of the liver (dotted line). Larvae were treated with (frrs1c, hmox1a) or without (fabp10) 100 µm DEM and analyzed by WISH before sectioning. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the liver. Asterisk denotes the basal expression in the intestine. Scale bar, 100 µm.
(TIF)
Induction of Nrf2 target genes by sulforaphane. 5-dpf larvae were treated with or without 40 µM sulforaphane for indicated hours and expression of seven Nrf2 target genes was analyzed by WISH. Lateral and ventral views. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the nose, gill and liver. Asterisks denote basal expression in the intestine.
(TIF)
Oligonucleotide primers used for RT-PCR analyses.
(DOC)
Oligonucleotide primers used for plasmid construction.
(DOC)
Identification of DEM-inducible genes in zebrafish (1).
(DOC)
Identification of DEM-inducible genes in zebrafish (2).
(DOC)
Acknowledgments
We thank Y. Kikuchi (Hiroshima University) for the fabp10 probe, S. Hosoyamada, T. Kinoshita, H. Niu, T. Shimokoube, R. Sakurazawa and N. Tsuruta for help in fish maintenance, and L. Li for discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (MK, MY) and the Japan Science and Technology Corporation (ERATO) (MY). Zebrafish microarrays were supported by Consortium R&D Project for Regional Revitalization from the Ministry of Economy, Trade and Industry of Japan (YT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Holland R, Fishbein JC. Chemistry of the cysteine sensors in Kelch-like ECH-associated protein 1. Antioxid Redox Signal. 2010;13:1749–1761. doi: 10.1089/ars.2010.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 6.Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, et al. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Takagi Y, Kobayashi M, Li L, Suzuki T, Nishikawa K, et al. MafT, a new member of the small Maf protein family in zebrafish. Biochem Biophys Res Commun. 2004;320:62–69. doi: 10.1016/j.bbrc.2004.05.131. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Kobayashi M, Kaneko H, Nakajima-Takagi Y, Nakayama Y, et al. Molecular Evolution of Keap1: Two Keap1 molecules with distinctive intervening region structures are conserved among fish. J Biol Chem. 2008;283:3248–3255. doi: 10.1074/jbc.M708702200. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, et al. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, et al. Pi-class glutathione S-transferase genes are regulated by Nrf2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J. 2005;388:65–73. doi: 10.1042/BJ20041860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 15.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 16.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, et al. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 17.Wild AC, Moinova HR, Mulcahy RT. Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 18.Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278:2361–2369. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- 19.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, et al. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 20.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 21.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 22.Whanger PD. Selenoprotein expression and function-selenoprotein W. Biochim Biophys Acta. 2009;1790:1448–1452. doi: 10.1016/j.bbagen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Vargas JD, Herpers B, McKie AT, Gledhill S, McDonnell J, et al. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim Biophys Acta. 2003;1651:116–123. doi: 10.1016/s1570-9639(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 24.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 25.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 27.Kelly VP, Suzuki T, Nakajima O, Arai T, Tamai Y, et al. The distal sequence element of the selenocysteine tRNA gene is a tissue-dependent enhancer essential for mouse embryogenesis. Mol Cell Biol. 2005;25:3658–3669. doi: 10.1128/MCB.25.9.3658-3669.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Kwok AM, Chan JY. The p65 Isoform of Nrf1 Is a Dominant Negative Inhibitor of ARE-mediated Transcription. J Biol Chem. 2007;282:24670–24678. doi: 10.1074/jbc.M700159200. [DOI] [PubMed] [Google Scholar]
- 34.Sankaranarayanan K, Jaiswal AK. Nrf3 negatively regulates antioxidant-response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. J Biol Chem. 2004;279:50810–50817. doi: 10.1074/jbc.M404984200. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhakshinamoorthy S, Jaiswal AK. Small maf (MafG and MafK) proteins negatively regulate antioxidant response element-mediated expression and antioxidant induction of the NAD(P)H:Quinone oxidoreductase1 gene. J Biol Chem. 2000;275:40134–40141. doi: 10.1074/jbc.M003531200. [DOI] [PubMed] [Google Scholar]
- 37.Dhakshinamoorthy S, Jaiswal AK. c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction. Oncogene. 2002;21:5301–5312. doi: 10.1038/sj.onc.1205642. [DOI] [PubMed] [Google Scholar]
- 38.Kimura M, Yamamoto T, Zhang J, Itoh K, Kyo M, et al. Molecular basis distinguishing the DNA binding profile of Nrf2-Maf heterodimer from that of Maf homodimer. J Biol Chem. 2007;282:33681–33690. doi: 10.1074/jbc.M706863200. [DOI] [PubMed] [Google Scholar]
- 39.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, et al. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown SL, Sekhar KR, Rachakonda G, Sasi S, Freeman ML. Activating transcription factor 3 is a novel repressor of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)-regulated stress pathway. Cancer Res. 2008;68:364–368. doi: 10.1158/0008-5472.CAN-07-2170. [DOI] [PubMed] [Google Scholar]
- 41.Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 42.Levy S, Forman HJ. c-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Kemadjou JR, Zinsmeister C, Bauer M, Legradi J, et al. Transcriptional profiling reveals barcode-like toxicogenomic responses in the zebrafish embryo. Genome Biol. 2007;8:R227. doi: 10.1186/gb-2007-8-10-r227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marvin M, O'Rourke D, Kurihara T, Juliano CE, Harrison KL, et al. Developmental expression patterns of the zebrafish small heat shock proteins. Dev Dyn. 2008;237:454–463. doi: 10.1002/dvdy.21414. [DOI] [PubMed] [Google Scholar]
- 45.Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol. 2006;2:231–240. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- 46.Scholz S, Fischer S, Gundel U, Kuster E, Luckenbach T, et al. The zebrafish embryo model in environmental risk assessment–applications beyond acute toxicity testing. Environ Sci Pollut Res Int. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- 47.Sharma MK, Liu RZ, Thisse C, Thisse B, Denovan-Wright EM, et al. Hierarchical subfunctionalization of fabp1a, fabp1b and fabp10 tissue-specific expression may account for retention of these duplicated genes in the zebrafish (Danio rerio) genome. FEBS J. 2006;273:3216–3229. doi: 10.1111/j.1742-4658.2006.05330.x. [DOI] [PubMed] [Google Scholar]
- 48.Sekhar KR, Rachakonda G, Freeman ML. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol Appl Pharmacol. 2010;244:21–26. doi: 10.1016/j.taap.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M. Harnessing the antioxidant power with ARE-inducing compounds. Chem Biol. 2010;17:419–420. doi: 10.1016/j.chembiol.2010.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of Gsta family proteins. Amino acid sequences of full-length proteins were analyzed. The tree was constructed by the neighbor-joining method using the ClustalW program (http://clustalw.ddbj.nig.ac.jp/top-j.html). c, chicken; h, human; m, mouse; r, rat; xt, Xenopus tropicalis; z, zebrafish.
(TIF)
Phylogenetic tree of Mgst3 family proteins. Amino acid sequences of full-length proteins were analyzed. ci, Ciona intestinalis; x, Xenopus laevis.
(TIF)
Phylogenetic tree of SepW family proteins. Amino acid sequences of full-length proteins were analyzed. d, Drosophila melanogaster.
(TIF)
Phylogenetic tree of Bcat family proteins. Amino acid sequences of full-length proteins were analyzed. ce, Caenorhabditis elegans.
(TIF)
Phylogenetic tree of Prdx family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Frrs family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Fth family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Gclc family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Gclm family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Phylogenetic tree of Hmox family proteins. Amino acid sequences of full-length proteins were analyzed.
(TIF)
Expression of frrs1c and hmox1c in the liver. Transverse sections of 5-dpf larvae through the trunk at the level of the liver (dotted line). Larvae were treated with (frrs1c, hmox1a) or without (fabp10) 100 µm DEM and analyzed by WISH before sectioning. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the liver. Asterisk denotes the basal expression in the intestine. Scale bar, 100 µm.
(TIF)
Induction of Nrf2 target genes by sulforaphane. 5-dpf larvae were treated with or without 40 µM sulforaphane for indicated hours and expression of seven Nrf2 target genes was analyzed by WISH. Lateral and ventral views. Red and white arrowheads indicate positive and negative expression, respectively, of each gene in the nose, gill and liver. Asterisks denote basal expression in the intestine.
(TIF)
Oligonucleotide primers used for RT-PCR analyses.
(DOC)
Oligonucleotide primers used for plasmid construction.
(DOC)
Identification of DEM-inducible genes in zebrafish (1).
(DOC)
Identification of DEM-inducible genes in zebrafish (2).
(DOC)